Abstract
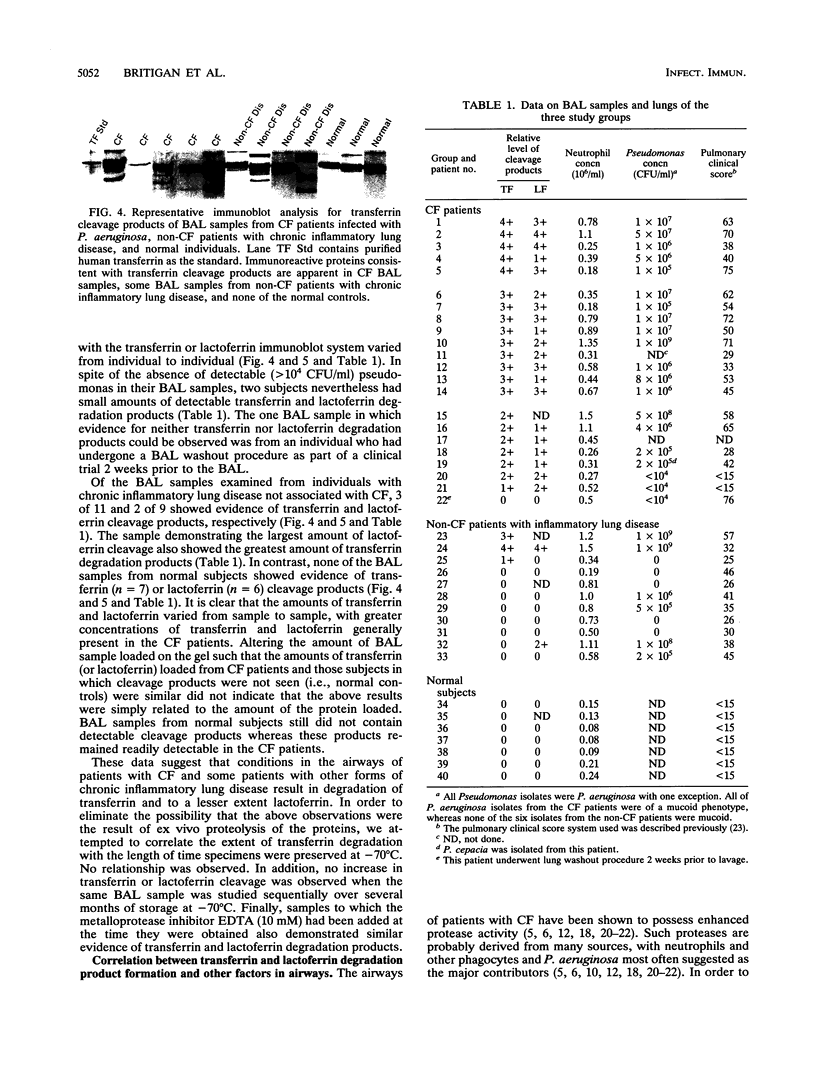
Bacterium- and neutrophil-derived proteases have been suggested to contribute to tissue injury at sites of Pseudomonas aeruginosa infection. Pseudomonas elastase cleavage of transferrin enhances in vitro iron removal from this protein by the P. aeruginosa siderophore pyoverdin. This cleavage also generates new iron chelates which, in contrast to iron bound to transferrin, are able to catalyze formation of the highly cytotoxic hydroxyl radical from neutrophil-derived superoxide and hydrogen peroxide via the Haber-Weiss reaction. In order to determine whether this cleavage occurs in vivo, a chemiluminescence immunoblot system was developed to detect the presence of proteolysis products of transferrin or the related iron-binding protein, lactoferrin. Using this immunoblot system, we detected transferrin and lactoferrin cleavage products in bronchoalveolar lavage (BAL) samples from 21 of 22 and 20 of 21 cystic fibrosis (CF) patients, respectively. Three of eleven and two of nine BAL samples from individuals with other forms of chronic inflammatory lung disease had transferrin and lactoferrin cleavage products, respectively. Each patient in whom such products were detected was also infected with P. aeruginosa. No such products were detected in normal individuals. In the CF patients, there was no clear correlation between the extent of transferrin or lactoferrin cleavage and BAL neutrophil or P. aeruginosa concentration or the disease status of the patient. In contrast, in the non-CF patients with chronic inflammatory lung disease, transferrin and lactoferrin cleavage products were detected only in those BAL samples which contained the greatest concentration of both neutrophils and P. aeruginosa. These data provide evidence that P. aeruginosa- and/or human-derived protease cleavage of transferrin and lactoferrin occurs in vivo in the airways of individuals with CF and other forms of chronic lung disease, suggesting that this process could contribute to P. aeruginosa-associated lung injury in these patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bluard-Deconinck J. M., Williams J., Evans R. W., van Snick J., Osinski P. A., Masson P. L. Iron-binding fragments from the N-terminal and C-terminal regions of human lactoferrin. Biochem J. 1978 May 1;171(2):321–327. doi: 10.1042/bj1710321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines R. D., Brock J. H. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim Biophys Acta. 1983 Sep 13;759(3):229–235. doi: 10.1016/0304-4165(83)90317-3. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Edeker B. L. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J Clin Invest. 1991 Oct;88(4):1092–1102. doi: 10.1172/JCI115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. C., Poncz L., Klinger J. D., Stern R. C., Tomashefski J. F., Jr, Dearborn D. G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985 Sep;132(3):529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- Döring G., Pfestorf M., Botzenhart K., Abdallah M. A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun. 1988 Jan;56(1):291–293. doi: 10.1128/iai.56.1.291-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Brock J. H. The effect of trypsin digestion on the structure and iron-donating properties of transferrins from several species. Biochim Biophys Acta. 1980 Apr 25;622(2):297–307. doi: 10.1016/0005-2795(80)90040-9. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams J. Studies of the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978 Aug 1;173(2):543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- GIBSON L. E., COOKE R. E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959 Mar;23(3):545–549. [PubMed] [Google Scholar]
- Hornick D. B., Fick R. B., Jr The immunoglobulin G subclass composition of immune complexes in cystic fibrosis. Implications for the pathogenesis of the Pseudomonas lung lesion. J Clin Invest. 1990 Oct;86(4):1285–1292. doi: 10.1172/JCI114836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem E., Reisman J., Corey M., Canny G. J., Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992 Apr 30;326(18):1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- Meyer K. C., Lewandoski J. R., Zimmerman J. J., Nunley D., Calhoun W. J., Dopico G. A. Human neutrophil elastase and elastase/alpha 1-antiprotease complex in cystic fibrosis. Comparison with interstitial lung disease and evaluation of the effect of intravenously administered antibiotic therapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):580–585. doi: 10.1164/ajrccm/144.3_Pt_1.580. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Tegner H., Ohlsson K., Desgrandchamps D., Waldvogel F. A. Levels of free granulocyte elastase in bronchial secretions from patients with cystic fibrosis: effect of antimicrobial treatment against Pseudomonas aeruginosa. J Infect Dis. 1986 May;153(5):902–909. doi: 10.1093/infdis/153.5.902. [DOI] [PubMed] [Google Scholar]
- Suter S. The imbalance between granulocyte neutral proteases and antiproteases in bronchial secretions from patients with cystic fibrosis. Antibiot Chemother (1971) 1989;42:158–168. doi: 10.1159/000417616. [DOI] [PubMed] [Google Scholar]
- Taussig L. M., Kattwinkel J., Friedewald W. T., Di Sant'Agnese P. A. A new prognostic score and clinical evaluation system for cystic fibrosis. J Pediatr. 1973 Mar;82(3):380–390. doi: 10.1016/s0022-3476(73)80110-6. [DOI] [PubMed] [Google Scholar]
- Thompson A. B., Bohling T., Payvandi F., Rennard S. I. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990 Feb;115(2):148–158. [PubMed] [Google Scholar]