Abstract
TLR2 plays a central role in the activation of innate immunity in response to Ft, the causative agent of tularemia. We reported previously that Ft LVS elicited strong, dose-dependent NF-κB reporter activity in TLR2-expressing human embryo kidney 293 T cells and that Ft LVS-induced murine macrophage proinflammatory cytokine gene and protein expression is TLR2-dependent. We demonstrated further that Ft can signal through TLR2 from within the phagosome and that phagosomal retention of Ft leads to greatly increased expression of a subset of proinflammatory genes. The two adaptor proteins associated with TLR2-mediated signaling are MyD88 and TIRAP. Although MyD88 is absolutely required for the Ft-induced macrophage cytokine response, the requirement for TIRAP can be overcome through retention of Ft within the phagosome. TIRAP-independent signaling was observed whether Ft was retained in the phagosome as a result of bacterial mutation (LVSΔiglC) or BFA-mediated inhibition of phagosome acidification. The requirement for TIRAP in TLR2 signaling could also be overcome by increasing the concentrations of synthetic bacterial TLR2 agonists. Taken together, these data suggest that prolonging or enhancing the interaction between TLR2 and its agonist overcomes the “bridging” function ascribed previously to TIRAP.
Keywords: macrophage, cytokine, bacterial infection, TLR2, MyD88
Introduction
Ft, the etiologic agent of tularemia [1,2,3], is an aerobic, nonspore-forming, Gram-negative coccobaccillus, that replicates in macrophages and other host cells after it escapes from the phagosome [4, 5]. Ft is classified as a Category A agent as a result of its ability to be aerosolized, extremely low infectious dose, and potential to cause severe morbidity and mortality [3]. There is currently no licensed vaccine against tularemia. In the 1930s, a live attenuated Type B strain was used to vaccinate over 50 million people in the former Soviet Union and resulted in a greatly reduced incidence of tularemia in endemic areas [6, 7]. Ft LVS was derived from the original Soviet vaccine, and in 1956, ampules of Ft LVS were transferred to the United States [6, 8]. Although vaccination with Ft LVS has been used to protect against laboratory-acquired tularemia [9], the Ft LVS strain remains unlicensed in the United States, as the molecular basis for its attenuation is unknown [10], and Ft LVS confers only partial protection against some forms of WT infections [11, 12]. Although attenuated for humans, Ft LVS is highly virulent in mice by some routes of infection and causes a disease that resembles human tularemia [13].
The innate immune response provides pathogen-specific recognition that ultimately shapes the adaptive immune response. Germline-encoded host receptors recognize evolutionarily conserved structures that are present in microbes but not in the host [14]. Several structurally related “families” of receptors have been described, e.g., the membrane-anchored TLRs, the cytosolic nucleotide-binding domain, leucine-rich repeat-containing family (or nucleotide-binding oligomerization domain-like receptors), and the cytosolic retinoic acid-inducible gene I-like receptors (reviewed in refs. [15, 16]). We and others [17,18,19,20,21] have reported previously that TLR2 plays a central role in the recognition of Ft and is required for control of pulmonary Ft infection [22]. We reported recently that Ft signals through TLR2 from within the phagosome [23] and that macrophages infected with LVSΔiglC, a Ft LVS mutant that fails to escape from the phagosome, display a greatly increased expression of a subset of TLR2-dependent, proinflammatory genes [23]. However, another subset of TLR2-dependent genes was induced poorly by infection of macrophages with LVSΔiglC. Expression of this gene subset required both TLR2-dependent signaling and intracytosolic recognition of the bacteria [23]. The intracytosolic “sensor” leading to this additional signaling pathway has yet to be defined.
TLR2-dependent signaling has been associated with two adapter proteins: TIRAP, also called MyD88 adapter-like, and MyD88, which was the first adapter protein found to be essential for TLR-mediated NF-κB activation [24]; TIRAP has been proposed to function as a “bridging” adaptor for MyD88 [25, 26]. TIRAP localizes to the plasma membrane through its interaction with PIP2, where it facilitates the interaction of MyD88 with TLR2 or TLR4 upon ligand-mediated activation [25, 26]. Although MyD88 is used by all known TLRs except TLR3, TIRAP functions as a “bridging adaptor” for MyD88 recruitment to TLR2 and TLR4 only (reviewed in refs. [27, 28]).
The data presented herein demonstrate TIRAP-independent, TLR2-mediated signaling that is achieved by retention of Ft within the phagosome. TLR2-dependent, TIRAP-independent signaling can also be achieved by increasing the concentration of synthetic bacterial TLR2 agonists. These data strongly suggest the possibility that sustained TLR2 engagement by an agonist can overcome the requirement for TIRAP.
MATERIALS AND METHODS
Mice
WT C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). MyD88−/− and TIRAP−/− mice on a C57BL/6 background were a kind gift of Dr. Shuzio Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) and were bred at the University of Maryland Baltimore (Baltimore, MD, USA) and the University of Massachusetts Medical School (Worcester, MA, USA), respectively. Peritoneal macrophages were isolated from mice after i.p. administration of sterile thioglycollate and cultured as described [18]. Briefly, macrophages were plated in six-well plates (4×106 cells/well). After overnight incubation, cells were washed with PBS to remove nonadherent cells and were cultured in RPMI 1640, supplemented with 2% FBS and 2 mM L-glutamine as well as antibiotics when indicated. Treatments were carried out in duplicate. All animal experiments were conducted with institutional approval.
Reagents
BFA (EMD Chemicals, Germany) was dissolved in DMSO (vehicle) and used at a final concentration of 25 nM BFA. The final concentration of DMSO in the tissue-culture media was 0.1%. Cells were treated with DMSO only or BFA 1 h prior to infection of macrophages with Ft. Escherichia coli K235 LPS was prepared by a modification of the method of McIntire et al. [29] using two rounds of hot phenol-water extraction. The synthetic TLR2/1 ligand, Pam3Cys, and the synthetic TLR2/6 ligand, Pam2Cys, were purchased from EMC Microcollections (Germany).
Bacteria
Frozen aliquots of Ft LVS (ATCC 29684) were prepared as described [30]. Ft LVS was grown in Mueller Hinton broth (BD, Franklin Lakes, NJ, USA) supplemented with 1% IsoVitaleX (Becton Dickinson), 0.1% glucose, and Ferric PPi (Sigma-Aldrich, St. Louis, MO, USA). MHA was used as solid culture media [31,32,33,34]. Construction of LVSΔiglC, a Ft LVS mutant, in which both copies of iglC were deleted, was described previously [23].
Real-time PCR
Total RNA extraction from macrophage cultures and real-time PCR were carried out as described previously [18].
Western analysis
After washing cells twice in PBS, whole cell lysates of macrophages were obtained by the addition of lysis buffer (20 mM HEPES, 0.5% Triton X-100, 150 mM NaCl, 10 mM NaF, 1 mM PMSF). Cell lysates were clarified by centrifugation at 4°C for 10 min at 14,000 g. Total protein was boiled for 5 min in Laemmli buffer before being separated by electrophoresis in denaturing 10% SDS-PAGE in Tris/glycine/SDS buffer (25 mM Tris, 250 mM glycine, 0.1% SDS; Bio-Rad, Hercules, CA, USA). Proteins were then electrotransferred onto Immobilon-P transfer membranes (Millipore, Billerica, MA, USA) at 100 V for 1.5 h (4°C). After blocking for 1 h in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat milk, membranes were washed three times in TBST and probed overnight at 4°C with anti-ERK, anti-pERK, anti-pAKT, anti-pp65, or anti-pp38 (Cell Signaling Technology, Danvers, MA, USA), according to the manufacturer’s instructions. Blots were washed three times with TBST before being incubated with appropriate HRP-conjugated secondary antibody (Jackson Immunochemicals, West Grove, PA, USA). Blots were developed following incubation in ECL Plus Western blotting detection reagent (Cell Signaling Technology).
Peritoneal macrophage intracellular bacteria assay
Intracellular survival of Ft LVS was evaluated in thioglycollate-elicited peritoneal macrophages. Cells were infected with Ft LVS at a MOI of ∼40 for 2 h. After washing twice with PBS, infected cells were incubated for 45 min in media containing 50 μg/ml gentamicin (Sigma-Aldrich) to kill extracellular bacteria. Cells were washed twice with PBS and then incubated with medium. The time of the addition of the bacteria was defined as the zero time-point. At the indicated time-points, cells were washed twice with PBS prior to being lysed in 1 ml ice-cold 0.02% SDS (Teknova, Hollister, CA, USA) in PBS. Two to four replicates were examined at each time-point. Lysates were serially diluted and plated on MHA plates.
RESULTS
Infection of macrophages with LVSΔiglC leads to prolonged activation of downstream signaling intermediates
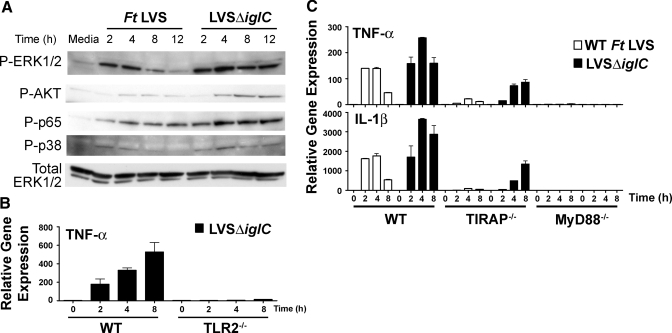
We showed previously that Ft-induced macrophage proinflammatory gene expression was wholly TLR2-dependent [17, 18], and using confocal microscopy, we demonstrated that phagosomal Ft LVS colocalized with TLR2 and MyD88 [17]. This suggested that Ft LVS was capable of intraphagosomal TLR2-mediated signaling. To confirm and extend this observation, primary peritoneal macrophages were infected with LVSΔiglC, a Ft LVS mutant that is retained within the phagosome [35, 36]. We hypothesized that if Ft LVS were capable of signaling from within the phagosome, retention of Ft in the phagosome would lead to greatly increased expression of TLR2-dependent, proinflammatory genes. Indeed, infection of macrophages with LVSΔiglC led to greatly increased expression of a subset of TLR2-dependent, proinflammatory genes [23] but sharply blunted expression of some TLR2-dependent genes whose expression also depends on activation of cytosolic pathways [23, 37, 38]. Figure 1A extends these findings by showing that phosphorylation of ERK-1/2, AKT, p65, and p38 was prolonged and/or enhanced in macrophages infected with LVSΔiglC compared with macrophages infected with WT Ft LVS. Thus, phagosomal retention of Ft leads to prolonged TLR2-mediated activation of these pathways.
Figure 1.
Effect of bacterial retention in the phagosome on Ft LVS-induced signaling and proinflammatory gene expression in macrophages. (A) Western analysis of cell lysates from WT macrophages treated with media alone or infected with WT Ft LVS or LVSΔiglC (MOI=5) was performed using anti-pERK, anti-pAKT, anti-pp65, anti-pp38, and anti-ERK antibodies. Cell lysates were collected at the time-points indicated. Results are from a single representative experiment (n=3). (B) Peritoneal macrophages from WT C57BL/6J or TLR2−/− mice were infected with LVSΔiglC (MOI=5) for 0–8 h. At the indicated time-points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. TNF-α mRNA expression is reported as relative gene expression compared with peritoneal macrophage-exposed medium only. Results shown represent the arithmetic mean ± sem from a single experiment. (C) Peritoneal macrophages from WT C57BL/6J, TIRAP−/−, or MyD88−/− mice were infected with Ft LVS or LVSΔiglC (MOI=5) for 0–8 h. At the indicated time-points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared with peritoneal macrophage-exposed medium only. Results shown represent the arithmetic mean ± sem from three separate experiments.
To insure that the previously observed, proinflammatory response to LVSΔiglC was fully TLR2-dependent, TLR2−/− macrophages were infected with LVSΔiglC. Infection with LVSΔiglC failed to induce TNF-α mRNA (or other proinflammatory genes or their secreted products; data not shown) expression in TLR2−/− macrophages (Fig. 1B).
Role of TIRAP and MyD88 in Ft LVS-induced TLR2-dependent signaling
The intracellular adaptor proteins, MyD88 and TIRAP, have been shown previously to play central roles in TLR2-mediated signaling (reviewed in ref. [39]). As TLR2 plays a key role in Ft LVS-induced production of proinflammatory cytokines, we sought to evaluate the roles of TIRAP and MyD88. Macrophages from WT or TIRAP−/− mice were infected with WT Ft LVS or LVSΔiglC (MOI=5) for up to 8 h, and TNF-α gene expression was measured. In WT macrophages, infection with LVSΔiglC resulted in enhanced expression of TNF-α, as we reported previously [23]. Although the lack of TIRAP resulted in greatly mitigated proinflammatory gene expression in response to infection with WT Ft, infection with the LVSΔiglC mutant led to significant induction of TNF-α gene expression in TIRAP−/− macrophages (Fig. 1C, upper panel). A similar pattern of gene expression was observed for the induction of other proinflammatory mediators including IL-β (Fig. 1C, lower panel) and cyclooxygenase-2 (data not shown). These data indicate that intraphagosomal signaling through TLR2 can proceed in a TIRAP-independent manner. In contrast to TIRAP−/− macrophages, proinflammatory gene expression was abrogated completely in macrophages derived from MyD88−/− mice, regardless of whether they were infected with WT Ft or LVSΔiglC (Fig. 1C). This indicates that the requirement for MyD88 is absolute, as the lack of this adaptor molecule could not be overcome by retention of Ft within the phagosome.
Uptake of Ft LVS by macrophages is not dependent on MyD88
Blander and Medzhitov [40] reported previously that bacterial phagocytosis was impaired in MyD88−/− and TLR2−/− × TLR4−/− macrophages. If this were also the case for Ft, then the observed failure of MyD88−/− and TLR2−/− macrophages to signal might be attributable to decreased bacterial uptake. Therefore, Ft LVS uptake by WT and MyD88−/− thioglycollate-elicited peritoneal macrophages was compared. In contrast to the findings of Blander and Medzhitov [40], macrophage uptake of Ft LVS was not statistically different in WT and MyD88−/− macrophages at 165 min (P=0.154) or 345 min (P=0.216) postinfection (Fig. 2). These data support an earlier report that WT and MyD88−/− bone marrow-derived macrophages exhibit comparable Ft LVS uptake and intracellular replication [41] and that no difference exists in the kinetics of Ft uptake by TLR2−/− and TLR2+/+ bone marrow-derived macrophages [22]. Taken together, these data indicate that the lack of a Ft-induced cytokine response in the MyD88−/− and TLR2−/− macrophages results from a signaling failure, rather than a defect in bacterial uptake.
Figure 2.
MyD88 is not required for macrophage uptake of Ft LVS. Peritoneal macrophages from WT C57BL/6J or MyD88−/− mice were infected with Ft LVS (MOI=40) for 2 h. After a subsequent 45-min incubation in medium containing 50 μg/ml gentamicin, infected cells were washed twice with PBS and then incubated with media alone. Cells were lysed, and the indicated times and lysates were serially diluted and plated on MHA plates. Data are presented as the mean ± sem of two to four replicates per time-point. A Student’s t-test revealed that bacterial uptake was not significantly different at 165 min (P=0.154) or 345 min (P=0.216) postinfection. Results represent a single representative experiment of three experiments of similar design and outcome.
Inhibition of phagosome acidification by BFA increases the macrophage proinflammatory response to WT Ft LVS and overcomes the requirement for TIRAP
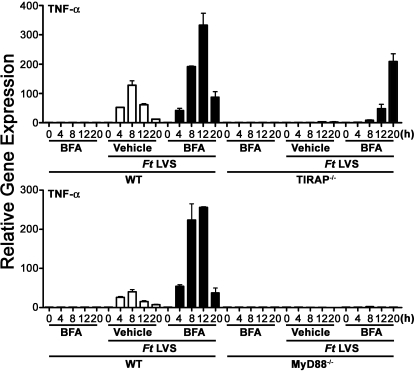
A recent paper by Santic et al. [42] reported that inhibition of phagosome acidification by BFA blocks rapid escape of Ft subsp. novicida from the phagosome into the cytosol. The authors concluded that transient phagosome acidification is essential for Ft escape into the cytosol. We therefore hypothesized that inhibition of phagosome acidification by BFA would mimic the effect of the ΔiglC mutation and preclude the organisms from escaping from the phagosome into the cytosol, thereby enabling a TLR2-dependent, TIRAP-independent macrophage proinflammatory response by WT Ft LVS. To test this hypothesis, macrophages derived from WT, TIRAP−/−, or MyD88−/− mice were pretreated with the vehicle, DMSO, or BFA for 1 h prior to infection with WT Ft (MOI=5). Compared with cells pretreated with vehicle only, macrophage pretreatment with BFA increased Ft-induced TNF-α mRNA dramatically in WT and TIRAP−/− macrophages (Fig. 3), and these results were similar to that observed upon infection of macrophages with LVSΔiglC. Although DMSO pretreatment of macrophages had no impact on Ft-induced IFN-β mRNA, pretreatment of macrophages with BFA completely inhibited Ft-induced macrophage expression of IFN-β mRNA in WT macrophages (data not shown). As phagosomal escape is required for Ft-induced macrophage production of IFN-β [23], this finding confirms that BFA pretreatment precludes bacterial escape from the phagosome into the cytosol. Importantly, BFA alone did not enhance macrophage expression of TNF-α significantly. Similar to the results shown in Figure 1C, BFA pretreatment did not enable Ft LVS-induced TNF-α mRNA expression in MyD88−/− macrophages (Fig. 3). This finding confirms that the requirement for MyD88 is absolute and cannot be overcome through bacterial retention in the phagosome.
Figure 3.
BFA treatment of macrophages enables Ft LVS-induced macrophage proinflammatory gene expression to TIRAP−/− but not MyD88−/− macrophages. Peritoneal macrophages from WT C57BL/6J, TIRAP−/−, or MyD88−/− mice were exposed to vehicle alone (open bars) or 25 nM BFA (solid bars) for 1 h prior to infection with Ft LVS (MOI=5) for 0–20 h. An additional control group of cells was exposed to 25 nM BFA for 1 h, followed by treatment with medium only. At the indicated time-points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared with peritoneal macrophages exposed to medium with vehicle only. Results represent a single representative experiment of two experiments of similar design and outcome.
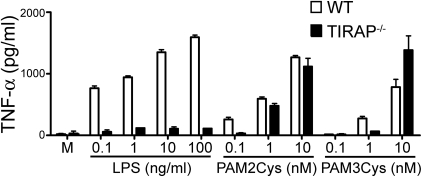
In an early study by Yamamoto et al. [25], the role of TIRAP in TLR2 signaling was established by exposing peritoneal macrophages from WT and TIRAP−/− mice to 3–30 ng/ml of the TLR2 agonist, MALP-2 [25]. Although macrophages from WT mice displayed dose-dependent production of IL-6, TNF-α, and IL-12 in response to MALP-2, macrophages from TIRAP−/− mice exhibited greatly impaired MALP-2-induced cytokine production [25]. As our Ft results indicated that increasing the interaction between Ft and TLR2 through retention in the phagosome overcomes the requirement for TIRAP, we sought to examine the effect that exposing peritoneal macrophages to high concentrations of the synthetic bacterial TLR2 agonists Pam2Cys and Pam3Cys would have on the requirement for TIRAP. In the case of both synthetic agonists, increasing their concentration eliminated the requirement for TIRAP for TNF-α secretion (Fig. 4). These data confirm and extend the findings of Santos-Sierra et al. [43], who reported recently that the requirement for TIRAP was eliminated at higher concentrations of Pam3Cys, and Kenny et al. [44], who demonstrated that TIRAP was not required for IL-6 induction or NF-κB activation at high concentrations of Pam3Cys or MALP-2. In contrast, TLR4-mediated, MyD88-dependent signaling is fully TIRAP-dependent, and this requirement could not be overcome by increasing the concentration of E. coli LPS (Fig. 4). Thus, TIRAP is required for TLR2-mediated signaling, only when the concentration of the TLR2 ligand is low. Moreover, this concentration-dependent effect occurred whether TLR2 formed a heterodimer with TLR1 (Pam3Cys) or TLR6 (Pam2Cys).
Figure 4.
Increasing the concentration of synthetic TLR2 agonists eliminates TIRAP dependence. Peritoneal macrophages from WT C57BL/6J or TIRAP−/− mice were exposed to media (M) or varying concentrations of E. coli LPS, Pam2Cys, and Pam3Cys. After 16 h, supernatants were collected and analyzed for TNF-α using ELISA. Data are presented as the mean ± sem (n=2).
DISCUSSION
Signaling adaptor proteins, MyD88 and TIRAP, have been shown previously to play central roles in TLR2-mediated signaling (reviewed in ref. [39]). TIRAP associates with the plasma membrane through PIP2 and has been proposed to serve as a bridging adaptor that recruits MyD88 to the growing TLR2 or TLR4 receptor complex (reviewed in refs. [27, 28]). To assess the roles of these adapters in the innate immune response to Ft, we infected WT, TIRAP−/−, and MyD88−/− macrophages with WT Ft LVS and LVSΔiglC. Neither strain induced proinflammatory gene expression in MyD88−/− macrophages, indicating an absolute reliance on MyD88 for Ft-dependent signaling. In contrast, although WT Ft LVS induced little TLR2-dependent, proinflammtory gene expression in TIRAP−/− macrophages, infection with LVSΔiglC, a mutant that is retained in the phagosome, induced strong proinflammatory gene expression (Fig. 1C). This suggested that prolonging or enhancing the contact between an agonist and TLR2 could overcome the requirement for TIRAP in TLR2 signaling.
There are other possible explanations for the MyD88-dependent, TIRAP-independent macrophage proinflammatory signaling observed after LVSΔiglC infection. One possible explanation is that when the Ft is retained within the phagosome, the bacteria are able to engage a receptor other than TLR2, which is MyD88-dependent but TIRAP-independent. Although MyD88 is used by all known TLRs except TLR3, TIRAP functions as a bridging adaptor for TLR2 and TLR4 only (reviewed in refs. [27, 28]). It was shown recently that TLR7-mediated recognition of group B streptococcus occurs within phagolysosomes of conventional dendritic cells and leads to production of IFN-β [45]. When we first identified TLR2 as essential for Ft-mediated signaling, we found that human embryo kidney 293 T cells expressing TLR2, but not other TLRs, responded [18]. Nevertheless, to exclude the role of other receptors in Ft-induced intraphagosomal signaling, we infected TLR2−/− macrophages with LVSΔiglC. The lack of TLR2 eliminated macrophage proinflammatory gene expression indicating that LVSΔiglC-induced cytokine production was entirely TLR2-dependent. It is also possible that IL-1 or IL-18 acts in an autocrine manner to drive the observed increase in MyD88-dependent, proinflammatory gene expression. Although we have shown previously that the level of IL-1 mRNA is increased greatly by retention of Ft within the phagosome, no secreted protein could be detected [23]. Processing of IL-1 and IL-18 into mature, secreted proteins is dependent on the activation of the inflammasome, which in turn, recruits and activates caspase-1 [46,47,48,49]. Thus, it is unlikely that the observed MyD88-dependent increase in the proinflammatory response induced by the LVSΔiglC mutant or by WT Ft after BFA pretreatment of macrophages is a result of autocrine action of IL-1 or IL-18, since IFN-β and therefore, active IL-1β are not produced in response to infection with phagosomally retained bacteria [23].
Blander and Medzhitov [40] reported previously that bacterial phagocytosis was impaired in MyD88−/− and TLR2−/− × TLR4−/− macrophages. However, in contrast to these findings, uptake of Ft LVS by MyD88−/− (Fig. 2) and TLR2−/− [22] macrophages was not impaired. Although we do not at this time understand why uptake of Ft is MyD88-independent, this finding is important, since it excludes the possibility that impaired signaling seen in the MyD88−/− macrophages is the consequence of impaired bacterial uptake.
Santic et al. [42] reported recently that inhibition of phagosome acidification by BFA blocks rapid escape of Ft subsp. novicida from the phagosome into the cytosol. This finding offered an alternate approach for examining bacterial retention within the phagsome distinct from bacterial mutation. Compared with cells pretreated with the vehicle only, pretreatment with BFA increased Ft-induced TNF-α mRNA dramatically in WT and TIRAP−/− macrophages (Fig. 3). BFA treatment induced a cytokine response to WT Ft LVS, which was similar to that seen after infection of WT and TIRAP−/− macrophages with LVSΔiglC; while BFA-treated cells responded to WT Ft LVS with increased signaling, BFA pretreatment failed to overcome the requirement for MyD88 for Ft LVS-induced proinflammatory gene expression. Thus, retention of the bacteria in the phagosome, achieved by deletion of the iglC genes or pretreatment with an agent that blocks acidification of the phagosome, enhances TLR2-dependent gene expression, possibly by sustaining the interaction of Ft with TLR2, even in TIRAP-deficient cells. Therefore, our data support the hypothesis that prolongation of the interaction between TLR2 and its Ft agonist in the phagosome overcomes the requirement for TIRAP in TLR2-dependent signaling by creating signaling “platforms” that are permissive for MyD88 recruitment without the bridging function normally ascribed to TIRAP.
Finally, we reasoned that increasing the concentration of synthetic TLR2 agonists might similarly increase or sustain the period over which TLR2 was engaged. Similar to a previous report [25], lower concentrations of Pam2Cys and Pam3Cys induced TNF-α secretion, which was entirely TIRAP-dependent; however, at higher concentrations of synthetic ligands, TNF-α secretion was equivalent in WT and TIRAP−/− macrophages. These observations indicate that for the TLR2/1 or TLR2/6 dimers, TIRAP independence can be achieved by increasing the concentration of ligand and imply that the interaction of MyD88 with TLR2 can be achieved even without facilitation by TIRAP. In contrast, LPS signaling through TLR4 was TIRAP-dependent at all concentrations tested. This suggests that in the absence of TIRAP, TLR4 is unable to recruit MyD88, and the bridging function ascribed to TIRAP previously, although dispensable at higher ligand concentrations for TLR2, is essential for TLR4-mediated signaling. Our findings also support previous studies that suggest that MyD88 interacts through different protein TIR domain surfaces with TLR2 and TLR4 [50,51,52].
In conclusion, this study provides new insights into the roles that TIRAP and MyD88 play in the macrophage response to the intracellular pathogen Ft LVS and synthetic bacterial agonists. Low concentrations of Pam2Cys and Pam3Cys, as well as WT Ft LVS (that escapes rapidly from the phagolysosome into the cytosol), require TIRAP and MyD88 for induction of macrophage proinflammatory gene expression. However, high concentrations of synthetic TLR2 agonists or retention of Ft LVS in the phagosome restored proinflammatory signaling in TIRAP−/− macrophages. The requirement for MyD88 was absolute and could not be overcome by retention of the bacteria in the phagosome. These findings suggest that under conditions that favor prolonged or enhanced interaction of TLR2 and its agonist, MyD88 recruitment occurs in the absence of TIRAP.
AUTHORSHIP
L. E. C., K. A. F., and S. N. V. carried out study design; L. E. C., M. H. W. L, A. Seekatz., Z. J., K. A. S., and K. A. F. performed experiments; A. Santiago., and E. B. provided crucial reagents; and L. E. C., K. A. F., and S. N. V. prepared the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health, National Institute of Allergy and Infectious Diseases Mid-Atlantic Regional Center of Excellence, grant U54 AI-157168 (E. B. and S. N. V.), AI-1 8797 (S. N. V.), and AI-067497 (K. A. F.). The authors acknowledge the thoughtful advice of Dr. Karen Elkins and Dr. Swamy Polumuri.
Footnotes
Abbreviations: BFA=bafilomycin A1, Ft=Francisella tularensis, iglC=intracellular growth locus C, LVS=live vaccine strain, MALP-2=macrophage-activating lipopeptide-2, MHA=Mueller Hinton agar, MOI=multiplicity of infection, P=phospho, Pam2Cys=S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-Cys-Ser-Lys4-OH, Pam3Cys=S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-Ser-Lys4-OH, PIP2=phosphatidylinositol 4,5 bisphosphate, TIRAP=Toll/IL-1R domain-containing adaptor protein, WT=wild-type
References
- Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- Ellis J, Oyston P C, Green M, Titball R W. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D T, Inglesby T V, Henderson D A, Bartlett J G, Ascher M S, Eitzen E, Fine A D, Friedlander A M, Hauer J, Layton M, Lillibridge S R, McDade J E, Osterholm M T, O'Toole T, Parker G, Perl T M, Russell P K, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H, Golovliov I, Baranov V, Ernst R K, Telepnev M, Sjostedt A. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53:953–958. doi: 10.1099/jmm.0.45685-0. [DOI] [PubMed] [Google Scholar]
- Cross A S, Calia F M, Edelman R. From rabbits to humans: the contributions of Dr. Theodore E. Woodward to tularemia research. Clin Infect Dis. 2007;45:S61–S67. doi: 10.1086/518150. [DOI] [PubMed] [Google Scholar]
- Sjostedt A, Tarnvik A, Sandstrom G. Francisella tularensis: host-parasite interaction. FEMS Immunol Med Microbiol. 1996;13:181–184. doi: 10.1111/j.1574-695X.1996.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Sandstrom G. The tularaemia vaccine. J Chem Technol Biotechnol. 1994;59:315–320. doi: 10.1002/jctb.280590402. [DOI] [PubMed] [Google Scholar]
- Burke D S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Titball R W, Petrosino J F. Francisella tularensis genomics and proteomics. Ann N Y Acad Sci. 2007;1105:98–121. doi: 10.1196/annals.1409.015. [DOI] [PubMed] [Google Scholar]
- Hornick R B, Eigelsbach H T. Aerogenic immunization of man with live tularemia vaccine. Bacteriol Rev. 1966;30:532–538. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrumb F R. Aerosol infection of man with Pasteurella tularensis. Bacteriol Rev. 1961;25:262–267. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K L, Cowley S C, Bosio C M. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Koyama S, Ishii K J, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve D A, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Cole L E, Shirey K A, Barry E, Santiago A, Rallabhandi P, Elkins K L, Puche A C, Michalek S M, Vogel S N. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75:4127–4137. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L E, Elkins K L, Michalek S M, Qureshi N, Eaton L J, Rallabhandi P, Cuesta N, Vogel S N. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–6899. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- Katz J, Zhang P, Martin M, Vogel S N, Michalek S M. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakran S, Li H, Lavine C L, Miller M A, Bina J E, Bina X R, Re F. Identification of Francisella tularensis lipoproteins that stimulate the Toll-like receptor (TLR) 2/TLR1 heterodimer. J Biol Chem. 2008;283:3751–3760. doi: 10.1074/jbc.M706854200. [DOI] [PubMed] [Google Scholar]
- Li H, Nookala S, Bina X R, Bina J E, Re F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukoc Biol. 2006;80:766–773. doi: 10.1189/jlb.0406294. [DOI] [PubMed] [Google Scholar]
- Malik M, Bakshi C S, Sahay B, Shah A, Lotz S A, Sellati T J. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L E, Santiago A, Barry E, Kang T J, Shirey K A, Roberts Z J, Elkins K L, Cross A S, Vogel S N. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol. 2008;180:6885–6891. doi: 10.4049/jimmunol.180.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signaling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Kagan J C, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Brikos C, O'Neill LA. Signalling of Toll-like receptors. Handb Exp Pharmacol. 2008;2008:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- Kenny E F, O'Neill L A. Signaling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- McIntire F C, Sievert H W, Barlow G H, Finley R A, Lee A Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Elkins K L, Winegar R K, Nacy C A, Fortier A H. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- Santiago A E, Cole L E, Franco A, Vogel S N, Levine M M, Barry E M. Characterization of rationally attenuated Francisella tularensis vaccine strains that harbor deletions in the guaA and guaB genes. Vaccine. 2009;27:2426–2436. doi: 10.1016/j.vaccine.2009.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C N, Hollis D G, Thornsberry C. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985;22:212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A H, Slayter M V, Ziemba R, Meltzer M S, Nacy C A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K L, Leiby D A, Winegar R K, Nacy C A, Fortier A H. Rapid generation of specific protective immunity to Francisella tularensis. Infect Immun. 1992;60:4571–4577. doi: 10.1128/iai.60.11.4571-4577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X H, Golovliov I, Sjostedt A. Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb Pathog. 2004;37:225–230. doi: 10.1016/j.micpath.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Santic M, Molmeret M, Klose K E, Jones S, Kwaik Y A. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol. 2005;7:969–979. doi: 10.1111/j.1462-5822.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss D S, Dixit V M, Monack D M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Brotcke A, Weiss D S, Thompson L J, Monack D M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill L A, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J Endotoxin Res. 2003;9:55–59. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- Blander J M, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Collazo C M, Sher A, Meierovics A I, Elkins K L. Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect. 2006;8:779–790. doi: 10.1016/j.micinf.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect Immun. 2008;76:2671–2677. doi: 10.1128/IAI.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sierra S, Deshmukh S D, Kalnitski J, Kuenzi P, Wymann M P, Golenbock D T, Henneke P. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 2009;28:2018–2027. doi: 10.1038/emboj.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny E F, Talbot S, Gong M, Golenbock D T, Bryant C E, O'Neill L A. MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J Immunol. 2009;183:3642–3651. doi: 10.4049/jimmunol.0901140. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- Kostura M J, Tocci M J, Limjuco G, Chin J, Cameron P, Hillman A G, Chartrain N A, Schmidt J A. Identification of a monocyte specific pre-interleukin 1 β convertase activity. Proc Natl Acad Sci USA. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R A, Kronheim S R, Sleath P R. Activation of interleukin-1 β by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Cerretti D P, Kozlosky C J, Mosley B, Nelson N, Van Ness K, Greenstreet T A, March C J, Kronheim S R, Druck T, Cannizzaro L A. Molecular cloning of the interleukin-1 β converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Dunne A, Ejdeback M, Ludidi P L, O'Neill L A, Gay N J. Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adaptors Mal and MyD88. J Biol Chem. 2003;278:41443–41451. doi: 10.1074/jbc.M301742200. [DOI] [PubMed] [Google Scholar]
- Toshchakov V U, Basu S, Fenton M J, Vogel S N. Differential involvement of BB loops of Toll-IL-1 resistance (TIR) domain-containing adapter proteins in TLR4- versus TLR2-mediated signal transduction. J Immunol. 2005;175:494–500. doi: 10.4049/jimmunol.175.1.494. [DOI] [PubMed] [Google Scholar]
- Toshchakov V Y, Fenton M J, Vogel S N. Cutting edge: differential inhibition of TLR signaling pathways by cell-permeable peptides representing BB loops of TLRs. J Immunol. 2007;178:2655–2660. doi: 10.4049/jimmunol.178.5.2655. [DOI] [PubMed] [Google Scholar]