Abstract
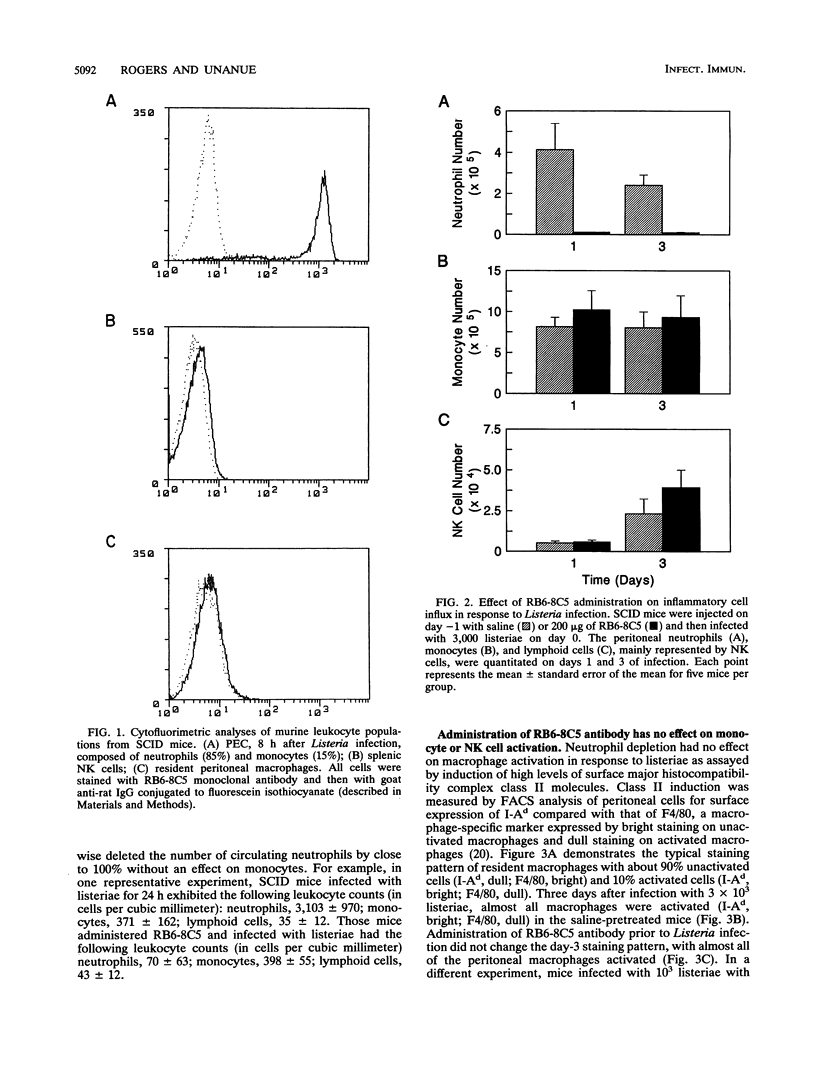
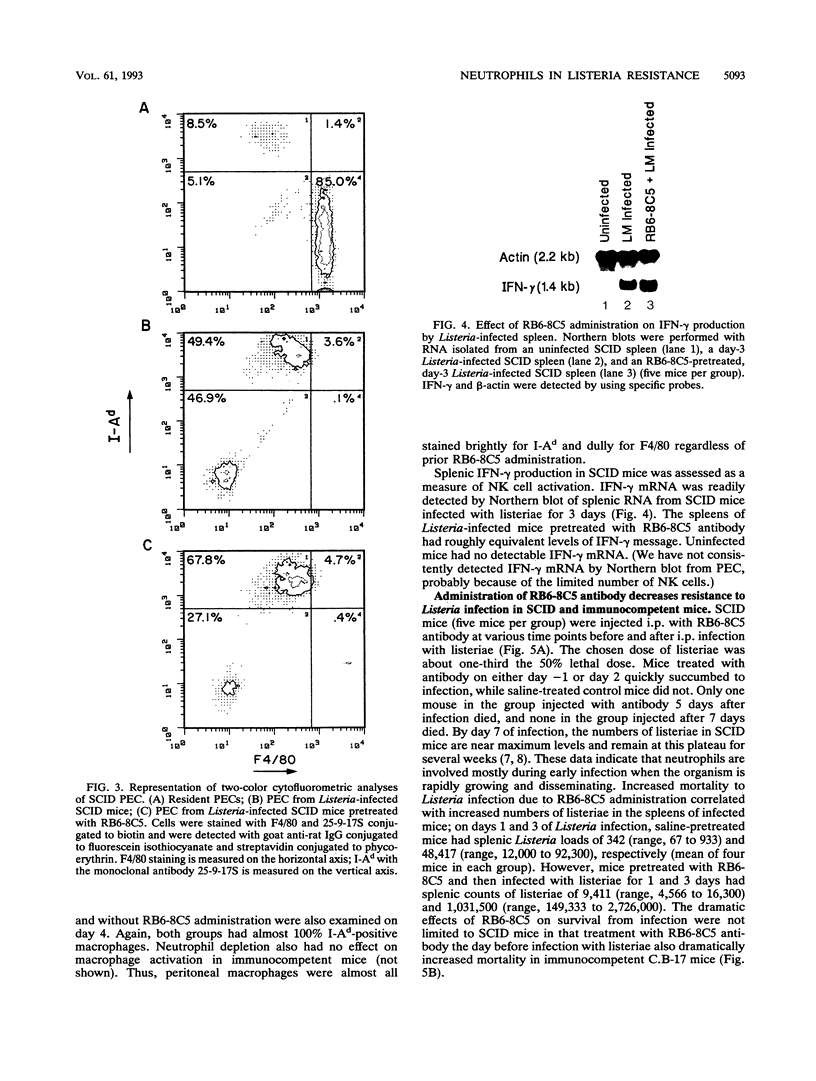
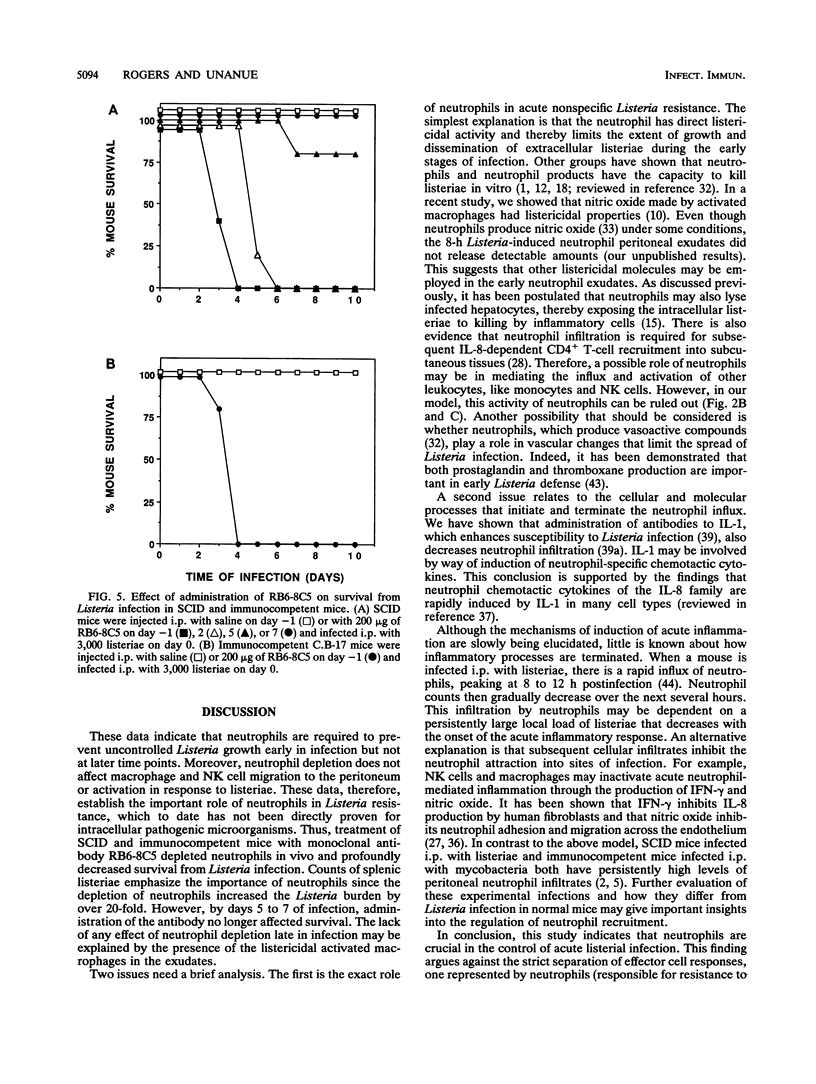
The importance of neutrophils in killing extracellular, pyogenic bacteria has long been established. However, there is only indirect evidence for a role for neutrophils in resistance against intracellular organisms. In this study, we directly demonstrate the involvement of neutrophils in defense against Listeria monocytogenes in normal C.B-17 immunocompetent and C.B-17 SCID mice. Because of the lack of sterilizing T-cell immunity, SCID mice are unable to completely eliminate listeriae systemically and become chronically infected. Both immunocompetent and SCID mice treated with a specific neutrophil-depleting monoclonal antibody during the early stages of Listeria infection were rendered remarkably sensitive to the organism, with a high level of mortality resulting from enhanced bacterial growth. At a late stage of infection in SCID mice, however, administration of neutrophil-depleting antibody did not affect mortality. In spite of the neutrophil depletion, other parameters of nonspecific immune function were normal. Macrophage infiltration to the site of infection and macrophage expression of major histocompatibility complex class II molecules were unaffected. Moreover, NK cell functions were normal as measured by infiltration to an infection site and gamma interferon production. These data demonstrate an important role for neutrophils in controlling the acute phase of Listeria infection, cooperating with, and yet independent of, macrophages and NK cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. E., Amaral E., Campbell P. A. Listericidal activity of human neutrophil cathepsin G. J Gen Microbiol. 1990 Jun;136(6):997–100. doi: 10.1099/00221287-136-6-997. [DOI] [PubMed] [Google Scholar]
- Appelberg R. Interferon-gamma (IFN-gamma) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992 Aug;89(2):269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J Leukoc Biol. 1992 May;51(5):472–477. doi: 10.1002/jlb.51.5.472. [DOI] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. M., Silva M. T. Host and bacterial factors control the Mycobacterium avium-induced chronic peritoneal granulocytosis in mice. Clin Exp Immunol. 1991 Feb;83(2):231–236. doi: 10.1111/j.1365-2249.1991.tb05620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Silva M. T. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989 Dec;78(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Baldridge J. R., Barry R. A., Hinrichs D. J. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990 Mar;58(3):654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Beckerman K. P., Rogers H. W., Corbett J. A., Schreiber R. D., McDaniel M. L., Unanue E. R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993 Feb 1;150(3):888–895. [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Vandenbroucke-Grauls C. M., van Asbeck B. S., Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987 Dec;55(12):3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Effects of purified anti-Lyt-2 mAb treatment on murine listeriosis: comparative roles of Lyt-2+ and L3T4+ cells in resistance to primary and secondary infection, delayed-type hypersensitivity and adoptive transfer of resistance. Immunology. 1990 Sep;71(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Recombinant murine interleukin-1 alpha enhancement of nonspecific antibacterial resistance. Infect Immun. 1987 Sep;55(9):2061–2065. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Canono B. P., Campbell P. A. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol. 1992 Jul;52(1):70–79. doi: 10.1002/jlb.52.1.70. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Campbell J. D., Schreiber R. D. Identification of a functionally important sequence in the C terminus of the interferon-gamma receptor. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11706–11710. doi: 10.1073/pnas.89.24.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg R. F., Sun L. H., Ordahl C. P., Frankel F. R. Identification of glucocorticoid-induced genes in rat hepatoma cells by isolation of cloned cDNA sequences. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5042–5046. doi: 10.1073/pnas.80.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P. L., Marchal G., Milon G. Transfer of both protection and delayed-type hypersensitivity against live Listeria is mediated by the CD8+ T cell subset: a study with Listeria-specific T lymphocytes recovered from murine infected liver. Int Immunol. 1992 May;4(5):591–598. doi: 10.1093/intimm/4.5.591. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo C., Araki A., Matsushima K., Sendo F. Inhibition of IL-8-induced W3/25+ (CD4+) T lymphocyte recruitment into subcutaneous tissues of rats by selective depletion of in vivo neutrophils with a monoclonal antibody. J Immunol. 1991 Oct 1;147(7):2196–2201. [PubMed] [Google Scholar]
- Kurlander R. J., Hoffman M., Kratz S. S., Gates J. Comparison of the effects of IL-1 alpha and TNF-alpha on phagocyte accumulation and murine antibacterial immunity. Cell Immunol. 1989 Oct 1;123(1):9–22. doi: 10.1016/0008-8749(89)90264-5. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Virgin H. W., 4th, Unanue E. R. Relationship of macrophage Ia and membrane IL 1 expression to antigen presentation. J Immunol. 1985 Dec;135(6):3652–3654. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. R., Oppenheim J. J. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992 May;13(5):169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira I. C., Sciavolino P. J., Lee T. H., Vilcek J. Downregulation of interleukin 8 gene expression in human fibroblasts: unique mechanism of transcriptional inhibition by interferon. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9049–9053. doi: 10.1073/pnas.89.19.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Active and memory immunity to Listeria monocytogenes infection in mice is mediated by phenotypically distinct T-cell populations. Immunology. 1989 Sep;68(1):93–95. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. W., Sheehan K. C., Brunt L. M., Dower S. K., Unanue E. R., Schreiber R. D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Mieno M., Udono H., Yamaguchi K., Usui T., Hara K., Shiku H., Nakayama E. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon gamma in listerial infection. J Exp Med. 1990 Apr 1;171(4):1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Kumar V., Bennett M. Rejection of bone marrow cell allografts by natural killer cell subsets: 5E6+ cell specificity for Hh-1 determinant 2 shared by H-2d and H-2f. Eur J Immunol. 1991 Nov;21(11):2821–2828. doi: 10.1002/eji.1830211125. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Needleman P., Unanue E. R. Indomethacin in vivo increases the sensitivity to Listeria infection in mice. A possible role for macrophage thromboxane A2 synthesis. J Clin Invest. 1987 Feb;79(2):399–403. doi: 10.1172/JCI112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Unanue E. R., Needleman P. Monocyte migration explains the changes in macrophage arachidonate metabolism during the immune response. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9655–9659. doi: 10.1073/pnas.83.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Wout J. W., Van der Meer J. W., Barza M., Dinarello C. A. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. Eur J Immunol. 1988 Jul;18(7):1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]



