Abstract
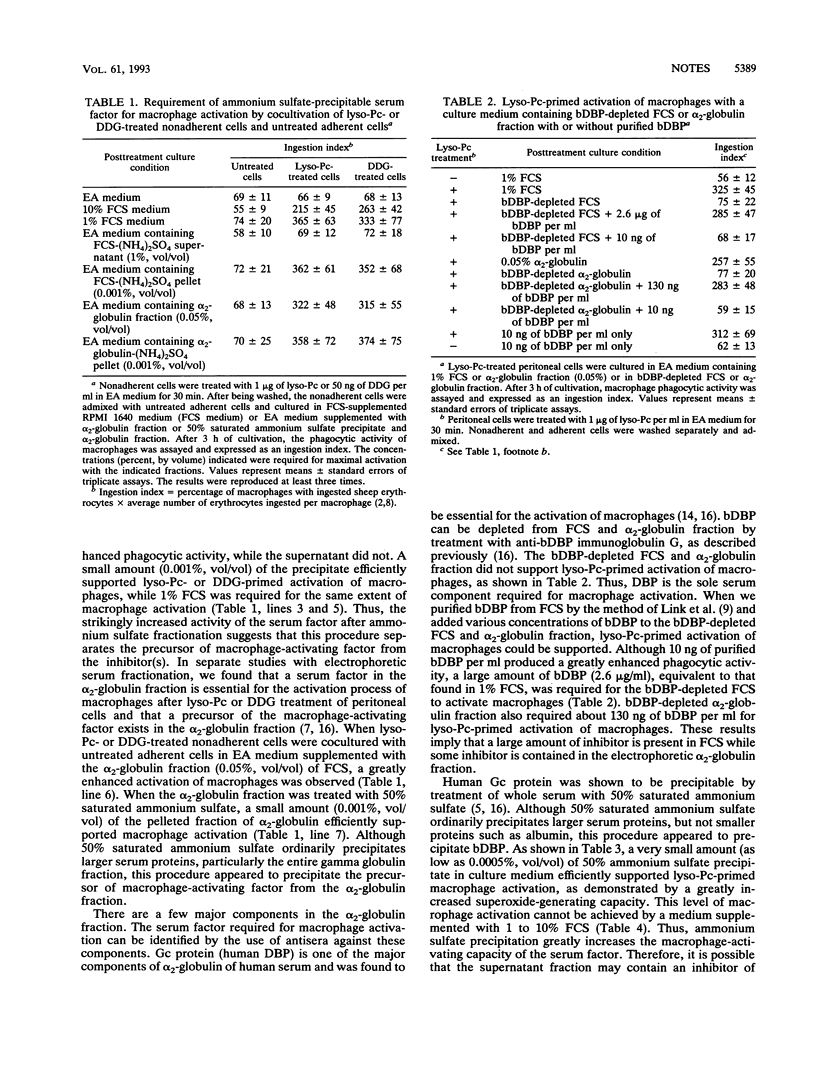
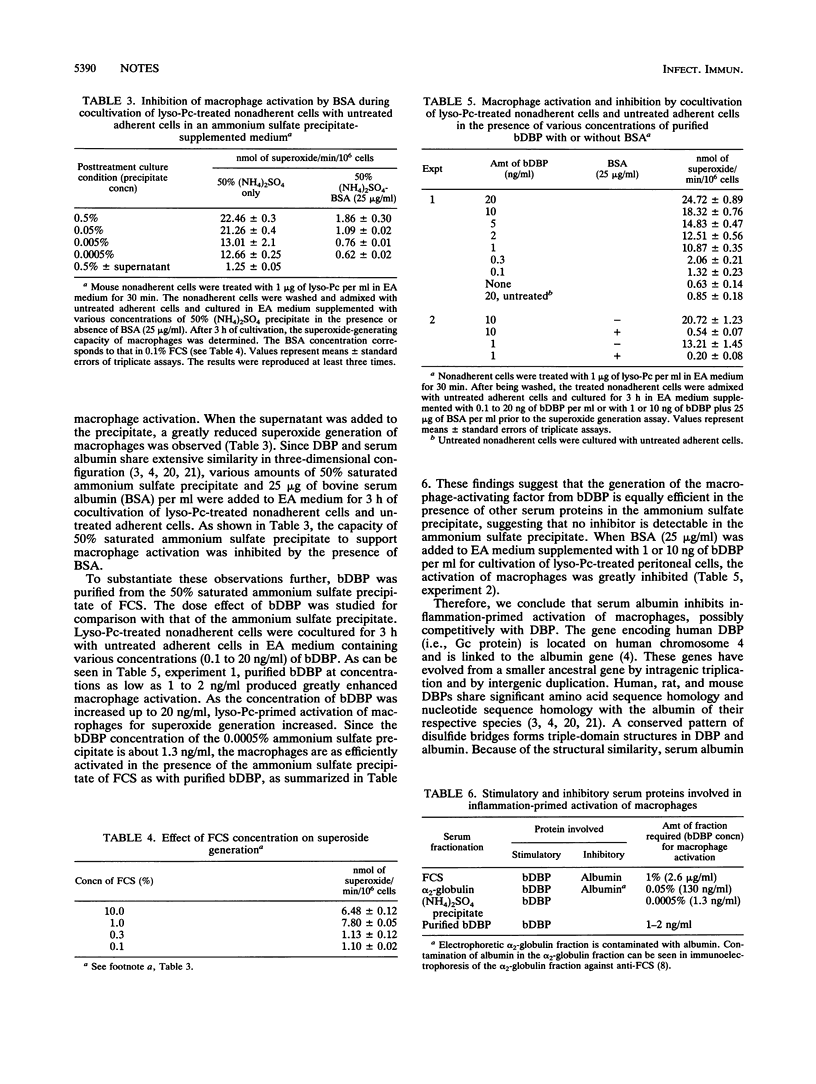
A very small amount (0.0005 to 0.001%) of an ammonium sulfate [50% saturated (NH4)2SO4]-precipitable protein fraction of alpha 2-globulin efficiently supported inflammation-primed activation of macrophages. This fraction contains vitamin D3-binding protein essential for macrophage activation. Comparative macrophage activation studies with fetal calf serum, alpha 2-globulin fraction, 50% (NH4)2SO4 precipitate, and purified bovine vitamin D3-binding protein revealed that fetal calf serum and alpha 2-globulin fraction appear to contain an inhibitor for macrophage activation while ammonium sulfate precipitate contains no inhibitor. This inhibitor was found to be serum albumin. When bovine serum albumin (25 micrograms/ml) was added to a medium supplemented with 0.0005 to 0.05% (NH4)2SO4 precipitate or 1 to 10 ng of vitamin D3-binding protein per ml, activation of macrophages was inhibited.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke N. E., Haddad J. G. Vitamin D binding protein (Gc-globulin). Endocr Rev. 1989 Aug;10(3):294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- Cooke N. E. Rat vitamin D binding protein. Determination of the full-length primary structure from cloned cDNA. J Biol Chem. 1986 Mar 5;261(7):3441–3450. [PubMed] [Google Scholar]
- Coppenhaver D. H., Sollenne N. P., Bowman B. H. Post-translational heterogeneity of the human vitamin D-binding protein (group-specific component). Arch Biochem Biophys. 1983 Oct 1;226(1):218–223. doi: 10.1016/0003-9861(83)90287-4. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., White J. G., Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974 Mar 22;248(446):347–348. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- Homma S., Millman I., Yamamoto N. A serum factor for macrophage activation after in vitro dodecylglycerol treatment of mouse lymphocytes. Immunol Cell Biol. 1990 Apr;68(Pt 2):137–142. doi: 10.1038/icb.1990.19. [DOI] [PubMed] [Google Scholar]
- Homma S., Yamamoto N. Activation process of macrophages after in vitro treatment of mouse lymphocytes with dodecylglycerol. Clin Exp Immunol. 1990 Feb;79(2):307–313. doi: 10.1111/j.1365-2249.1990.tb05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link R. P., Perlman K. L., Pierce E. A., Schnoes H. K., DeLuca H. F. Purification of human serum vitamin D-binding protein by 25-hydroxyvitamin D3-Sepharose chromatography. Anal Biochem. 1986 Sep;157(2):262–269. doi: 10.1016/0003-2697(86)90624-x. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Activation of peritoneal macrophages by lysophosphatidylcholine. Biochim Biophys Acta. 1985 Mar 29;839(1):9–15. doi: 10.1016/0304-4165(85)90175-8. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Contribution of lysophosphatidylcholine-treated nonadherent cells to mechanism of macrophage activation. Proc Soc Exp Biol Med. 1990 Feb;193(2):118–124. doi: 10.3181/00379727-193-43011. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Homma S., Haddad J. G., Kowalski M. A. Vitamin D3 binding protein required for in vitro activation of macrophages after alkylglycerol treatment of mouse peritoneal cells. Immunology. 1991 Nov;74(3):420–424. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Homma S., Millman I. Identification of the serum factor required for in vitro activation of macrophages. Role of vitamin D3-binding protein (group specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J Immunol. 1991 Jul 1;147(1):273–280. [PubMed] [Google Scholar]
- Yamamoto N., Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z. Activation of mouse peritoneal macrophages by lysophospholipids and ether derivatives of neutral lipids and phospholipids. Cancer Res. 1987 Apr 15;47(8):2008–2013. [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z., Sery T. W., Pieringer R. A. Activation of macrophages by ether analogues of lysophospholipids. Cancer Immunol Immunother. 1987;25(3):185–192. doi: 10.1007/BF00199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., St Claire D. A., Jr, Homma S., Ngwenya B. Z. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988 Nov 1;48(21):6044–6049. [PubMed] [Google Scholar]
- Yang F., Bergeron J. M., Linehan L. A., Lalley P. A., Sakaguchi A. Y., Bowman B. H. Mapping and conservation of the group-specific component gene in mouse. Genomics. 1990 Aug;7(4):509–516. doi: 10.1016/0888-7543(90)90193-x. [DOI] [PubMed] [Google Scholar]
- Yang F., Brune J. L., Naylor S. L., Cupples R. L., Naberhaus K. H., Bowman B. H. Human group-specific component (Gc) is a member of the albumin family. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7994–7998. doi: 10.1073/pnas.82.23.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]


