Abstract
Since brain metabolism is accompanied by heat production, measurement of brain temperature offers a method for assessing global alterations in metabolic neural activity. This approach, high-resolution (5-s bin) temperature recording from the nucleus accumbens (NAcc), temporal muscle, and facial skin, was used to study motivated drinking behavior in rats. Experienced animals were presented with a cup containing 5-ml of Coca-Cola® (Coke) beverage that resulted, within certain latencies, in initiation of a continuous chain of licking until all liquid was fully consumed. While cup presentation induced rapid, gradual NAcc temperature increase peaking at the start of drinking, temperatures slowly decreased during Coke consumption, but phasically increased again in the post-consumption period when rats were hyperactive, showing multiple interactions with an empty cup. Muscle temperatures followed a similar pattern, but the changes were weaker and delayed compared to those in the brain. Skin temperature rapidly dropped after cup presentation, steadily maintained at low levels during consumption, and slowly restored during the post-consumption period. Substitution of the expected Coke with either sugar-free Diet Coke® or water resulted in numerous drinking attempts but ultimately no consumption. During these tests, locomotor activation was much greater and more prolonged, brain and muscle temperatures increased monophasically, and their elevation was significantly greater than that with regular Coke tests. Food deprivation decreased drinking latencies, did not change the pattern of temperature fluctuations during Coke consumption, but temperature elevations were greater than in controls. Our data suggest sustained neural activation triggered by appetitive stimuli and associated with activational (seeking) aspects of appetitive motivated behavior. This seeking-related activation is rapidly ceased following consumption, suggesting this change as a neural correlate of reward. In contrast, inability to obtain an expected reward maintains neural activation and seeking behavior, resulting in larger deviations in physiological parameters.
Keywords: brain metabolism, brain temperature, vasoconstriction, neural activation, appetitive motivated behavior, consumption, reward
1. Introduction
While it is often believed that brain temperature is a stable, tightly regulated homeostatic parameter, relatively rapid and strong brain temperature fluctuations have been reported in animals following exposure to biologically relevant environmental stimuli and during different types of motivated behavior (Abrams and Hammel, 1964; Delgado and Hanai, 1966; Hayward and Baker, 1968; Kiyatkin, 2005). Brain temperature is also an important index of CNS activity state, varying ~4°C within the normal physiological continuum. For example, in male rats, temperature in the nucleus accumbens (NAcc), a ventrally located deep brain structure, falls tonically to 34.5–35.0°C during deep sleep in highly habituated conditions (Smirnov and Kiyatkin, 2008) and peaks phasically above 39.5°C at the ejaculation points during copulatory behavior (Kiyatkin and Mitchum, 2003). Although temperature fluctuations analyzed on a slow time scale are generally parallel in various brain structures and accompanied by similar fluctuations in body temperature, the brain sites show several important differences in high-speed temperature dynamics, suggesting metabolic neural activity as its major contributor (Kiyatkin, 2009). Although cerebral circulation is the primary means for heat dissipation from the brain, thus affecting brain temperatures, heat cannot be brought to the brain from the periphery because the temperature of arterial blood supply is always lower than brain tissue temperature (Feitelberg and Lampl, 1935; Hayward and Baker, 1968; Kiyatkin et al., 2002; McElligott and Melzack, 1967; Nybo et al., 2002).
Despite a classic finding of a ~1°C brain temperature increase during food consumption in hungry dogs and its attenuation during satiety (Schiff, 1872; cited by James, 1892) and William James' pioneering idea (1892) that brain temperature monitoring could be a valuable tool for correlating brain activity with psychic functions, the potential of this dynamic physiological parameter for studying behavior-associated alterations in neural activity has never been properly tested or recognized (see, however, Delgado and Hanai, 1966; McElligott and Melzack, 1967; Moser et al., 1993; Moser and Mathiesen, 1996). In our previous thermorecording studies of cocaine self-administration, we found that brain temperature increases following exposure to cocaine-related sensory cues and during drug-seeking behavior but decreases transiently after intravenous (iv) drug infusion (Kiyatkin and Brown, 2003, 2004). While these data could be interpreted as a reflection of metabolic neural activation associated with drug-seeking behavior and a rapid, transient interruption of this activation as a neural correlate of cocaine reward, the fine dynamics of temperature fluctuations associated with natural motivated behavior have not been examined previously. In this study, high-speed thermorecording from the NAcc, nonlocomotor head muscle (musculus temporalis) and facial skin, together with conventional monitoring of locomotion, were employed to study motivated drinking behavior in male rats. The NAcc is a deep brain structure that is heavily implicated in regulation of motivational processes (Parent and Hazrati, 1995; Wise and Bozarth, 1987). Moreover, this structure has been used in our previous thermorecording studies of feeding, sexual, and drug-taking behaviors, allowing a comparison of common features and differences in brain temperature fluctuations associated with different forms of motivated behavior.
As a basic model, we used presentation to and consumption of a palatable sugar-containing drink (caffeine-free Coca-Cola Classic® or Coke®). Naive rats learn rapidly to consume Coke following several days of exposure and this behavior is maintained for many consecutive daily sessions without water or food deprivation. Therefore, we first examined changes in temperatures and locomotor activity associated with a presentation of a Coke-filled container, subsequent drinking behavior, and removal of the empty container from the cage. Basic drinking sessions (regular Coke consumption) were inter-mixed with three unusual sessions, which were preceded and followed by regular Coke sessions; first, instead of regular Coke, rats were presented with an equal portion of caffeine- and calorie-free Diet Coke®; second, instead of regular Coke, rats were presented with an equal portion of water; third, similar to regular sessions, rats were presented with Coke but after 24-hour food deprivation.
Our immediate goal was to examine the pattern of central and peripheral temperature fluctuations and locomotor activity associated with different aspects of motivated drinking behavior and its variants associated with either the change in the nature of the reinforcer (water or Diet Coke vs. regular Coke) or in the animals' motivational state (hunger vs. satiety). Based on these data, our general goal was to learn more about basic mechanisms underlying motivated appetitive behavior.
2. Results
2.1. Data sample
Data shown in this study were obtained in six rats during 40 daily sessions (80 tests with beverage presentations). During five tests, we experienced technical complications with either temperature or locomotor recording; these data were excluded from group analyses. In addition to the six rats providing these data, four more rats were tested for Coke drinking in pilot experiments and rejected due to unstable drinking behavior. Two more rats underwent surgery, but were rejected from further testing because of problems in either temperature recording or drinking behavior.
2.2. Tonic temperature changes during motivated Coke-drinking behavior
All rats included in this data sample showed consistent Coke-drinking behavior following each of the two drink presentations during each experimental session. Within the whole group (n=33 Coke presentations during 18 sessions), latency to start drinking following the first Coke presentation varied between 20 and 105 s with a mean of 46.5±7.1 s and for the second Coke presentation varied between 15 and 310 s (mean 67.6±19.0 s). The duration of drinking also varied: between 105 and 335 s (mean 209.6±15.6 s) for the first drink and between 155 and 390 s (mean 238.1±17.9 s) for the second drink. While both the latency to start drinking and drinking duration were larger for the second drink, differences vs. the first drink were not statistically significant.
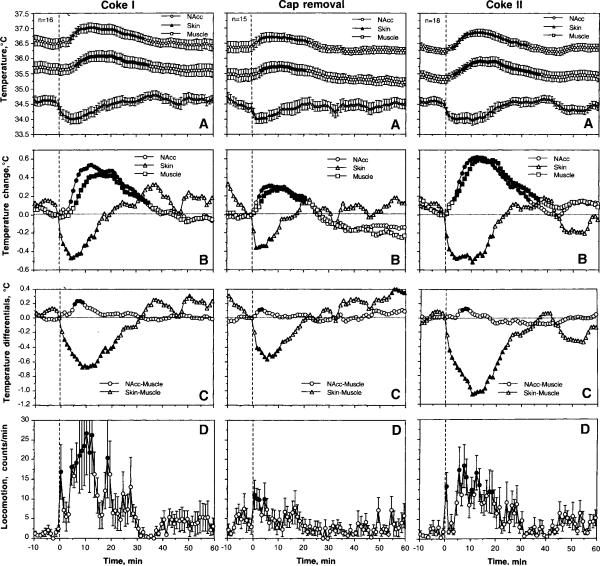
As can be seen in Fig. 1 (left panel), the presentation of a Coke-containing cup with subsequent Coke consumption caused significant increases in NAcc and muscle temperatures (~0.5°C), decreases in skin temperature (~0.5°C), and locomotor activation. Decreases in skin temperature were the most rapid, strong, and short-lived compared to increases in brain and muscle temperatures, which showed longer onset latencies and longer durations (B). Being analyzed with 1-min bins, mean temperatures in the NAcc and muscle first reached a statistical significance at 5 and 7 min, approximately at the time when all rats finished drinking (282 s or 4.7 min). In contrast, skin temperature became significantly lower than baseline at the second minute after cup presentation. As can be seen in Fig. 1B and C, temperature in the NAcc increased more rapidly and was stronger than in muscle, resulting in a significant increase in NAcc-muscle differential; this parameter peaked at 7–8 min, at the time when all rats finished drinking. Because of the opposite changes in the muscle and skin, the temperature differential between these locations became strongly negative for ~25 min after Coke presentation. Locomotor activation increased at the first minute after Coke presentation, decreased during the next three min during Coke consumption, but then increased again for ~15–20 min.
Fig. 1.
Mean changes in absolute (A) and relative temperatures (B), temperature differentials (C), and locomotion (D) following the first (left panel) and second (right panel) presentations of a Coke-filled cup, and removal of an empty cup (middle panel). n shows numbers of averaged tests and filled symbols show values significantly different from baseline. One-way ANOVA with repeated measures was used for statistical evaluation of temperatures and locomotion. F values for the first Coke test are: NAcc − F15,495=11.93; Skin − 6.50; Muscle − 11.79; locomotion − 1.56; at least p<0.05 each. F values for the second Coke test are: NAcc − F17,557=15.57; Skin − 9.00; Muscle − 14.14; locomotion − 1.82; at least p<0.01 each. F values cup removal are: NAcc − F14.464=6.36; Skin − 3.87; Muscle − 4.67; locomotion − 1.60; at least p<0.05 each.
The second Coke presentation followed by its consumption induced a similar pattern of temperature fluctuations and a similar biphasic locomotor response (Fig. 1, right panel). Slightly larger relative increases in brain and muscle temperature changes during the second Coke presentation (compare B on right and left panels) could be related to slightly lower basal temperatures at the moment of second cup presentation (A). However, these differences were not statistically significant.
Removal of an empty cup (after the first drink; see middle panel in Fig. 1) also induced a significant temperature response and locomotor activation, which were weaker in amplitude than those seen with full cup presentations. However, in contrast to full cap presentation, increases in brain and muscle temperatures were more rapid in this case and the locomotor response was monophasic.
NAcc temperature increases induced by both cup presentation and cup removal were dependent upon basal NAcc temperatures (Fig. 2). The increases were greatest at low basal temperatures and decreased progressively at higher basal temperatures (r=0.46 and 0.64 for cup presentation and removal, respectively; p<0.01). Both regression lines intersected with the line of no effect (=0), suggesting that temperature responses disappeared at high brain temperatures. These values of basal NAcc temperatures were higher for cup presentation (~38.8°C) than for cup removal (~37.75°C).
Fig. 2.
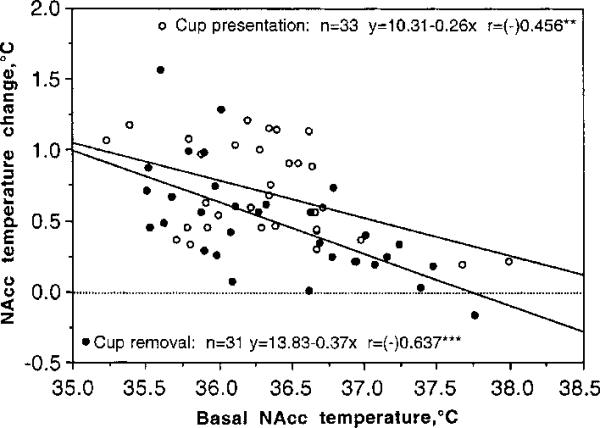
The relationships between basal NAcc temperatures and their changes induced by cup presentation and its removal. For this analysis, all tests (n) with Coke presentation and removal (two from each session) were combined, and peak increases induced by both stimuli were represented vs. basal temperatures. In each case, amplitude of temperature elevation was negatively dependent on basal temperature. Both regression lines intersect with the line of no effect (=0, hatched) at higher brain temperature (~37.75 and 38.80°C for cup removal and presentation, respectively). r is a coefficient of correlation, which is highly significant for both tests.
2.3. Phasic temperature fluctuations during motivated Coke-drinking behavior
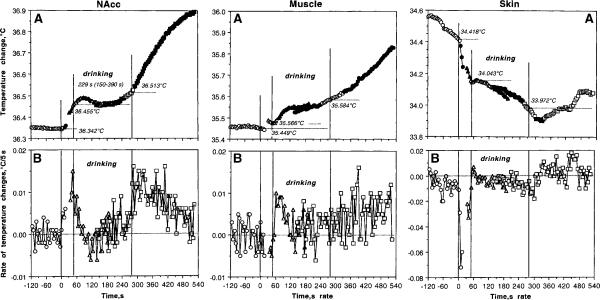
Figure 3 shows phasic changes in temperatures analyzed with respect to three events of motivated drinking behavior. For this analysis, both Coke tests (n=33) were combined and temperature dynamics were evaluated at 5-s time resolution with respect to the moments of cup presentation, initiation of drinking, and its end time. This fine time-course analysis was done for each recording location; both the changes in absolute temperature and rate of temperature changes (differences between each consecutive 5-s temperature values) are shown for 2 min before and 9 min after cup presentation (time=0 s).
Fig. 3.
Phasic changes in brain (left panel), muscle (central panel), and skin (right panel) temperatures associated with motivated drinking behavior. Top graphs (A) show absolute temperatures and bottom graphs (B) show rate of temperature changes. Three vertical lines in each graph mark the moments of full cup presentation, initiation of drinking behavior, and its end. Horizontal hatched lines in A show mean values of temperatures at the moment of each behavioral event. The hatched line in B shows zero change in temperature, i.e., no difference between each consecutive temperature value. Statistical evaluation was done separately for each of three events, with plus (following) and minus (preceding) time comparisons. Filled symbols show values statistically significant from reference points (either a 5-s value immediately preceding the start and end of drinking or the first 5-s value after these events). F values for cup presentation are: NAcc F32,164=4.88; p<0.001; Muscle=0.58, NS; Skin=52.42, p<0.001. F values for initiation of drinking: (1) preceding drinking onset: NAcc F32,164=36.46, p<0.001; Muscle=1.73, NS; Skin=16.18, p<0.001; (2) following drinking onset: NAcc F32,989=2.36; Muscle=15.05, Skin=4.28, each p<0.001. F values for changes following the end of drinking: (1) preceding end of drinking: NAcc F32,956=5.01; Muscle =6.34, Skin=9.44 (each p<0.001), p=0.59; skin-muscle differential =15.31 (p<0.001); (2) following drinking offset: NAcc F32,1022=57.13 (p<0.001), Muscle =20.17, Skin =2.93 (each p<0.001). While most changes are significant, note quantitative differences in F values that show the strength of the effect.
As can be seen, NAcc temperature began to increase rapidly following full cup presentation, becoming statistically significant at the third 5-s value (10–15 s). This increase continued gradually until the rat initiated drinking (see significant pre-drinking increase before the second vertical line in Fig. 3A). At this moment, NAcc temperature increased on average for 0.11°C (36.46±0.10 vs. 36.35±0.10) within ~53 s (mean latency) that corresponds to a high-rate gradual increase (~0.13°C/min; see also B). When the rat initiated drinking, NAcc temperature, after some weak increase, decreased but then increased slightly again at the final stage of drinking. While the durations of drinking were consistently longer than their onset latencies (range 105–390 s, mean 229 s), NAcc temperature at the end of drinking remained at levels only slightly higher than at its start (0.058°C for 229 s, or 0.015°C/min). Precise NAcc temperature dynamics are evident in B, which shows that the rate of temperature increase drops immediately at the start of drinking but still remains positive within subsequent 25–30 s. Then, the rates fluctuate around zero but increase slightly at the final periods of drinking. Finally, NAcc temperatures showed a strong increase that began immediately after finishing drink consumption and continued during post-drinking behavioral activation (see Fig. 3A and 1D). The maximal acceleration of temperature increase occurred immediately after the end of drinking and then returned slowly toward baseline (see B); the rate of temperature elevation for the first minute post-drinking (~0.138°C/min) was identical to that seen after cup presentation and preceding the initiation of drinking (~0.13°C/min).
Changes in muscle temperature had some common properties and important differences compared to those seen in the brain (Fig. 3, middle panel). In contrast to the NAcc, muscle temperature virtually did not change during cup presentation and before the initiation of drinking (+0.03°C/min), slightly increased during drinking, and continued to increase slowly but gradually during the post-drinking behavioral activation. In contrast to the exceptionally rapid temperature increase seen the NAcc after the end of drinking (significant at 10–15 s), an increase in temporal muscle was much slower, becoming significant only at 40–45 s. The rate of muscle temperature changes was lower and more variable than in the brain, except a brief period of stable increase at the start of drinking (B).
Temperature changes in the skin (right panel in Fig. 3, A and B) differed from those seen in both the NAcc and muscle. First, skin temperature decreased sharply immediately after cup presentation; the decrease became significant during the second data point (5–10 s) and its rate (−0.42°C/min) greatly exceeded the rate of temperature increases seen in the NAcc and muscle. This rapid decrease weakened during drinking (−0.02°C/min), became stronger again at the end of drinking, but inverted into a weaker increase from 25–30 s after the end of drinking.
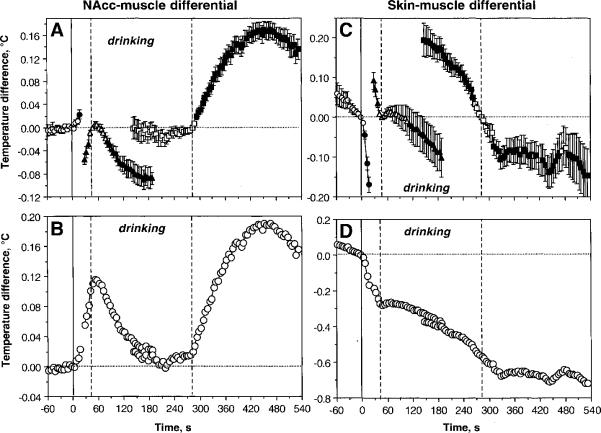
Figure 4 shows changes in NAcc-muscle and skin-muscle differentials analyzed with respect to key events of motivated drinking behavior. As can be seen, NAcc-muscle differential (A and B) increased gradually following cup presentation until the start of drinking, decreased to baseline during drinking, but re-appeared again, at a larger pace, during post-drinking behavioral activation. In contrast, skin-muscle differential (C and D) decreased rapidly and strongly following cup presentation, continued to decrease at a slower pace during drinking, and remained at relatively stable low levels for some time after the end of drinking.
Fig. 4.
Mean changes in NAcc-muscle (A and B) and skin-muscle (C and D) temperature differentials during motivated drinking behavior. The changes are shown in two ways: with respect to the reference point of the behavioral event (Coke-containing cup presentation, initiation of drinking, and its end) set as zero (A and C) and with respect to the starting point (cup presentation) (B and D). Three vertical lines show, respectively, the moments of Coke-containing cup presentation (solid line=0 s), initiation of drinking (first hatched line), and its end (second hatched line). Similar to data shown in Fig. 3, statistical evaluation was done separately for each of three events, with plus (following) and minus (preceding) time comparisons. Filled symbols show values statistically significant from reference points. F values for cup presentation are: NAcc-muscle differential F32,164=5.88; skin-muscle differential = 57.40 (both p<0.001). F values for initiation of drinking: (1) preceding drinking onset: NAcc-muscle differential F32,164=23.99, skin-muscle differential =16.18 (both p<0.001); (2) following drinking onset: NAcc-muscle differential F32,956=22.46; skin-muscle differential 4.67 (both p<0.001). F values for changes following the end of drinking: (1) preceding end of drinking: NAcc-muscle differential =F32,956=0.59, p=0.59; skin-muscle differential =15.31 (p<0.001); (2) following drinking offset: NAcc-muscle differential F32,1022=57.13 (p<0.001), skin-muscle differential =2.86 (p<0.01). While most changes are significant, note quantitative differences in F values that show the strength of the effect.
High-speed temporal resolution was also used to analyze the pattern of temperature changes associated with removal of the empty cup from the cage (Fig. 5). For this analysis, all artifact-free tests with cup removal in regular Coke sessions were combined in one group (n=31) and temperature changes are shown with 5-s bins for one minute before and seven minutes after cup removal. These data were compared to those seen after the presentation of a Coke-containing cup that was followed by drinking behavior. As shown in Fig. 5A and B, in contrast to the presentation of a full cup, brain and muscle temperatures increased gradually within the entire post-removal interval. This increase was about the same in both groups during the first minute but significant between-group differences later emerged because the rats in one group began drinking and temperature decreased transiently, but the rats of the second group (empty cup removal) continued to be behaviorally active and temperatures continued to increase. Eventually, from 5–7 min brain and muscle temperature in both groups became inverted, with stronger increases seen in drinking rats. Changes in skin temperature were similar in both groups but the decrease was stronger and more prolonged in rats that consumed Coke. There were also small but significant differences in brain-muscle differentials (C). While in rats consuming Coke there were two increases, during pre-drinking and post-drinking periods, only one, more tonic peak was seen after removal of an empty cup.
Fig. 5.
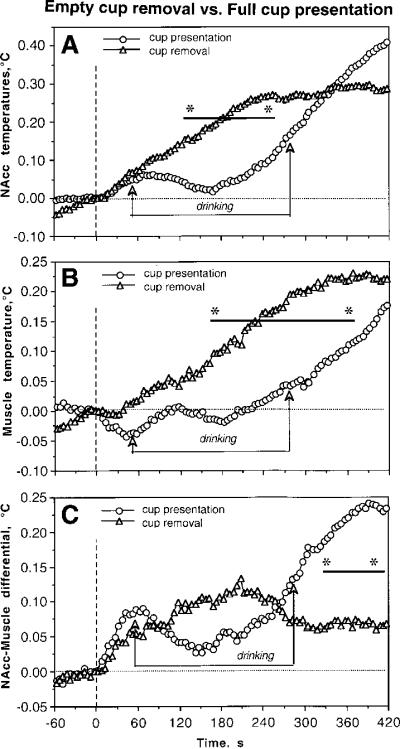
Differences in high-speed temperature dynamics in the NAcc (A), temporal muscle (B), and NAcc-muscle temperature differentials (C) associated with presentation of Coke-filled cup and subsequent removal of the empty cup. Vertical hatched line at time=0 s shows the moments of cup presentation and removal. Two lines with arrows show the duration of drinking after full cup presentation. Bold lines with asterisks show intervals where between-group differences in each parameter were significant (p<0.05, Student's t-test).
2.4. Tonic and phasic temperature fluctuations following substitution of a regular Coke to Diet Coke
When the rats with previous experience of Coke consumption were first presented with Diet Coke, they made a first attempt to drink the new beverage with the same latencies (range: 15–215 s; mean 70.7±26.9 s) as in controls (15–310 s and 53.3±8.4 s, respectively). However, in no case did rats entirely consume Diet Coke. Typically, several attempts at consumption were made, but a prolonged period of drinking never occurred and most of the drink volume remained in the cup. These attempts continued for up to 30–40 min and they were associated with a large increase in locomotor activity (see Fig. 6A). In contrast, rats returned to normal drinking pattern when regular Coke was presented as a second beverage. In this case, rats demonstrated a typical range of latencies to start drinking (25–170 s, mean 81.0±26.1 s) and durations of drinking (from 151 to 260 s, mean 190.4±19.1 s) as in controls (127 to 390, or 229.1±11.6 s).
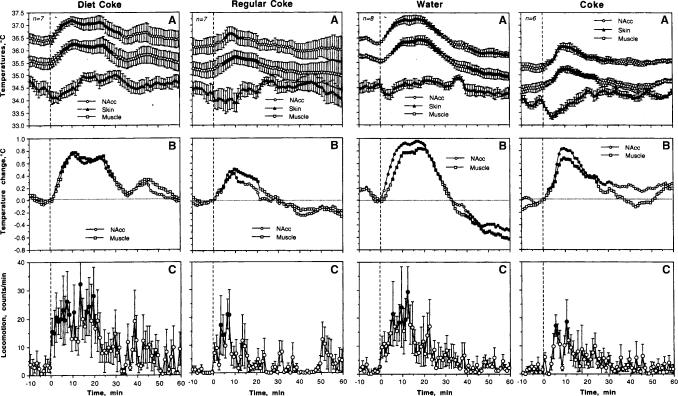
Fig. 6.
Mean changes in absolute (A) and relative (B) temperatures, and locomotion (C) following presentation of Diet Coke with a subsequent test with regular Coke (two left panels) and following presentation of water with a subsequent test with regular Coke (two right panels). n shows numbers of averaged tests and filled symbols show values significantly different from baseline. One-way ANOVA with repeated measures was used for statistical evaluation of temperatures and locomotion. F values for Diet Coke presentation were: NAcc - F6,216=2.94; Skin=10.78, Muscle=2.57; each at least p<0.01; locomotion=0.79, NS. F values for Coke presentation after a Diet Coke: NAcc - F4.154=2.49, Muscle=2.89, Skin=2.80, p<0.01 each; locomotion=1.55, p<0.05. F values for water presentation: NAcc - F7,247=8.77; Skin=2.08, Muscle=9.17; each at least p<0.001; locomotion=1,16, NS. F values for Coke presentation after water: NAcc - F5.185=12.34, Muscle=6.14, Skin=6.38, p<0.001 each; locomotion=1.40, p=0.09.
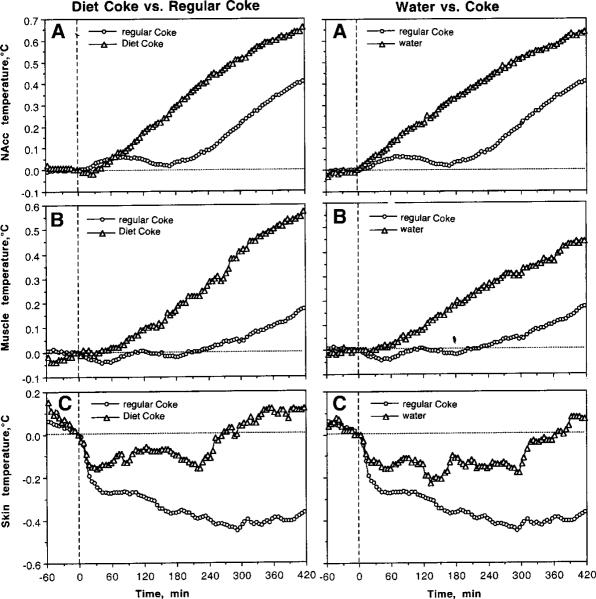
Although temperature changes associated with Diet Coke presentation mimicked a general pattern seen with a regular Coke presentation, they had several important differences (Fig. 6; two left panels). First, increases in brain and muscle temperature as well as locomotor activation after Diet Coke were stronger and more prolonged (A and C). Being calculated for a 30-min period post-stimulation, mean increases seen following Diet Coke were 0.54±0.04 and 0.56±0.04°C for NAcc and muscle, respectively, while they were only 0.19±0.03 and 0.27±0.03°C for regular Coke presentation (p<0.001 in both cases; compare B in Fig. 6). Locomotor activation evaluated for the first 20 min post-stimulation was about twice as large following Diet Coke vs. regular Coke (20.19±1.15 vs. 8.21±1.26 counts/min; p<0.001; compare D and H in Fig. 5). Finally, between-condition differences are especially evident with high temporal resolution analysis (Fig. 7; left panel). While NAcc and muscle temperatures decreased transiently when the rats consumed Coke, they continue to grow rapidly when the rats repeatedly tried to drink Diet Coke (A and B). Skin temperature decreased similarly after cup presentation in both conditions. During regular Coke drinking, skin temperatures further decreased but then stabilized and increased above baseline when the rats interacted with Diet Coke.
Fig. 7.
Differences in phasic changes in brain (A), muscle (B), and skin (C) temperatures associated with Coke substitution to Diet Coke (left panel) or water (right panel). Each graph shows two curves, representing relative temperature changes (5-s bins) following presentation of a regular Coke and its substitute. Hatched lines show zero time and zero temperature change.
2.5. Tonic and phasic temperature fluctuations following substitution of water for regular Coke
When the rats with previous experience of Coke drinking were first presented with water, they made a first drinking attempt within 15–120 s (mean 53.4±12.4 s), similar as in controls (20–310 s, mean 65.6±12.30 s). As with Diet Coke, rats made several (between three and 12) attempts to drink water but in no case did they consumed water to a significant extent. These attempts continued for up to 20–30 min and were associated with increased locomotor activity (see Fig. 6C; right panel). Rats returned to normal drinking patterns when regular Coke was presented as a second beverage. In this case, rats showed a typical range of latencies before the start of drinking (15–60 s), with a mean (32.9±6.17 s) slightly shorter than in controls. The duration of Coke drinking after water substitution (from 171 to 280 s, mean 219.4±12.1 s) was similar to that seen in controls (127 to 390, or 229.1±11.6 s).
Similar to Diet Coke, substitution of water for regular Coke resulted in more rapid and stronger increases in brain and muscle temperatures as well as greater locomotor activity (Fig. 6; right panels). Being calculated for a 30-min period, mean increases in NAcc and muscle temperatures after water presentation (0.67±0.05 and 0.55±0.05°C for NAcc and muscle, respectively) were significantly larger than those after Coke presentation (0.47±0.04 and 0.37±0.04; p<0.05 for both locations). Locomotor activation evaluated for the first 20 min post-stimulation was about twice as strong following water vs. regular Coke (14.81±1.32 vs. 7.95±1.15 counts/min; p<0.001; compare C in Fig. 6). Finally, high temporal resolution analysis revealed differences similar to those seen with Diet Coke (Fig. 7, right panel). While NAcc and muscle temperatures decreased transiently when the rats consumed Coke, they continued to grow rapidly when the rats tasted water (A and B). Skin temperature decreased similarly after cup presentation in both conditions, but it stabilized and grew when the rats interacted with water, while decreasing further during regular Coke drinking.
2.6. Tonic and phasic temperature fluctuations during motivated Coke-drinking after food deprivation
Consistent with our previous findings (Smirnov and Kiyatkin, 2008), rats after 24-h food deprivation had lower basal temperatures in the NAcc (35.97±0.26°C), muscle (35.23±0.24°C), and skin (34.13±0.24°C) than in control (36.34±0.10, 35.45±0.12 and 34.42±0.10, respectively). These between-state differences were maximal in the brain but had not reached statistical significance in each recording location.
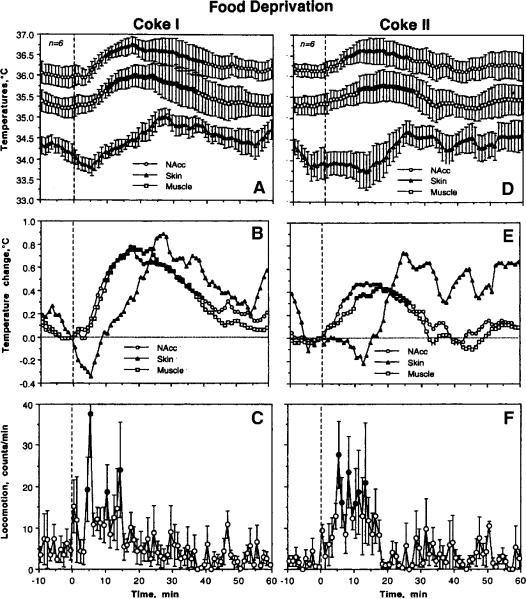
After the first presentation of a Coke-containing cup, hungry rats rapidly initiated drinking (latencies: range 10–70 s, mean 25.8±9.1 s, p<0.05 vs. that in controls), showing robust and prolonged increases in brain and muscle temperatures (Fig. 8). Although the pattern of temperature changes following the second Coke presentation was similar, the latencies to drinking became longer (range: 10–290 s; mean 73.7±43.9 s) and increases in brain and muscle temperatures were significantly weaker (NAcc: 0.33±0.03 vs. 0.50±0.05°C; muscle: 0.29±0.02 vs. 0.50±0.05°C; p<0.01 in both locations). In both cases, hungry animals showed a clearly biphasic change in skin temperature with a weaker initial decrease and a powerful rebound-like increase. There were no changes in locomotor activation following both Coke presentations in hungry rats (see D in Fig. 7; 11.94±1.58 vs. 12.44±1.79 counts/min). Similarly, durations of drinking were similar for both Coke presentations: 204.8±14.8 and 207.0±24.1 s, with no significant differences vs. controls.
Fig. 8.
Mean changes in absolute (A and D) and relative temperatures (B and E) in each recording location as well as in locomotion (C and F) following the first (left panel) and second (right panel) presentations of Coke-filled cup in food-deprived conditions. n show the numbers of averaged tests and filled symbols show values significantly different from baseline. One-way ANOVA with repeated measures was used for statistical evaluation of temperatures and locomotion. F values for the first Coke presentation: NAcc - F5.185=2.62, Muscle=3.32, Skin=10.28, p<0.01 each; locomotion=2.18, p<0.01. F values for the second Coke presentation: NAcc - F5.185=1.70, Muscle=1.67, Skin=6.40, p<0.02 each; locomotion=1.99, p<0.01.
High temporal resolution revealed some differences in temperature dynamics in hungry rats (Fig. 9). In contrast to controls, the first Coke presentation in hungry conditions induced a stronger drinking-related decrease in NAcc temperature but this decrease was less evident with the second Coke presentation (A). Differences in muscle temperature (B) were less evident and the drinking-associated decrease seen in control conditions was not evident with both Coke presentations in food-deprived conditions. While decrease in skin temperature with the first Coke presentation in hungry conditions was similar to that in controls, the decrease was not evident with the second Coke presentation (C).
Fig. 9.
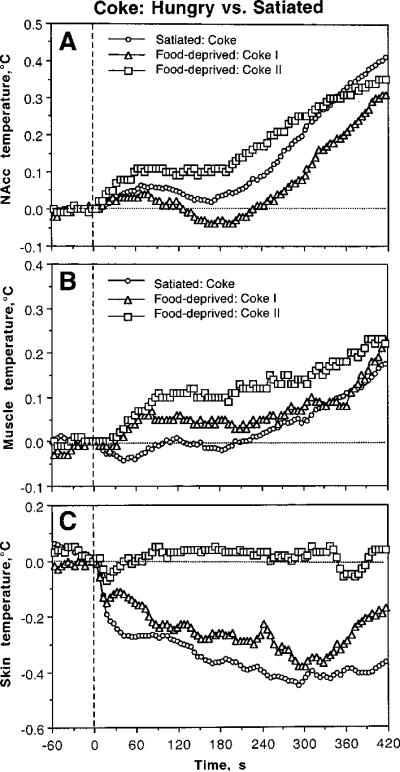
Differences in phasic changes in brain (A), muscle (B), and skin (C) temperatures associated with Coke presentations in food-deprived and satiated conditions. Each graph shows three curves, representing relative temperature changes (5-s bins) after two (I and II) Coke presentations in food-deprived conditions and regular Coke presentation in control, satiated conditions. Hatched lines show zero time and zero temperature change.
3. Discussion
3.1. Model of Motivated Drinking Behavior
Coca-Cola is a sugar-containing beverage that is widely consumed by humans because of its taste and high caloric value. As shown in this study, naive rats quickly develop Coke-drinking behavior after a relatively short exposure. Therefore, Coke could be viewed as a positive reinforcer and our behavioral paradigm could be viewed as a model of learned, motivated behavior. In contrast to food or water, which acts as a state-dependent reinforcer typically inducing eating or drinking in food- or water-deprived conditions, Coke consumption is maintained without food or water deprivation, suggesting that the reinforcing properties of Coke are independent of deprivation state. People also widely consume Diet Coke, a sugar- and calorie-free beverage, which was developed to mimic the sensory properties of a regular Coke. However, rats trained to drink Coke made several attempts to drink Diet Coke but never successfully consumed it. While this finding suggests that Diet Coke cannot substitute regular Coke as a reinforcer, it also indicates that this beverage has different sensory properties, which are rapidly recognized by rats that are expecting to receive Coke. A similar case is true with respect to water, which is a classic natural reinforcer in water-deprived conditions but was refused consistently by rats expecting to obtain Coke. In both cases of Coke substitution rats showed stronger increases in locomotor activity and larger temperature fluctuations. These temperature fluctuations, moreover, had profound differences in their pattern and time-course compared to those seen in control conditions.
While there is enormous volume of experimental and theoretical work aimed at understanding motivated behavior and its underlying mechanisms, most of this work is focused on specific brain structures and neurochemical systems that are critical for developing and regulating this behavior. Moreover, majority of these studies are in psychological domain. Because of the nature of the recorded parameters, our discussion is more focused on behavior-related changes in physiological activity that are often common or non-specific for different types of motivated behavior as well as other forms of an organism's adaptive activities. This focus placed the natural limits in our discussion, leaving many otherwise important studies out of the scope of the present consideration.
3.2. The sources and mechanisms of brain, muscle, and skin temperature fluctuations
Metabolism-related heat production appears to be a primary factor determining brain temperature increases occurring under behavioral conditions (McElligott and Malzack, 1967; Moser and Mathiesen, 1996; Siesjo, 1978; Trubel et al., 2006). Although circulation is the primary means of heat dissipation from the brain and a contributor to brain temperature fluctuations, heat cannot be delivered to the brain from the periphery because of a constant positive temperature gradient between brain tissue and arterial blood arriving to the brain (Delgado and Hanai, 1966; Feitelberg and Lampl, 1935; Hayward and Baker, 1968; Kiyatkin et al., 2002; McElligott and Melzack, 1967; Nybo et al., 2002). Brain temperature increases induced by environmental challenges, moreover, have consistently shorter latencies and are stronger than those occurring in arterial blood (Kiyatkin et al., 2002) and non-locomotor muscles (Bae at al., 2007 and the present study), suggesting metabolic neural activity as a primary underlying factor and a force behind more delayed and weaker increases in body temperature.
Although not as specific as other measures of neural activity (i.e., single-unit and multi-unit electrical discharges, EEG) or brain metabolism (oxygen or glucose consumption), brain temperature is a dynamic parameter that reflects both generalized and structure-specific changes in metabolic neural activity (see Introduction). In contrast to general beliefs on high stability, this study demonstrates that NAcc temperature varies widely within the normal physiological continuum (range: 35.23–38.20°C within regular Coke drinking sessions), showing significant fluctuations occurring within a 5–10 s time scale (see Fig. 3). While this time scale is slower relative to the electric activity of nerve cells, it is surprisingly rapid for temperature. In contrast to true metabolic or neuronal measures, which often require anesthesia and have a limited time window, temperatures may be recorded simultaneously with high precision and artifact-free in several brain and body sites in behaving animals during chronic experiments. This technical advantage was crucial for the goals of this study. Using the same animals recorded during several daily sessions, we were able to evaluate the pattern of fluctuations in central and peripheral temperatures associated with motivated Coke-drinking behavior and its modifications induced by the reinforcer's substitution (Diet Coke or water vs. expected regular Coke) and food deprivation.
Because temperatures in facial skin and temporal muscle depend upon two variables, the state of vessels (vasoconstriction/vasodilatation) and the temperature of arterial blood inflow, these two locations were important for evaluating the source of brain temperature fluctuations and its underlying mechanisms. By tracking temperature responses in different brain and body sites with a short collection interval, one is able to follow the dynamics of heat generation and flows within the organism. For example, more rapid and stronger temperature increases in the NAcc than in the muscle (i.e., increased NAcc-muscle temperature differentials) occurring consistently after cup presentation and its removal clearly point at the brain as a primary source of heat generation. In contrast to the NAcc, where temperatures phasically decreased during Coke consumption, a transient increase was seen in the temporal muscle during the initial period of drinking (see Fig. 3). Although temporal muscle is not a motor muscle and its temperature increases occur primarily due to the inflow of warmer arterial blood, this change may suggest that this muscle is involved in licking behavior and/or is affected by local heat inflow from the neighboring mm. masseter. In contrast to increases in brain and muscle temperatures, skin temperature decreased rapidly and strongly in association with cup presentation and further decreased during drinking. Although skin temperature also depends upon both the temperature of arterial blood inflow, its rapid drop (evident on a second time scale) reflects acute vasoconstriction, a known centrally mediated organism's response that is triggered by various arousing stimuli (Altschule, 1951; Baker et al., 1976; Solomon et al., 1964) and aimed at heat retention under conditions of potential danger. However, skin cooling is a transient response, which is eventually inverted into a rebound-like skin warming, suggesting enhanced heat dissipation after a period of its retention. Since rapid skin temperature decrease due to vasoconstriction is opposed by a slow temperature increase due to inflow of warmer blood, skin-muscle temperature differentials were instrumental for better representation of peripheral vasoconstriction and organism's heat retention and loss.
3.3. Appetitive stimulus, arousal, and motivation
Presentation of a Coke-containing cup to an experienced rat results, with a certain variable latency, in initiation of drinking behavior. The same appetitive stimulus also induces locomotor activation and a typical triad of changes in central and peripheral temperatures: increases in brain and muscle temperatures, more rapid and stronger temperature acceleration in the brain vs. muscle, and an ultra-fast, profound decrease in skin temperature. The same immediate response also occurred when the usual content of the cup was substituted to water or Diet Coke, resulting in tasting of these beverages within the same range of latencies. Since these changes not only followed a stimulus presentation but also preceded the initiation of drinking (see Fig. 3), they could be viewed as a physiological correlate of motivation, a driving force for this searching behavior. However, as shown in our previous studies (see Kiyatkin, 2009 for review), the same temperature response associated with locomotor activation occurs in naive rats when exposed to various salient or arousing somato-sensory stimuli (tail-pinch, placement in a new cage, social interaction with another animal, etc.). Therefore, an increased arousal (or a generalized neural activation) could be viewed as both a pre-condition for and a correlate of seeking aspects of motivated behavior (motivated search). Despite different causes and underlying mechanisms, brain and body hyperthermia coupled with decreased skin temperatures is homologous to fever, the natural organism's response to increase body temperature by enhancing heat production and limiting its dissipation (Schmidt-Nielsen, 2002; see, however, Romanovsky, 2004 for review).
Food deprivation (starvation, fasting) is known to actualize feeding motivation (Barbano and Cador, 2005), enhancing an organism's responsiveness to food and food-related conditioned stimuli. Although food-deprived rats had lower basal temperatures in each recording location, obviously reflecting decreased brain and whole body metabolism (Dumas et al., 2004; Overton et al., 2001; Munch et al., 1993) and peripheral vasoconstriction that limits heat dissipation to the external environment, they showed a significantly shorter latency to initiate Coke drinking and much greater brain and muscle temperature elevations associated with drinking behavior. Therefore, food deprivation, despite lowering basal metabolism, increases behavioral and central metabolic responses to the same appetitive stimulus.
3.4. Drinking behavior, consumption and reward
Although NAcc temperatures gradually increased preceding the initiation of Coke drinking, temperature dynamics sharply changed after drinking began (see Fig. 3). Although absolute temperature still increased during the first 25–30 s of Coke consumption, the rate of increase dropped within the first 5 s and then inverted into a decrease, resulting in lowering brain temperatures vs. their values at the start of drinking. Such decreases never occurred following Coke substitution to either Diet Coke or water despite multiple tasting attempts. In contrast, NAcc temperature continued to increase gradually in these conditions (see Fig. 8). While these data could suggest that Coke consumption is associated with rapid cessation of preceding neural activation, conceptually this finding could be interpreted differently. If Coke consumption in experienced rats engaged in motivated search is viewed as `reward', this abrupt, transient cessation of preceding activation could be viewed as its neural correlate. On the other hand, the same change could be viewed as a correlate of the consummatory (satisfying) phase of motivated behavior, which follows its activation-related seeking phase. This change could also reflect disappearance of motivational excitation that results from the sensory experience of interaction with Coke as a desired satisfier of this motivated behavior. Finally, this neural change could signify a match between expected and really obtained behavioral outcomes. Despite differences in conceptual interpretation, the surprisingly rapid inversion of NAcc temperature dynamics following initiation of drinking suggests that sensory stimulation associated with Coke consumption, not metabolic consequences of consumed sugars, is the primary factor underlying this change. Much more time is necessary for sugars to be absorbed, interact with receptor sites, and affect brain activity. Therefore, this change could be also viewed as a correlate of sensory or “hedonic” but not metabolic reward (Barbano and Cador, 2007).
Although simple physical calculations suggest that consumption of 5 ml of room temperature Coke could decrease “the whole body” temperatures about 0.15°C, it is unlikely that this physical factor could contribute significantly to the relative decreases in NAcc temperature that occur during drinking. In contrast to what can be expected if this factor is viewed as a leading cause, fluctuations in brain temperature tightly correlate with behavioral events (cup presentation, initiation, and termination of drinking) and motor activity and have quite different dynamics (see Fig. 4). Following the initiation of licking behavior, NAcc temperatures rapidly decrease but slowly increase during the final stages of drinking when the impact of local gastric cooling should be maximal. An exceptionally rapid (5–10 s) and strong increase occurs immediately after the cup is emptied and the rat re-initiates searching behavior. If a slow gastric cooling in fact results in a significant decrease in arterial blood temperature, then the temporal muscle that receives the arterial blood supply from the same source as the brain and is not thermogenic per se should be gradually cooled during drinking. In contrast, muscle temperature rapidly increases at the start of drinking, with subsequent stabilization (but no decrease) and slowly increases during post-drinking behavioral activation. Although cooling of arterial blood should accelerate heat dissipation from the brain, thus decreasing its temperature, it is unclear whether this cooling could occur during intra-gastric liquid delivery and what its dynamics could be. These important issues could be clarified in special experiments with either direct monitoring of arterial blood temperature during drinking or monitoring of brain temperatures following passive intra-gastric liquid delivery.
3.5. Coke-seeking activity (motivated search) and its physiological correlates
When Diet Coke and water were substituted for Coke, rats with the same latency tasted the presented drink, but refused to consume it. Most animals made several drinking attempts, which were distributed within 20–30 min, showing on average a two-fold larger locomotor activation that continued for a longer time when compared to regular Coke presentation (see Fig. 7). While NAcc temperatures during these tests showed the same rapid changes until the first drinking attempt, there was no subsequent temperature decrease. In contrast, temperatures continue to increase gradually, peaking at ~15–30 min; the mean amplitudes and durations of temperature elevation were significantly larger than those during regular Coke presentation. Therefore, it appears that the inability to obtain expected Coke (or satisfy existing motivation, no reward) is associated with sustained neural activation, enhanced intra-brain heat production and its accumulation in the brain, manifesting at the behavioral level as a continuous searching activity.
However, the second activation peak also consistently occurred after the rats finished Coke consumption and made multiple returns to the empty cup, trying to lick out all remaining drops of liquid. These back-and-forth attempts and licking of an empty cup made it difficult to define the exact moment when drinking was finished, possibly contributing to a slight increase in measured brain temperature at the final period of drinking. Nevertheless, when the cup became empty, rats' locomotion increased greatly and brain temperatures showed a more prolonged acceleration, which dissipated slowly within 5–7 min, when temperatures reached their absolute peaks. Despite a robust increase in locomotion, temperature in the muscle increased much slower than in the NAcc, showing no distinct difference in rate between the final stages of drinking and the post-drinking locomotor activation (see Fig. 4B). Therefore, the second peak of post-consumption neural activation (which is reflected most clearly in NAcc-muscle differentials; see Fig. 5) could be related to the reappearance of a motivated search that dissipated slowly over time because of an inability to find a satisfier (or satisfy this motivation). Our pilot experiments revealed that rats are able to consume much larger volumes of the drink, suggesting that the presented 5-ml sample is unable to satisfy them fully. Although the post-drinking increases in brain and muscle temperatures are larger in amplitude than those occurring before drinking, this difference results from a variation in time intervals. While after the presentation of a Coke-filled cup, the rat began drinking within a relatively short time interval (<1 min), hyperactivity and search after emptying the cup continues much longer (5–15 min), resulting in larger absolute temperature increase despite slower acceleration (see Fig. 4B). While the rate of NAcc temperature increase immediately after emptying the cup was about the same as before the start of drinking, it weakened gradually within the next several minutes until inverting into a gradual decrease that slowly returned temperatures from their peaks to baseline levels. At this stage, animals were relaxed and locomotor activity also returned to baseline.
3.6. Common features of temperature dynamics during different forms of motivated behavior
Temperature changes associated with Coke-drinking behavior have several important similarities as well as certain differences to those occurring during sexual (Mitchum and Kiyatkin, 2003) and feeding (Smirnov and Kiyatkin, 2008) behaviors, two other basic motivated activities of living beings. Similar to this study, locomotor activation and gradual increases in NAcc temperature seen in hungry rats following presentation of a food container and in sexually experienced male rats after presentation of a female partner. Although the rate of increase was similar in each case of motivated search, the absolute increases varied due to differences in experimental protocols. In this study, rats initiated drinking within ~1 min after cup presentation and absolute temperature increase was relatively small, but NAcc temperatures increased up to 1.5–2.0°C when males were able to see and perceive the receptive female, but were unable to interact directly with her and initiate copulatory behavior for 30 min until the removal of a separating barrier. Similarly, increases were stronger and more prolonged when hungry rats saw and perceived food but were unable to retrieve it from a closed container. A similar gradual increase in brain and muscle temperatures also occurred in rats trained to self-administered cocaine following exposure to drug-related sensory cues and preceding the first-in-session drug self-injection (Kiyatkin and Brown, 2003). Absolute temperature increases in this case were also directly dependent upon the timing when the animal made the lever-press and received the drug. When the lever-presses were not reinforced by cocaine, brain temperature continued to increase, correlating with hyperactivity and multiple lever-presses.
In contrast to a transient hyperactivity that preceded Coke consumption, male copulatory behavior is highly energetic and accompanied by further gradual increases in brain temperatures when the rate of mounts and intromissions increased, sharply peaking at ejaculation (Mitchum and Kiyatkin, 2003). After this event, the male's motor activity decreased rapidly and brain temperatures dropped sharply, mimicking the pattern seen during Coke consumption. However, in contrast to Coke-drinking behavior, where sensory stimulation associated with Coke consumption appears to be a rewarding event, that rapidly changes brain temperature dynamics, neural activation triggered by sexually relevant stimuli appears to persist during the entire copulatory behavior, with ejaculation being an acute rewarding event or a “true” satisfier of this motivated behavior. In contrast to Coke-drinking behavior, brain and muscle temperature fluctuations during sexual behavior were larger in amplitude, with both sharper temperature increases during copulatory behavior and stronger post-ejaculatory temperature decreases. These pre-ejaculatory increases (up to 39.5–40.0°C in the NAcc and medial-preoptic hypothalamus) were maximal in terms of amplitude among all types of physiological and behavioral activity in rats (see Kiyatkin, 2009 for review). Although cocaine is a drug reinforcer and iv self-administration is unnatural motivated behavior, brain temperature also decreased rapidly but transiently after each regular cocaine self-injection relative to its peaks associated with lever presses and drug infusions (Kiyatkin and Brown, 2003). In this case, the decrease was more delayed (20–30 s), obviously reflecting the time necessary for the drug to reach its brain receptor sites (Fowler et al., 1998). Within the cycle of repeated cocaine self-injections, NAcc temperatures increased gradually during cocaine-seeking activity, peaked at the moment of self-injection, and decreased rapidly after iv drug infusion. These rapid fluctuations were superimposed on a moderate tonic increase, which was quickly developed at the session start and slowly disappeared when drug access was terminated. Therefore, it appears that different motivated behaviors, despite different manifestations, share some basic common pattern of alterations in metabolic brain activity and other homeostatic physiological parameters.
3.7. Perspectives and significance
This study demonstrates that brain temperature is a sensitive physiological parameter that shows relatively rapid and large fluctuations during appetitive motivated behavior. Although brain temperature in this study was represented by the NAcc, a deep brain structure that is heavily implicated in regulation of motivational processes (Parent and Hazrati, 1995; Wise and Bozarth, 1987), based on our previous data, it could be reasonably assumed that generally similar temperature fluctuations occur in other brain structures. Although there are significant structural differences in basal temperatures as well as minor but significant differences in temperature changes elicited by various arousing stimuli and occurring during different motivated behaviors, behavior-related temperature fluctuations are correlative, suggesting its generality for the brain as a whole (see Kiyatkin 2005, 2009 for review). However, similar to this study, these fluctuations were highly specific with respect to the nature of the stimulus, its arousing potential, and key events of motivated behavior. Therefore, it appears that brain temperature provides a valuable measure of non-specific arousal components common to different types of motivated behavior, though this measure is weaker in revealing structural specificity of neuronal activity.
4. Experimental Procedures
4.1. Subjects
Twelve Long-Evans male rats (Taconic, Germantown, NY), weighing 400–460 g were used in this study. They were housed individually under a 12 h light cycle (lights on at 07:00), with food and water provided ad libitum. Prior to surgery, rats were put through a pilot trial of the Coke drinking procedure for both training and selective purposes (see below). Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publication 865-23) and were approved by the Animal Care and Use Committee, National Institute on Drug Abuse – Intramural Research Program.
4.2. Surgery
All animals were implanted with three thermocouple electrodes as described previously (Kiyatkin and Brown, 2003). Animals were anesthetized intraperitoneally with 3.3ml/kg of Equithesin (sodium pentobarbital, 32.5 mg/kg and chloral hydrate, 145 mg/kg) and mounted in a stereotaxic apparatus. Four holes were drilled through the skull: three for securing screws and one for thermocouple insertion over the NAcc shell (1.2 mm anterior to bregma, 0.9 mm lateral to bregma) using the coordinates of Paxinos and Watson (1998). The dura mater was retracted and the thermocouple probe was lowered slowly to the desired target depth (7.4 mm, measured from the skull surface). A second thermocouple probe was implanted subcutaneously along the nasal ridge with the tip approximately 15 mm anterior to bregma. Although the tail is the primary organ of heat dissipation in rats, facial skin has dense vascularization and this location provides mechanical stability of the electrode, an essential condition for long-term, artifact-free temperature recordings. A third thermocouple probe was implanted in the deep temporal muscle (musculus temporalis), which has a similar arterial blood supply as the brain and is not directly involved in locomotion or motor activity. Because temperature fluctuations in skin and muscle depend upon two variables, the state of vessels (vasoconstriction/vasodilatation) and the temperature of arterial blood inflow, these two locations were important for evaluating the source of brain temperature fluctuations and its underlying mechanisms. By tracking temperature responses in different brain and body sites with a short collection interval, one is able to follow the dynamics of heat generation and flows within the organism. The probes were secured with dental cement to the three stainless steel screws threaded into the skull. Rats were allowed three days recovery and two more days of habituation (6 h sessions) to the testing environment before the start of testing.
4.3. Experimental Protocol
During pilot trials that preceded surgeries, rats were trained to consume caffeine-free Coca-Cola Classic® (Coke). During the first training session, rats were food-deprived over-night (19:00–9:00) and, after a two to three hour habituation period in the experimental chamber, they were presented with a small plastic bottle cup (~3.0×1.6 cm, fixed on a metal plate for stability) containing 5-ml of Coke. This drink contains 11% sugar, with 1.97 calories or 0.55 g of sugar in a 5-ml portion. The liquid was de-carbonated by intense shaking and warmed to room temperature (~23°C). The Coke-containing cup was presented for one hour and the procedure was repeated twice during the ~6-hour session. During the next two training sessions, rats were not food-deprived but had no food or water except two Coke drinks of the same size, each presented for 1-hour intervals. If the rats learned to consume the beverage eagerly and entirely, they were selected for surgery.
All tests occurred inside a Plexiglas chamber (32×32×32 cm) placed inside a sound- and light-attenuated plastic box (60 × 56 × 70 cm) under continuous weak white light insulation (15 Wt) and in view of a small USB camera mounted above the cage. These chambers were equipped with four infrared motion detectors (Med Associates, Burlington, VT, USA), allowing for the monitoring of locomotor activity. Rats were brought to the testing chamber at ~09:30 AM and attached via a flexible cord and electrical commutator to thermal recording hardware (Thermes 16, Physitemp, Clifton, NJ, USA). Temperatures were recorded with a 5-s time resolution and movement was recorded as the number of infrared beam breaks per 1 min. Room temperature was maintained at 23–24°C and controlled by another thermocouple located inside of the recording chamber.
Each experimental rat underwent 10 to 12 recording sessions. During the first two to three recording sessions, each rat was exposed to a similar basic testing protocol, which included two presentations of regular Coke beverages. After a two- to three-hour habituation period and stabilization of basal temperatures, a Coke-containing cup was first presented to rats resulting, with some onset latency, in drinking behavior. The empty cup was always removed from the cage at least one hour after its presentation (range: 60–75 min). The same procedure was repeated in the afternoon, about two to three hours after the first Coke presentation. The moments of cup introduction, cup removal, and behavioral events (start of drinking, end of drinking) were recorded for further analyses. Independently of animal location in the chamber, the cup was placed in the same location right below the view of the video camera, allowing visual observation of animal behavior from another room without any influence from the experimenter.
In addition to two to three identical initial sessions with the presentation of regular Coke beverages, each rat experienced three unusual sessions that were performed in a stochastic order. To exclude the expectancy factor, each unusual session was always preceded and followed by at least one regular Coke-drinking session.
During the first unusual session, after a similar period of habituation, rats were presented an equal amount of caffeine-free Diet Coke® (5 ml) in place of the first beverage. This beverage was developed to mimic the sensory properties of regular Coke, but it is virtually sugar- and calorie-free (<0.02 calories and 0.002 g sugars in a 5-ml volume). Similar to the basic session, the second presentation was a regular Coke. The second unusual session included presentation of a cup containing an equal amount of water (5 ml) in place of the first beverage; the second presentation was a regular Coke. Finally, the third unusual session was identical to the basic session (two regular Coke presentations) but the rats were food-deprived for 24 hours (10:00–10:00) prior to the session.
4.5. Histology and Data Analysis
When recording was completed, all rats were anesthetized, decapitated, and had their brains removed for sectioning and confirmation of probe placement. Brains were cut on a cryostat into 50μm slices and placed on glass slides. All probes were located within the medial portion of the ventral striatum (NAcc shell), as described in Paxinos and Watson (1998).
Temperature data were analyzed with 5-s and 60-s time bins and were presented as both absolute and relative changes with respect to the moment of cup presentation, cup removal, and key behavioral events (start and end of drinking). Locomotor activity was analyzed with 60-s bins. One-way ANOVA with repeated measures, followed by post-hoc Fisher tests, was used for statistical evaluation of temperature and locomotion changes preceding and following events of interest. Student's t-test was used for comparisons of between-site and between-condition differences in temperature and locomotion. Between-state differences were evaluated based on statistical comparisons of basal temperatures and locomotion averaged for 6 min preceding the container's presentation. The use of the words “increase” or “decrease” as well as “significant” refers to the presence of a statistically significant change in the parameter or differences between the compared groups or conditions (with at least p <0.05) revealed by either ANOVA or Student's t-test.
Because rats showed different latencies to initiation of drinking and different durations of drinking and, in some cases, they refused to drink other beverages or their drinking was transient or intermittent, different types of statistical analyses were used to accurately represent temperature dynamics associated with the events of interest. First, we analyzed mean temperatures and mean locomotor activity preceding the events of interest. For this analysis, temperature and locomotor data were averaged for different specified durations preceding these events. Second, we analyzed temperatures and locomotor activity following presentations of a beverage-containing cup and its removal from the cage one hour later. This slow time-course analysis was performed using consecutive 1-min values of recorded parameters. This analysis provided a general representation of tonic changes in temperature and locomotion induced by different stimuli, allowing between-stimulus comparison. To reveal fine time-course dynamics of temperature fluctuations in different conditions, this analysis was also performed with 5-s temporal resolution. Finally, high temporal resolution (5-s bins) was used to evaluate changes associated with drinking behavior. Because latency to drinking as well as duration of drinking varied widely in each rat and session, we analyzed temperature dynamics with respect to the initiation of drinking and the end of drinking. In this case, these two events were set at zero and temperature values preceding and following these events were averaged for all rats within specified timing intervals and statistically evaluated with respect to these events. For example, for evaluating temperature changes associated with drinking behavior, which had different durations, we calculated mean changes following the start of drinking and mean changes preceding the end of drinking. A similar strategy was used for statistical evaluation of highly variable timing intervals between cup presentation and initiation of drinking behavior.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIDA. We wish to thank Dr. Mary Pfeiffer and Jeremy Tang for language editing of this manuscript.
Abbreviations
- ANAVA
analysis of variance
- Coke
Coca-Cola
- Diet Coke
Diet Coca-Cola
- iv
intravenous
- NAcc
nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams R, Hammel HT. Hypothalamic temperature in unanesthetized albino rats during feeding and sleeping. Am. J. Physiol. 1964;206:641–646. doi: 10.1152/ajplegacy.1964.206.3.641. [DOI] [PubMed] [Google Scholar]
- Altschule MD. Emotion and circulation. Circulation. 1951;3:444–454. doi: 10.1161/01.cir.3.3.444. [DOI] [PubMed] [Google Scholar]
- Bae DB, Brown PL, Kiyatkin EA. Procedure of rectal temperature measurement affects brain, muscle, skin and body temperatures and modulates the effects of intravenous cocaine. Brain Res. 2007;1154:61–70. doi: 10.1016/j.brainres.2007.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Cronin M, Mountjoy D. Variability of skin temperature in the waking monkey. Am. J. Physiol. 1976;230:449–455. doi: 10.1152/ajplegacy.1976.230.2.449. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Various aspects of feeding behavior can be partially dissociated in the rat by the incentive properties of food and the physiological state. Behav. Neurosci. 2005;119:1244–1253. doi: 10.1037/0735-7044.119.5.1244. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Delgado JMR, Hanai T. Intracerebral temperatures in free-moving cats. Am. J. Physiol. 1966;211:755–769. doi: 10.1152/ajplegacy.1966.211.3.755. [DOI] [PubMed] [Google Scholar]
- Dumas JF, Roussel D, Simard G, Douay O, Foussard F, Maltheiry Y, Ritz P. Food restriction affects energy metabolism in rat liver mitochondria. Biochem. Biophys. Acta. 2004;1690:126–131. doi: 10.1016/j.bbagen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Feitelberg S, Lampl H. Warmetonung der Grosshirnrinde bei Erregung und Ruhe. Functionshemmung. Arch exp Path Pharmak. 1935;177:726–736. (in German) [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding Y-S, Wang G-J. Measuring dopamine transporter occupancy by cocaine in vitro: radiotracer considerations. Synapse. 1968;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Baker MA. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am. J. Physiol. 1968;215:389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- James W. Briefer Course. Henry Holt; New York: 1892. Psychology. [Google Scholar]
- Kiyatkin EA. Brain hyperthermia as physiological and pathological phenomena. Brain Res. Rev. 2005;50:27–56. doi: 10.1016/j.brainresrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: Physiological fluctuations and pathological shifts. Front. Biosci. 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. Eur. J. Neurosci. 2002;16:164–168. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Fluctuations in neural activity during cocaine self-administration: Clues provided by brain thermorecording. Neuroscience. 2003;116:525–538. doi: 10.1016/s0306-4522(02)00711-x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain temperature fluctuations during passive vs. active cocaine administration: clues for understanding the pharmacological determination of drug-taking behavior. Brain Res. 2004;1005:101–116. doi: 10.1016/j.brainres.2004.01.038. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Mitchum R. Fluctuations in brain temperature during sexual behavior in male rats: An approach for evaluating neural activity underlying motivated behavior. Neuroscience. 2004;119:1169–1183. doi: 10.1016/s0306-4522(03)00222-7. [DOI] [PubMed] [Google Scholar]
- McElligott JC, Melzack R. Localized thermal changes evoked in the brain by visual and auditory stimulation. Exp. Neurol. 1967;17:293–312. doi: 10.1016/0014-4886(67)90108-2. [DOI] [PubMed] [Google Scholar]
- Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate filed potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- Moser EI, Mathiesen J. Relationships between neuronal activity and brain temperature in rats. NeuroReport. 1996;7:1876–1880. doi: 10.1097/00001756-199607290-00038. [DOI] [PubMed] [Google Scholar]
- Munch IC, Markussen NH, Oristsland NA. Resting oxygen consumption in rats during food deprivation, starvation and refeeding. Acta Physiol. Scand. 1993;148:335–340. doi: 10.1111/j.1748-1716.1993.tb09564.x. [DOI] [PubMed] [Google Scholar]
- Nybo L, Scher NH, Nielson B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J. Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME. Central leptin infusion attenuates the cardiovascuilar and metabolic effects of fasting in rats. Hypertension. 2001;37:663–669. doi: 10.1161/01.hyp.37.2.663. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortical-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paxinos J, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Romanovsky AA. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am. J. Physiol. 2004;287:R992–R995. doi: 10.1152/ajpregu.00068.2004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Adaptation and Environment. 5th Ed. Cambridge Univ Press; Cambridge: 1989. Animal Physiology. [Google Scholar]
- Siesjo B. Brain Energy Metabolism. Wiley; New York: 1978. [Google Scholar]
- Smirnov MS, Kiyatkin EA. Fluctuations in central and peripheral temperatures associated with feeding behavior in rats. Am. J. Physiol. 2008;295:R1415–R1424. doi: 10.1152/ajpregu.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GF, Moos RH, Stone GC, Fessel WJ. Peripheral vasoconstriction induced by emotional stress in rats. Angiology. 1964;15:362–365. doi: 10.1177/000331976401500806. [DOI] [PubMed] [Google Scholar]
- Trubel HKF, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J. Cereb. Blood Flow Metab. 2006;26:68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]