Abstract
Lipopolysaccharide (LPS) extracted from three strains of Salmonella typhimurium, i.e., the rough Re mutant SL1102, the rough Ra mutant TV119, and the smooth strain SH4809, was first electrodialyzed (eLPS) and then divalent cation deprived by EDTA treatment and finally made monomeric by deoxycholate solubilization. The removal of excess detergent by extensive dialysis in the absence of mineral cations resulted in the reassociation of LPS subunits into monodisperse micelles of reduced aggregation number (dLPS) as estimated by electron microscopy and gel filtration chromatography. For all LPS chemotypes tested, the developed procedure reproducibly results in stable and clear solutions of dLPS in concentrations of up to 100 mg/ml. The dLPS and eLPS preparations possessed the same reactivity with monoclonal antibodies (MAbs) raised against different LPS domains. The 100% lethal dose in galactosamine-sensitized mice of 0.01 microgram for the smooth eLPS was from 10- to 100-fold lower than that of dLPS at 0.1 to 1.0 microgram. dLPS from both the smooth strain and the Ra mutant had a significantly reduced capacity to activate the proenzyme cascade in the Limulus amoebocyte lysate assay in comparison with the slightly reduced activity of dLPS from the Re mutant. In contrast, dLPS as well as the deoxycholate-dispersed and then diluted eLPS from the smooth strain had a higher mitogenic activity on splenocytes than eLPS. The results indicate that the biological and endotoxic properties of LPS are significantly influenced by the physical state of its aggregates in aqueous solutions. The approach developed for production of a stable and dispersed form of LPS should further assist in investigation of LPS properties and interpretation of the data of endotoxic research.
Full text
PDF


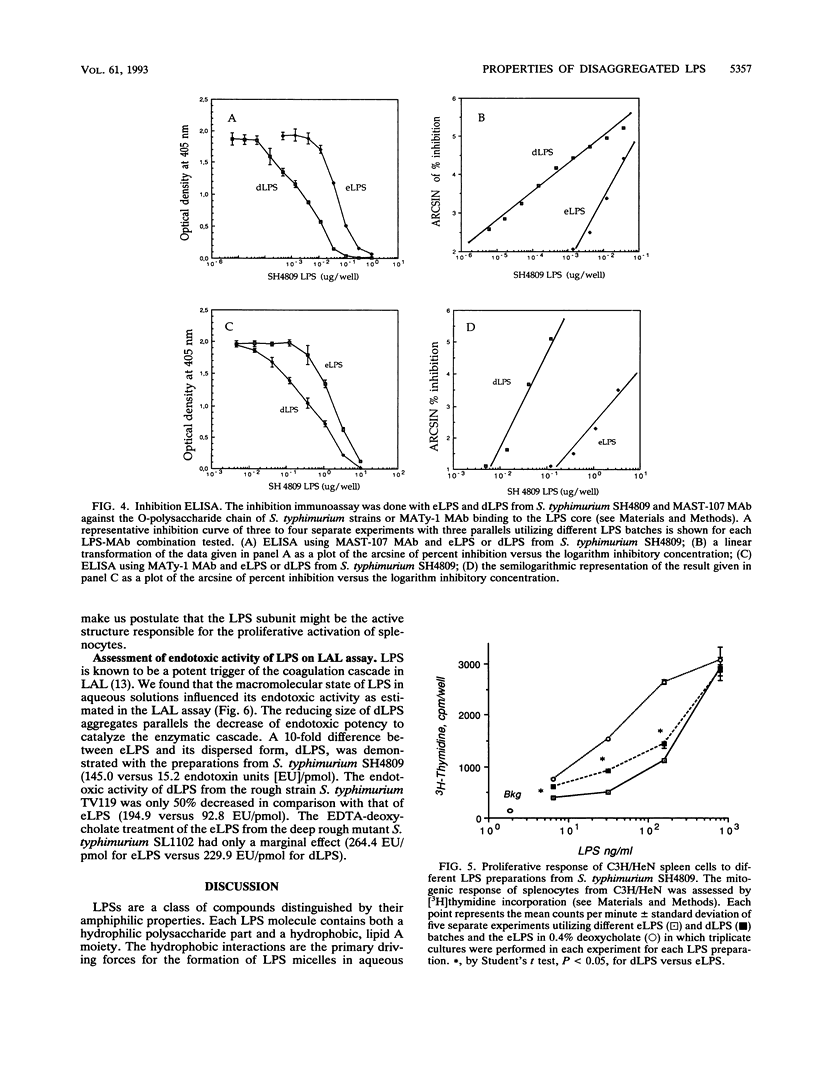
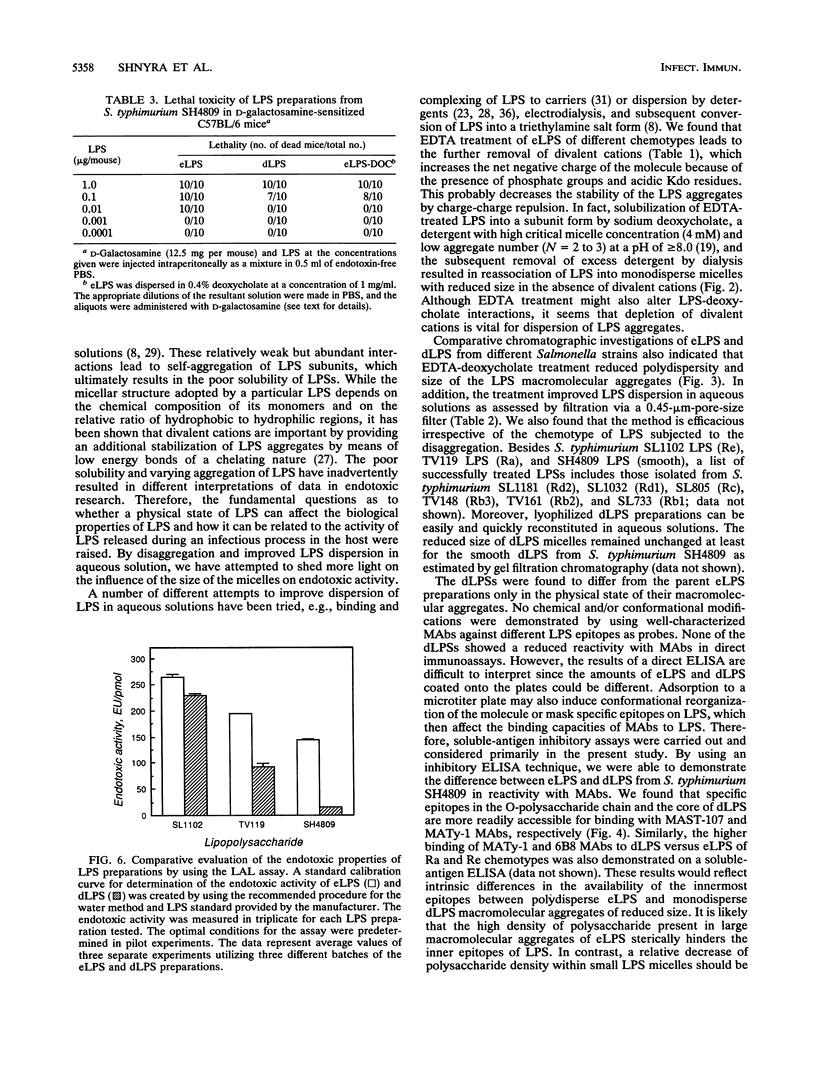


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gagliardi N. C., Nolan J. P., Moreno Feind D., DeLissio M. A rapid sensitive monoclonal assay for lipid A in solution. J Immunol Methods. 1986 Jul 24;91(2):243–247. doi: 10.1016/0022-1759(86)90485-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Matsuura M., Coumbos A. Hypersensitivity to endotoxin and mechanisms of host-response. Prog Clin Biol Res. 1988;272:295–308. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Luk J. M., Lind S. M., Tsang R. S., Lindberg A. A. Epitope mapping of four monoclonal antibodies recognizing the hexose core domain of Salmonella lipopolysaccharide. J Biol Chem. 1991 Dec 5;266(34):23215–23225. [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J. M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]
- Savory J., Wiggins J. W., Heintges M. G. Measurements of calcium and magnesium in serum and urine by atomic absorption spectrometry. Am J Clin Pathol. 1969 Jun;51(6):720–727. doi: 10.1093/ajcp/51.6.720. [DOI] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Chun P. W. The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J Biol Chem. 1980 Feb 10;255(3):1221–1226. [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Shnyra A. A., Kalantarov G. F., Vlasik T. N., Trakht I. N., Mayatnikov A. J., Tabachnik A. L., Borovikov D. V., Golubykh V. L. Monoclonal antibody to lipid A prevents the development of haemodynamic disorders in endotoxemia. Adv Exp Med Biol. 1990;256:681–684. doi: 10.1007/978-1-4757-5140-6_63. [DOI] [PubMed] [Google Scholar]
- Takayama K., Din Z. Z., Mukerjee P., Cooke P. H., Kirkland T. N. Physicochemical properties of the lipopolysaccharide unit that activates B lymphocytes. J Biol Chem. 1990 Aug 15;265(23):14023–14029. [PubMed] [Google Scholar]
- Tesh V. L., Vukajlovich S. W., Morrison D. C. Endotoxin interactions with serum proteins relationship to biological activity. Prog Clin Biol Res. 1988;272:47–62. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Interactions of bacterial lipopolysaccharides with serum proteins. Prog Clin Biol Res. 1988;272:309–318. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Conversion of lipopolysaccharides to molecular aggregates with reduced subunit heterogeneity: demonstration of LPS-responsiveness in "endotoxin-unresponsive" C3H/HeJ splenocytes. J Immunol. 1983 Jun;130(6):2804–2808. [PubMed] [Google Scholar]