Abstract
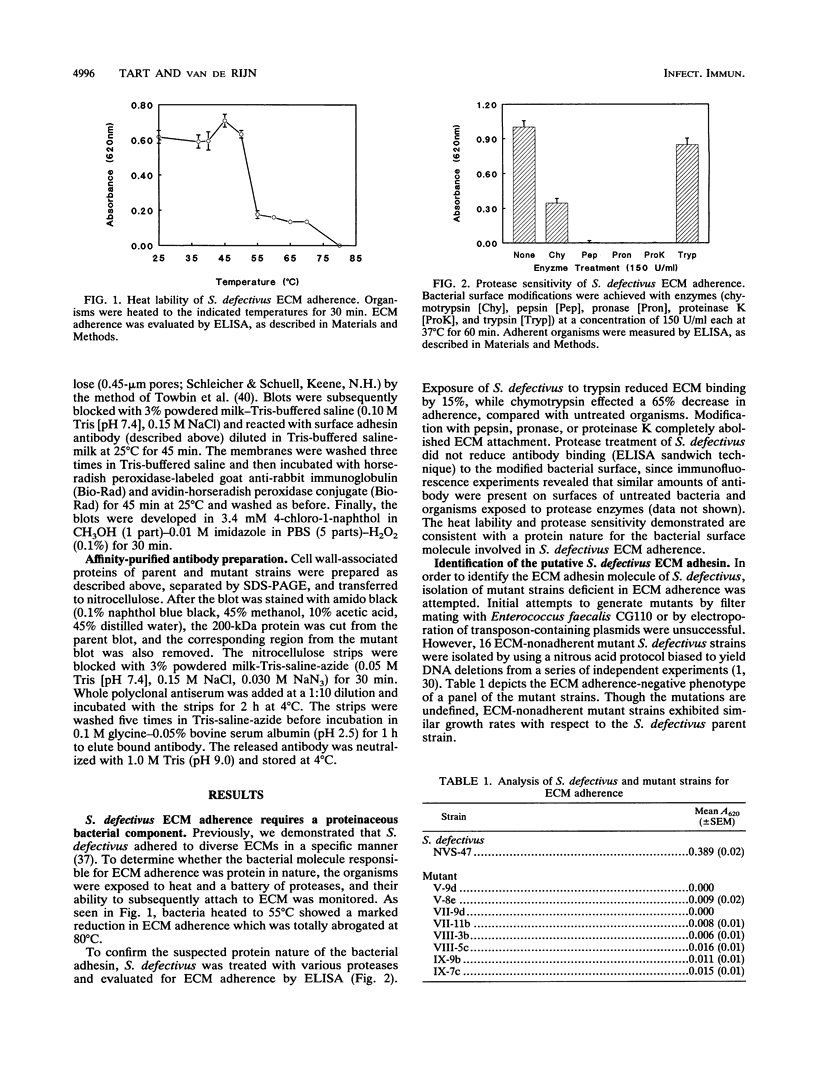
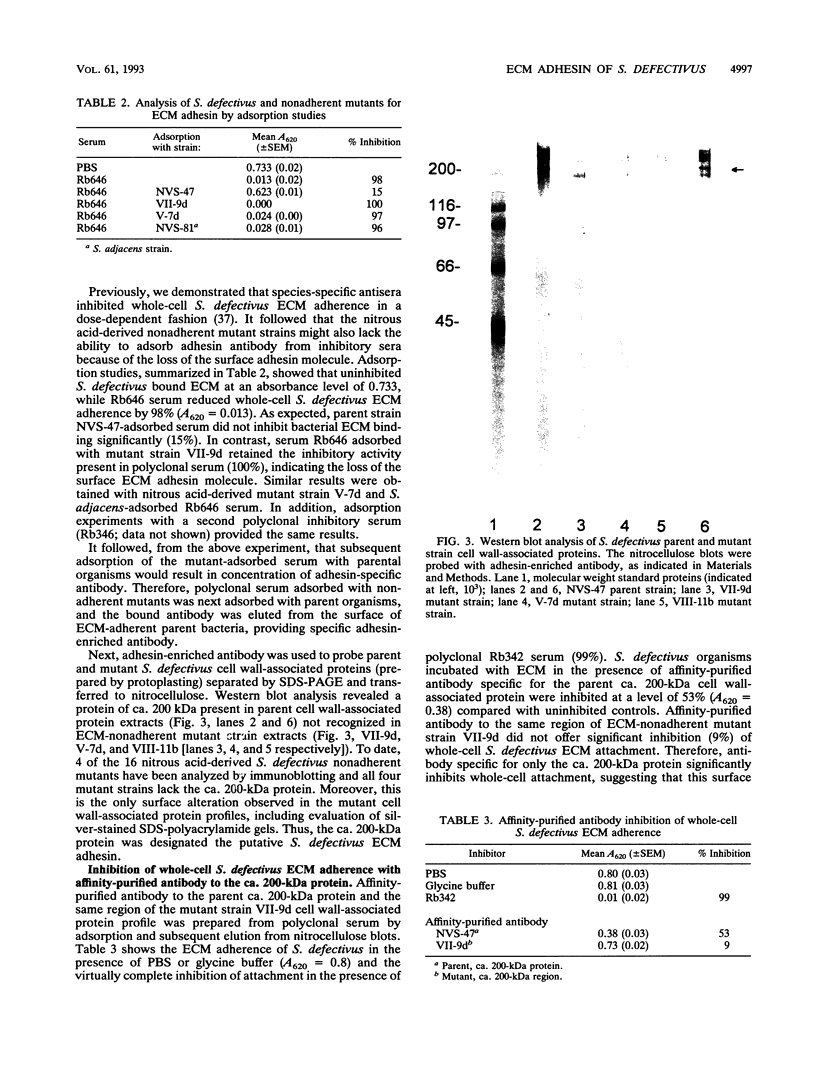
Bacterial attachment to host tissue is considered to be a crucial primary step in pathogen infection. Previous studies have shown that Streptococcus defectivus adheres specifically to cell-secreted extracellular matrix (ECM). Though generally not exposed in vivo, this host tissue is exposed at endothelial cell junctions and sites of tissue injury. In this report, we identify a ca. 200-kDa surface protein of S. defectivus involved in ECM adherence. Nitrous acid-derived mutant strains that were unable to bind ECM and which failed to adsorb adhesin-specific antibody from polyclonal inhibitory sera were isolated. A surface protein (ca. 200 kDa) was absent from ECM-nonadherent mutants, indicating its involvement in ECM attachment. Additionally, affinity-purified antibody to the ca. 200-kDa protein inhibited whole-cell S. defectivus ECM attachment, whereas antibody to the same region of the nonadherent mutant cell wall-associated protein profile did not. Furthermore, solubilized cell wall-associated protein extracts of parent but not mutant strains bound ECM, confirming the significance of this protein in ECM adherence. Therefore, we propose that the ca. 200-kDa protein is the major S. defectivus surface component that mediates the ECM attachment of these organisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Positive selection of mutants with deletions of the gal-chl region of the Salmonella chromosome as a screening procedure for mutagens that cause deletions. J Bacteriol. 1975 Jan;121(1):259–266. doi: 10.1128/jb.121.1.259-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Courtney H. S. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S475–S481. doi: 10.1093/clinids/9.supplement_5.s475. [DOI] [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Stephens D. S., Olsén A., Scott J. R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991 May;59(5):1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I., Russell C. Comparative adhesion of seven species of streptococci isolated from the blood of patients with sub-acute bacterial endocarditis to fibrin-platelet clots in vitro. J Appl Bacteriol. 1986 Feb;60(2):127–133. doi: 10.1111/j.1365-2672.1986.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Emödy L., Heesemann J., Wolf-Watz H., Skurnik M., Kapperud G., O'Toole P., Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989 Dec;171(12):6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock J. I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Hök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987 Aug;6(8):2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Haapasalo M., Müller K. H., Uitto V. J., Leung W. K., McBride B. C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992 May;60(5):2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Horwitz P. A., Caparon M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992 Dec;60(12):5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook M., McGavin M. J., Switalski L. M., Raja R., Raucci G., Lindgren P. E., Lindberg M., Signas C. Interactions of bacteria with extracellular matrix proteins. Cell Differ Dev. 1990 Dec 2;32(3):433–438. doi: 10.1016/0922-3371(90)90060-a. [DOI] [PubMed] [Google Scholar]
- Jönsson K., Signäs C., Müller H. P., Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991 Dec 18;202(3):1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Maca R. D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988 Sep;56(9):2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Kostrzynska M., Paulsson M., Schmidt K. H., Wadström T. Comparative studies on binding of vitronectin and fibronectin to groups A and C streptococci. Microbios. 1992;71(288-289):179–192. [PubMed] [Google Scholar]
- Kostrzynska M., Schalén C., Wadström T. Specific binding of collagen type IV to Streptococcus pyogenes. FEMS Microbiol Lett. 1989 May;50(1-2):229–233. doi: 10.1016/0378-1097(89)90492-8. [DOI] [PubMed] [Google Scholar]
- Kostrzynska M., Wadström T. Binding of laminin, type IV collagen, and vitronectin by Streptococcus pneumoniae. Zentralbl Bakteriol. 1992 Jun;277(1):80–83. doi: 10.1016/s0934-8840(11)80874-1. [DOI] [PubMed] [Google Scholar]
- Kuypers J. M., Proctor R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang O. D., Ascencio F., Fransson L. A., Wadström T. Binding of heparan sulfate to Staphylococcus aureus. Infect Immun. 1992 Mar;60(3):899–906. doi: 10.1128/iai.60.3.899-906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Baddour L. M., Simpson W. A. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Invest. 1990 Jul;86(1):7–13. doi: 10.1172/JCI114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Liang O. D., Ascencio F., Wadström T. Vitronectin-binding surface proteins of Staphylococcus aureus. Zentralbl Bakteriol. 1992 Jun;277(1):54–64. doi: 10.1016/s0934-8840(11)80871-6. [DOI] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V. A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992 Jul 24;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Schwartz D. O., Beckwith J. R. Mutagens which cause deletions in Escherichia coli. Genetics. 1969 Feb;61(2):371–376. doi: 10.1093/genetics/61.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. S., Nelson K. E. Endocarditis due to nutritionally deficient streptococci: therapeutic dilemma. Rev Infect Dis. 1987 Sep-Oct;9(5):908–916. doi: 10.1093/clinids/9.5.908. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989 Dec 15;264(35):21080–21086. [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tart R. C., van de Rijn I. Analysis of adherence of Streptococcus defectivus and endocarditis-associated streptococci to extracellular matrix. Infect Immun. 1991 Mar;59(3):857–862. doi: 10.1128/iai.59.3.857-862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect Immun. 1986 Jun;52(3):736–741. doi: 10.1128/iai.52.3.736-741.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin tetrapeptide is target for syphilis spirochete cytadherence. J Exp Med. 1985 Nov 1;162(5):1715–1719. doi: 10.1084/jem.162.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Doig P., Emödy L., Kienle Z., Wadström T., O'Toole P. High-affinity binding of the basement membrane proteins collagen type IV and laminin to the gastric pathogen Helicobacter pylori. Infect Immun. 1991 Dec;59(12):4398–4404. doi: 10.1128/iai.59.12.4398-4404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Visai L., Speziale P., Bozzini S. Binding of collagens to an enterotoxigenic strain of Escherichia coli. Infect Immun. 1990 Feb;58(2):449–455. doi: 10.1128/iai.58.2.449-455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Kuusela P., Risteli J., Risteli L., Vartio T., Rauvala H., Virkola R., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989 Mar;3(3):329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Winkler J. R., John S. R., Kramer R. H., Hoover C. I., Murray P. A. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect Immun. 1987 Nov;55(11):2721–2726. doi: 10.1128/iai.55.11.2721-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M., Bouvet A., Roberts R. B. Enzyme-linked immunosorbent assay for the detection of antibodies to nutritionally variant streptococci in patients with endocarditis. J Infect Dis. 1986 Jan;153(1):116–121. doi: 10.1093/infdis/153.1.116. [DOI] [PubMed] [Google Scholar]