Abstract
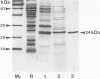
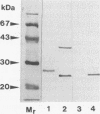
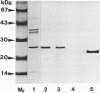
The superoxide dismutase (SOD) of Helicobacter pylori, a pathogenic bacterium which colonizes the gastric mucosa, evoking a marked inflammatory response, was purified and characterized, and the N-terminal amino acid sequence was determined. The enzyme consists of two identical subunits each with an apparent molecular weight of 24,000. Analysis of the primary structure and inhibition studies revealed that H. pylori possesses a typical procaryotic iron-containing enzyme. No other isoenzymes could be detected. Indirect gold immunostaining of H. pylori SOD with a polyclonal antibody directed against the iron-containing SOD of Escherichia coli showed a surface-associated localization of the enzyme. The H. pylori SOD gene was cloned by functional complementation of a SOD-deficient E. coli mutant. Sequencing and alignment revealed striking homology to the following facultative intracellular human pathogens: Listeria ivanovii, Listeria monocytogenes, Coxiella burnetti, Porphyromonas gingivalis, Legionella pneumophila, and Entamoeba histolytica. An open reading frame of 642 bp encoding 214 amino acids was determined. There was no leader sequence detectable. Cloning of the H. pylori SOD gene is one of the prerequisites to investigation of its pathophysiological role in the defense against antimicrobial mechanisms of polymorphonuclear granulocytes.
Full text
PDF


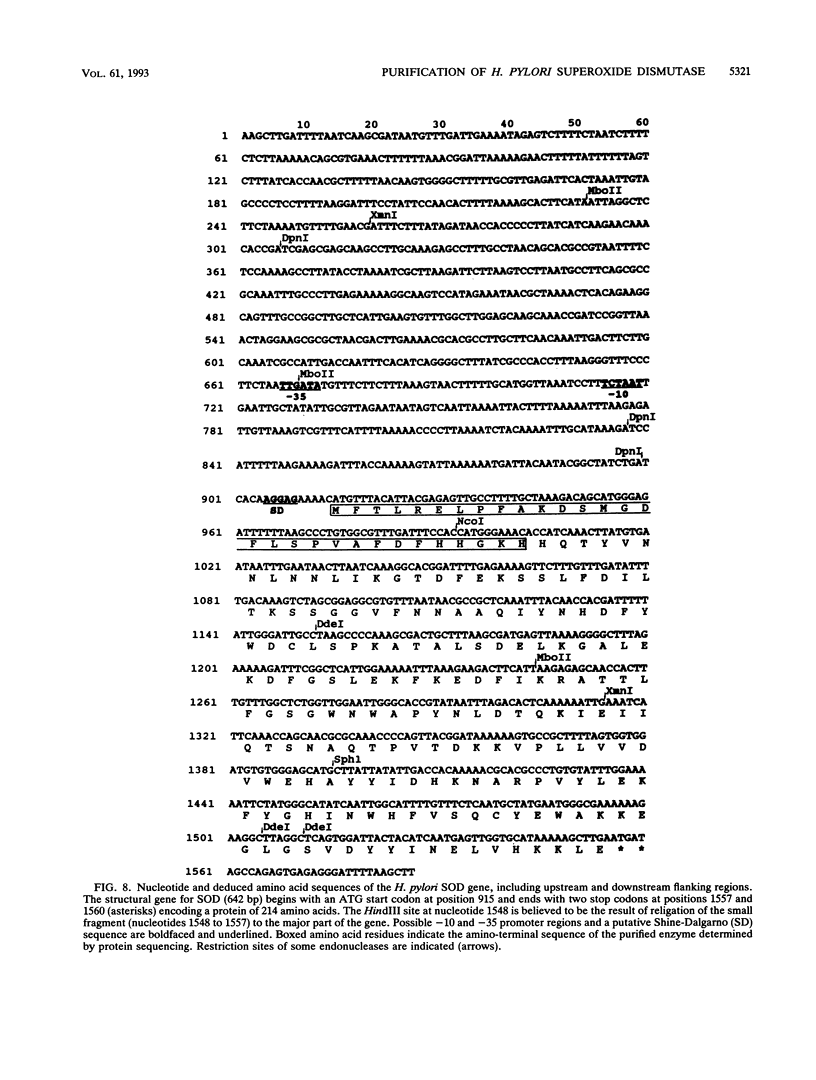
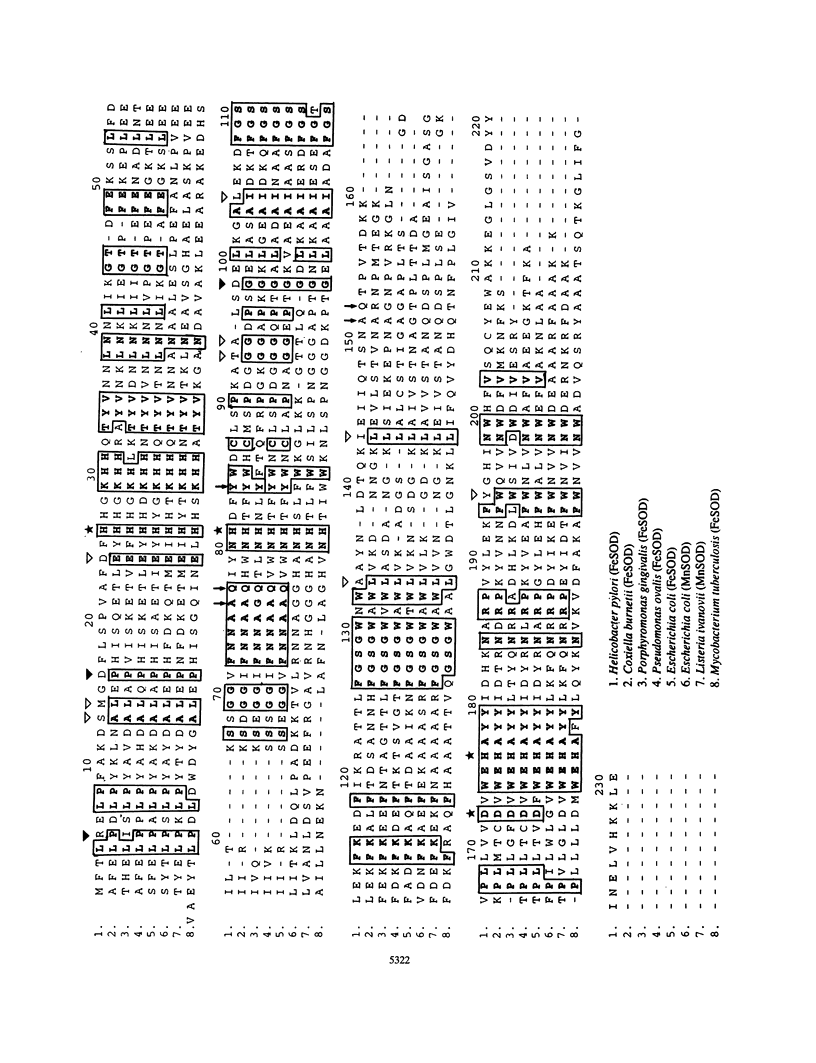



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano A., Ishimoto T., Tamagawa H., Shizukuishi S. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun. 1992 Feb;60(2):712–714. doi: 10.1128/iai.60.2.712-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen L. P., Blom J., Nielsen H. Survival and ultrastructural changes of Helicobacter pylori after phagocytosis by human polymorphonuclear leukocytes and monocytes. APMIS. 1993 Jan;101(1):61–72. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béji A., Mégraud F., Vincent P., Gavini F., Izard D., Leclerc H. GC content of DNA of Campylobacter pylori and other species belonging or related to the genus Campylobacter. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):527–534. doi: 10.1016/0769-2609(88)90152-4. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. L., Wren B. W., Mullany P., Topping A., Tabaqchali S. Molecular cloning and expression of Campylobacter pylori species-specific antigens in Escherichia coli K-12. Infect Immun. 1989 Feb;57(2):623–629. doi: 10.1128/iai.57.2.623-629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac V., Ferrero R. L., Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992 Apr;174(8):2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eschweiler B., Bohrmann B., Gerstenecker B., Schiltz E., Kist M. In situ localization of the 60 k protein of Helicobacter pylori, which belongs to the family of heat shock proteins, by immuno-electron microscopy. Zentralbl Bakteriol. 1993 Sep;280(1-2):73–85. doi: 10.1016/s0934-8840(11)80942-4. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Karjalainen T. K., Evans D. J., Jr, Graham D. Y., Lee C. H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993 Feb;175(3):674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzon V. L., Arondel J., Sansonetti P. J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990 Feb;58(2):529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier B. A., Pfeifer J. D., Russell D. G., Falk P., Olsén A. N., Hammar M., Westblom T. U., Normark S. J. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. J Bacteriol. 1993 Feb;175(4):966–972. doi: 10.1128/jb.175.4.966-972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):1–13. doi: 10.1007/BF01969526. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., McCulloch R. K., Armstrong J. A., Wee S. H. Unusual cellular fatty acids and distinctive ultrastructure in a new spiral bacterium (Campylobacter pyloridis) from the human gastric mucosa. J Med Microbiol. 1985 Apr;19(2):257–267. doi: 10.1099/00222615-19-2-257. [DOI] [PubMed] [Google Scholar]
- Haas A., Goebel W. Cloning of a superoxide dismutase gene from Listeria ivanovii by functional complementation in Escherichia coli and characterization of the gene product. Mol Gen Genet. 1992 Jan;231(2):313–322. doi: 10.1007/BF00279805. [DOI] [PubMed] [Google Scholar]
- Haas A., Goebel W. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radic Res Commun. 1992;16(3):137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- Hassett D. J., Cohen M. S. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989 Dec;3(14):2574–2582. doi: 10.1096/fasebj.3.14.2556311. [DOI] [PubMed] [Google Scholar]
- Hawtin P. R., Stacey A. R., Newell D. G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990 Oct;136(10):1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- Heinzen R. A., Frazier M. E., Mallavia L. P. Coxiella burnetii superoxide dismutase gene: cloning, sequencing, and expression in Escherichia coli. Infect Immun. 1992 Sep;60(9):3814–3823. doi: 10.1128/iai.60.9.3814-3823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. Structure-function relationships in Escherichia coli promoter DNA. Prog Nucleic Acid Res Mol Biol. 1990;38:137–164. doi: 10.1016/s0079-6603(08)60710-2. [DOI] [PubMed] [Google Scholar]
- Isobe T., Fang Y. I., Muno D., Okuyama T., Ohmori D., Yamakura F. Amino acid sequence of iron-superoxide dismutase from Pseudomonas ovalis. FEBS Lett. 1987 Oct 19;223(1):92–96. doi: 10.1016/0014-5793(87)80516-1. [DOI] [PubMed] [Google Scholar]
- Jones D. M., Lessells A. M., Eldridge J. Campylobacter like organisms on the gastric mucosa: culture, histological, and serological studies. J Clin Pathol. 1984 Sep;37(9):1002–1006. doi: 10.1136/jcp.37.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist M. Isolierung und Identifizierung von Bakterien der Gattungen Campylobacter und Helicobacter. Zentralbl Bakteriol. 1991 Dec;276(1):124–139. [PubMed] [Google Scholar]
- Kist M., Spiegelhalder C., Moriki T., Schaefer H. E. Interaction of Helicobacter pylori (strain 151) and Campylobacter coli with human peripheral polymorphonuclear granulocytes. Zentralbl Bakteriol. 1993 Sep;280(1-2):58–72. doi: 10.1016/s0934-8840(11)80941-2. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Harel J., Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for genetic analysis of Campylobacter jejuni. J Bacteriol. 1987 Nov;169(11):5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leying H., Suerbaum S., Geis G., Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992 Oct;6(19):2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nakayama K. The superoxide dismutase-encoding gene of the obligately anaerobic bacterium Bacteroides gingivalis. Gene. 1990 Nov 30;96(1):149–150. doi: 10.1016/0378-1119(90)90357-w. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. Negative staining of viruses. J Microsc. 1972 Dec;96(3):351–362. doi: 10.1111/j.1365-2818.1972.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992 Dec;103(6):1747–1753. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Logan S. M., Kostrzynska M., Wadström T., Trust T. J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991 Jan;173(2):505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schininà M. E., Maffey L., Barra D., Bossa F., Puget K., Michelson A. M. The primary structure of iron superoxide dismutase from Escherichia coli. FEBS Lett. 1987 Aug 31;221(1):87–90. doi: 10.1016/0014-5793(87)80357-5. [DOI] [PubMed] [Google Scholar]
- Sobala G. M., Crabtree J. E., Dixon M. F., Schorah C. J., Taylor J. D., Rathbone B. J., Heatley R. V., Axon A. T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991 Nov;32(11):1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Suerbaum S., Josenhans C., Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993 Jun;175(11):3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M., Yasui A., Oikawa A. Unique characteristics of superoxide dismutase of a strictly anaerobic archaebacterium Methanobacterium thermoautotrophicum. J Biol Chem. 1991 Aug 5;266(22):14151–14154. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]