Abstract
Background
High mobility group box-1 (HMGB1) protein, a critical mediator of inflammatory processes, is a novel predictor of adverse postinfarction clinical outcomes, being involved in the healing process after MI. Heart rate recovery (HRR), a marker of autonomic function defined as the fall in heart rate during the first minute after exercise, is a powerful predictor of mortality in postinfarction patients. The present study was designed to test the hypothesis that HMGB1 is associated with autonomic dysfunction in postinfarction patients.
Methods
Sixty-seven consecutive patients (mean age 59.3 years, 84% males) recovering from acute MI were included in the study protocol. All patients underwent Doppler-echocardiography, cardiopulmonary exercise and HMGB1 assay.
Results
HMGB1 levels were inversely correlated with peak oxygen consumption (VO2peak) (r=-0.449, P<0.001), with left ventricular ejection fraction (LVEF) (r=-0.360, P=0.003), and with HRR (r=-0.387, P<0.001). In a linear regression analysis adjusted for multiple confounders, we found a significant inverse association between HMGB1 levels and HRR independent of age, gender, body mass index, VO2peak, slope of increase in ventilation over carbon dioxide output (VE/VCO2slope), and presence of diabetes (β=-0.377, p=0.034).
Conclusions
This study provided the first evidence for a significant association between increased HMGB1 levels and autonomic dysfunction expressed by post-exercise slower HRR in postinfarction patients. The prognostic implication of such association needs to be explored as well as whether HMGB1 could represent a valid marker for risk stratification either during the acute phase or long-term after MI.
Keywords: High Mobility Group Box-1, Autonomic Dysfunction, Heart Rate Recovery, Cardiac Remodeling, Myocardial Infarction, Left Ventricular Remodeling
Introduction
Left ventricular (LV) remodeling after myocardial infarction (MI) is characterized by complex alterations in LV size and shape, continuing long after infarction healing, representing an important predictor of long-term mortality [1]. Inflammatory response and cytokine elaboration are integral components of the host response to tissue injury and play a particularly active role after MI [2].
High mobility group box-1 (HMGB1) is a ubiquitous nuclear protein, constitutively expressed in quiescent cells, where it is involved in several cellular functions, including determination of nucleosomal structure and stability, and binding of transcription factors to DNA sequences [3]. The evidence that HMGB1 is passively released from necrotic or damaged cells suggests HMGB1 as an effective stimulus triggering the inflammatory response [4].
Previous studies showed that loss of baroreflex sensitivity and increased sympathetic afferent activity determines an increase of total sympathetic nervous activity in heart failure [5]. The sympathetic nervous system negatively impacts the cardiovascular system in heart failure in several ways, including down-regulating beta1-receptors, exerting direct toxic effects on the myocardium, and contributing to myocardial remodeling and life-threatening arrhythmias [5].
Heart rate recovery (HRR), defined as the fall in heart rate during the first minute after exercise, is a marker of vagal tone which is a powerful predictor of mortality in patients with coronary artery disease (CAD) [6-8], independently from angiographic severity of CAD, left ventricular function, and exercise capacity [9].
Given the potential interplay between inflammatory response and autonomic dysfunction on cardiac remodeling, this study aimed at testing the hypothesis that higher HMGB1 is associated with autonomic dysfunction (as expressed by slower HRR) in patients after acute MI.
Methods
Study population
From October 2007 to November 2008, ninety-seven consecutive patients immediately after acute ST elevation myocardial infarction (first event) diagnosed according to American College of Cardiology/American Heart Association (ACC/AHA) guidelines [10] were screened for inclusion into the study. Patients were recruited at the Division of Cardiology, Department of Clinical Medicine, Cardiovascular and Immunological Sciences, University of Naples “Federico II”, Italy.
Patients with postinfarction residual myocardial ischemia, and pericarditis were excluded. Exclusion criteria were also age over 75 years, severe concomitant non-cardiac disease such as cancer, renal dysfunction (serum creatinine level over 3 mg/dl), and liver dysfunction (alanine aminotransferase/aspartate aminotransferase level >1.5 times the upper normal limit).
After exclusions, 67 postinfarction patients were enrolled into the study protocol. A number of assessments were performed in all postinfarction patients including: cardiovascular physical examination; body mass index (BMI, ratio between the weight and the square of the height); 12-lead electrocardiography; Doppler-echocardiography; symptom-limited cardiopulmonary exercise stress test with HRR evaluation; blood chemistry and HMGB1 assay. Age-, gender- and BMI-matched subjects (n=20) with no cardiac disease were included in the study protocol as control group.
The study was conducted according to the guidelines of the Declaration of Helsinki, and institutional ethical committee of the University of Naples “Federico II” (Italy) approved the protocol. The purpose of the protocol was explained to each subject, and written informed consent was obtained before beginning the study.
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing (CPX) was performed 3-4 weeks after MI. All patients underwent incremental CPX on a bicycle ergometer (Vmax 29C, Sensormedics, Yorba Linda, California) and autonomic function was evaluated by heart rate recovery (HRR, the difference between heart rate at peak exercise and heart rate at first minute of the cool-down period), as previously described [11,12].
Doppler-echocardiography
Doppler-echocardiography (Hewlett Packard Agilent Sonos 5500 phase-array scanner, Andover, MA, USA) was performed at a median of 1 day (range 0 to 3 days) after hospital admission as detailed elsewhere [13]. The Doppler-echocardiographic studies were all performed by the same physician who was blinded to the patient results of cardiopulmonary exercise stress testing and HMGB1 levels assay.
High Mobility Group Box -1 assay
HMGB1 levels were measured by an enzyme-linked immunosorbent assay (ELISA) kit assay according to the manufacturer's instructions (Shino-test Corporation, Kanagawa, Japan) [14]. Briefly, blood samples were collected from patients with acute MI within 3 hours after the hospital admission and immediately before thrombolysis or percutaneous transluminal coronary angioplasty (rescue or primary PTCA). After allowing the blood to coagulate, the serum was isolated by low-speed centrifugation at 4°C, and frozen/stored at -80°C until used to perform the ELISA test. The inter-assay as well as the intra-assay coefficient was <10%.
Statistics
Sample size determination was based on a t-test assuming a normal distribution with non-equal variance with the mean and standard deviation for the experimental group (postinfarction patients) of HMGB1 levels equal to 15 ± 7 and 2 ± 1 ng/dl for the control group derived from a previous study, respectively (14). The required sample size was calculated to be 20 subjects per group to detect on the size of one SD with α value of 0.05 (two-sided) and power (1 – β) of 0.8. Data are presented as mean, standard deviation and percentage. For continuous variables, unpaired t-test was used to compare means. The bivariate correlations procedure was used to compute Pearson's correlation coefficients. Multiple linear regression analysis (stepwise method) was performed with HMGB1 levels as dependent variable and with age, gender, body mass index, VO2peak, VE/VCO2slope, LVEF, presence of diabetes and HRR as independent variables. In assessing the suitability of the data for a linear regression model, the collinearity diagnostics were evaluated. A subgroup analysis was performed aiming at testing the differences in HMGB1 levels and HRR between non-diabetic and diabetic patients. Statistical significance was indicated at the level p<0.05. Statistical tests were performed with SPSS software, version 15.0.0.
Results
Clinical and demographic characteristics of the study population are shown in Table 1. Fifty-five percent of patients experienced anterior MI; 58% of the patients had a positive history of hypertension and 49% of dyslipidemia (Table 1). Thirty-two percent (n=16) of patients underwent primary PTCA (Table 1). Seventy-three percent of patients (n=49) underwent thrombolisis and rescue PTCA was performed in 16 out of 49 (32%) (Table 1). Sixty-eight percent (n=46) of patients were on angiotensin-converting enzyme inhibitor, 19% (n=13) of patients were on angiotensin receptor blocker; 65% (n=44) of patients were on beta-blockers and 43% (n=29) of patients were on statin therapy. HMGB1 levels, cardiopulmonary and Doppler-echocardiography data obtained in postinfarction patients were summarized in Table 2.
Table 1.
Demographic and clinical characteristics of the study population (n = 67).
| Age (years) (mean ± SD) | 59.3 ± 8.5 |
| Males (n, %) | 56 (84) |
| BMI (kg/m2) (mean ± SD) | 26.9 ± 2.9 |
| Anterior MI (n, %) | 39 (59) |
| Inferior MI (n, %) | 17 (25) |
| Other MI locations (n, %) | 11 (16) |
| PTCA (primary) (n, %) | 18 (27) |
| Thrombolysis (n, %) | 49 (73) |
| PTCA (rescue) (n, %) | 16 (32) |
| Peak CK-MB (U/L) | 277 ± 186 |
| Peak Troponin I (ng/ml) | 3.9 ± 16.5 |
| Hypertension (n, %) | 39 (58) |
| Dyslipidemia (n, %) | 33 (49) |
| Diabetes (n, %) | 13 (19) |
Data are expressed in mean ± standard deviation (SD) or percentage (%).
Abbreviations: BMI, body mass index; CK-MB, creatin kinase-MB; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Table 2.
HMGB1 levels, Doppler-echocardiography and cardiopulmonary parameters of the study population (n = 67).
| HMGB1 (ng/ml) | 14.6 ± 6.7 |
| LVIVSd (mm) | 10.8 ± 1.9 |
| LVPWTd (mm) | 11.2 ± 2.2 |
| LA dimension (mm) | 39.6 ± 2.4 |
| LVEDV (ml/m2) | 48.4 ± 6.9 |
| LVESV (ml/m2) | 31.3 ± 7.4 |
| LVEF (%) | 47.7 ± 6.9 |
| Peak E-wave velocity (cm/s) | 59.5 ± 7.0 |
| E/A ratio | 0.94 ± 0.2 |
| Systolic Blood Pressure (mmHg) | 120 ± 9 |
| Diastolic Blood Pressure (mmHg) | 76 ± 5 |
| Resting Heart Rate (beats/min) | 77 ± 5 |
| VO2peak (ml/kg/min) | 14.4 ± 4.1 |
| VE/VCO2slope | 31.9 ± 6.0 |
| Heart Rate Recovery (beats/min) | 20 ± 4 |
Data are expressed in mean ± standard deviation.
Abbreviations: HMGB1, high mobility group box-1 protein; LA, left atrium; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVIVSd, diastolic left ventricular interventricular septum; LVPWTd, left ventricular posterior wall thickness; VO2peak, oxygen consumption at peak exercise; VE/VCO2slope, slope of increase in ventilation over carbon dioxide output.
Control subjects (n=20) underwent medical history, physical examination, and complete blood chemistry. Each control was defined as age-, gender- and BMI- matched with postinfarction patients case when the differences between the case and control was less than 2 years and 1 kg/m2 for age and BMI, respectively. Five subjects reported a positive history of hypertension whereas 3 subjects reported mild dyslipidemia. HMGB1 levels in postinfarction patients were significantly higher compared to controls (14.6 ± 6.7 vs. 2.9 ± 1.4 ng/ml, p<0.0001).
In postinfarction patients, bivariate correlation analysis showed that age was not correlated either with HMGB1 levels (r=0.180, P=0.144) or HRR (r=-0.135, P=0.277). Bivariate correlation analysis showed that HMGB1 levels were inversely correlated with VO2peak (r=-0.449, P<0.001), LVEF (r=-0.360, P=0.003), and HRR (r=-0.387, P<0.001) (Figure 1).
Figure 1.
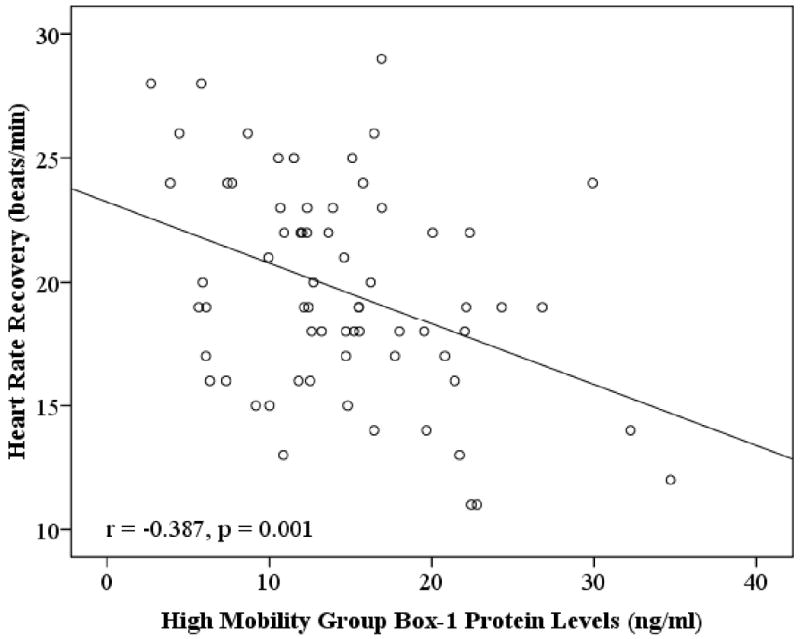
The relationship between High Mobility Group Box-1 Protein Levels (ng/ml) and post-exercise Heart Rate Recovery (beats/min). The best fit line is shown.
A subgroup analysis was performed in order to test the differences between diabetic (n = 13) and non-diabetic patients (n = 54). In postinfarction patients with diabetes, HMGB1 levels were significantly higher compared to postinfarction patients without diabetes (19.6 ± 7.0 vs. 13.4 ± 6.1 ng/ml, P=0.001). Postinfarction patients with diabetes showed slower HRR compared to postinfarction patients without diabetes (16.3 ± 4.0 vs. 20.4 ± 3.9 ng/ml, P=0.001). In diabetic patients, bivariate correlation analysis showed that HMGB1 levels were inversely correlated with HRR (r=-0.340, P=0.02).
We evaluated whether the association between HMGB1 levels and HRR was independent of age and gender. The association between HMGB1 levels and HRR was not influenced after adjusting for these two variables (Table 3, Model 1). Next, we attempted to identify potential mediators of the association between HMGB1 levels and HRR, by successively adding variables to the regression model. The association between HMGB1 levels and HRR remained unchanged after BMI was added to the model (Table 3, Model 2). Similarly, this association was not influenced by VO2peak (Table 3, Model 3), or by the addition of VE/VCO2slope (Table 3, Model 4). Finally, this association between HMGB1 levels and HRR remained unchanged after adjusting for LVEF (Table 3, Model 5), and presence of diabetes (Table 3, Model 6).
Table 3. Multivariate Models Examining the Relationship between High Mobility Group Box-1 Protein Levels and Heart Rate Recovery.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | B | SE | B | SE | B | SE | B | SE | B | SE | B | SE |
| Age | 0.106 | 0.087 | 0.155 | 0.084 | 0.040 | 0.086 | 0.030 | 0.084 | 0.049 | 0.081 | 0.045 | 0.079 |
| Gender | 1.507 | 2.070 | 0.146 | 2.014 | -0.195 | 1.882 | 0.084 | 1.828 | 0.001 | 1.753 | 0.099 | 1.744 |
| BMI | … | … | 0.756 | 0.261† | 0.652 | 0.246† | 0.555 | 0.242* | 0.464 | 0.235* | 0.474 | 0.229* |
| VO2peak | … | … | … | … | -0.590 | 0.184† | -0.523 | 0.181† | -0.476 | 0.174† | -0.452 | 0.189† |
| VE/VCO2slope | … | … | … | … | … | … | 0.257 | 0.116* | 0.245 | 0.111* | 0.252 | 0.119* |
| LVEF | … | … | … | … | … | … | … | … | -0.235 | 0.093* | -0.229 | 0.101* |
| Diabetes | … | … | … | … | … | … | … | … | … | … | 0.355 | 0.264* |
| Heart Rate Recovery | -0.575 | 0.182† | -0.558 | 0.172† | -0.496 | 0.162† | -0.427 | 0.160† | -0.396 | 0.154* | -0.377 | 0.156* |
p<0.05;
p<0.01.
For each Model, β-coefficient (B) and Standard Error (SE) are given. Model 1 = age and gender; Model 2 = Model 1 + BMI; Model 3 = Model 2 + VO2peak. Model 4 = Model 3 + VE/VCO2slope; Model 5 = Model 4 + LVEF; Model 6 = Model 5 + presence of diabetes.
Abbreviations: BMI, body mass index; LVEF, left ventricular ejection fraction; VO2peak, peak oxygen consumption; VE/VCO2slope, slope of increase in ventilation over carbon dioxide output.
Discussion
Several lines of evidence suggest that inflammatory response and autonomic function might potentially affect cardiac remodeling after MI. In this study, we showed a significant association between higher HMGB1 levels and slower HRR, a marker of autonomic dysfunction in postinfarction patients.
Previous studies showed that both exaggerate postinfarction inflammatory response [2,15-17] and autonomic function [6-9] are associated with LV remodelling and poor clinical outcomes. An efficient postinfarction inflammatory response leads to an appropriate infarct-healing process and the formation of a scar with tensile strength, resulting in prevention of infarct expansion. Despite the importance of the inflammatory response and healing process in postinfarction LV remodeling, the mechanisms that initiate and control these processes remain to be elucidated.
HMGB1 is quickly released extracellularly after ischemic injury, inducing the expression of pro-inflammatory cytokines and adhesion molecules as an inflammatory mediator [18]. A recent study showed that increased HMGB1 levels in patients with acute MI were associated with pump failure, cardiac rupture, and increased in-hospital cardiac death [19]. Interestingly, six months after MI, HMGB1 levels were positively correlated to serum C-reactive protein, suggesting that HMGB1 could be a predictor of adverse clinical outcomes and late-phase LV dysfunction after MI [19]. In addition, our research group found that increased HMGB1 levels in postinfarction patients were associated with impaired indices of cardiovascular functional capacity [14].
The pathophysiological role of HMGB1 in MI setting is controversial. Several lines of evidence have pointed out that HMGB1 might play a role in restoration of cardiac function after MI, probably by promoting stem cell recruitment and/or stimulating angiogenesis [20-22]. Exogenous administration of HMGB1 in the peri-infarcted LV might have therapeutic potential for the attenuation of LV remodeling in a permanent MI model through a mechanism that involves the activation of stem/progenitor cells [20-22]. In addition, the blockade of HMGB1 results in worsening of LV remodeling through impaired infarct healing and marked scar thinning, thus suggesting a key role of HMGB1 in the appropriate healing process and in preserving the structural integrity of the infarcted LV [19].
Conversely, other studies have underlined the negative effects exerted by HMGB1 on myocardial cells [23,24]. In particular, Tzeng et al. [23] recently found that HMGB1 might act as a novel myocardial depressant factor that may contribute to excessive and/or sustained inflammation and/or profound myocardial depression and myocardial collapse. Moreover, Hagiwara et al. [24] have demonstrated that this protein induces a negative inotropic effect in isolated rat hearts. Mechanisms by which HMGB1 might be involved in the negative effects on myocardial function have been partially explained with NF-κB, ERKs and PKCε suggested as potential cellular pathways [23,25].
It is clear that there is currently a disagreement regarding the role of HMGB1 in ischemic heart disease; the studies by Kitahara et al.[20] and Limana et al.[21] indicated that it is beneficial, whereas the study by Andrassy et al.[25] suggested the contrary. The reason for this discrepancy remains unclear at present; however, we should consider the differences in the experimental conditions of these studies. First, in the former two studies, permanent ligation of the left anterior descendent (LAD) coronary artery was performed, i.e. permanent MI was induced. In contrast, in the latter study, the heart was reperfused after a 30 min ligation of the LAD artery. There is a substantial difference between the pathophysiology of permanent MI and ischemia–reperfusion injury. Reperfusion is accompanied by the release of an excessive amount of oxygen-derived free radicals that cause reperfusion injury. The inflammatory responses, including neutrophils and macrophage infiltration, are much more severe in reperfusion injury than in infarction, suggesting that ischemia–reperfusion injury could enhance inflammatory cell activity.
The mechanisms by which HMGB1 might exert beneficial or deleterious effects on myocardium are not completely elucidated yet. It has been reported that HMGB1 administration is beneficial in low doses and detrimental in high doses, suggesting that the effects of HMGB1 are dose dependent [18]. In particular, HMGB1 secreted from the infarcted, necrotic myocardium might slightly increase its local concentrations, resulting in the proliferation of resident cardiac c-kit+ stem cells and their differentiation into myocytes [20]. Conversely, in postinfarction patients who are quickly reperfused with PTCA or thrombolysis (i.e. our study population), increased recruitment of inflammatory cells such as neutrophils and macrophages due to ischemia followed by reperfusion, might significantly increase local HMGB1 levels with consequent detrimental effects on cardiac function. Therefore, HMGB1 might act as a double-edged sword in postinfarction inflammatory response and have bidirectional effects on LV remodeling depending on the site, extent, and timing of HMGB1 modulation [26].
Several observations might constitute the pathophysiological basis for understanding the prognostic effects of sympatho-vagal activity after MI [11,12]. The most obvious beneficial effect of cardiac vagal activity is to decrease cardiac work by reducing resting heart rate and contractility [27]. The combination of this reduction in contractility with a reduction in cardiac work and myocardial oxygen demand may be advantageous in the context of coronary artery disease and left ventricular dysfunction. In addition, stimulation of the vagus nerve inhibits sympathetic nerve activity via peripheral pre- [28] and post-synaptic interactions [29]. Myocardial exposure to high levels of noradrenalin results in β receptor-mediated cytotoxic effects and apoptosis as well as α receptor-mediated hypertrophic effects [30,31].
The pathophysiological mechanisms underlying the association between HMGB1 and HRR are unclear. Cross-sectional studies have suggested that autonomic function is related to inflammatory markers [32]. Experimental studies reported that vagal nerve stimulation could modulate inflammatory cytokines through the cholinergic anti-inflammatory pathway [33,34], thus suggesting that HRR may be related to inflammatory markers through a cholinergic anti-inflammatory reflex. Interestingly, in patients with diabetes elevated levels of HMGB1 were significantly associated with slower HRR, which is strongly associated with cardiovascular outcome in these patients [35]. Indeed, the engagement of advanced glycation end (AGE) products with their receptor (RAGE) and subsequent signalling has been suggested as responsible of development of vascular complications in diabetic patients [36]. Moreover, in this patient population, HMGB1 can interact with RAGE or with Toll-like receptor on inflammatory cells thus activating inflammation-associated pathways which involve several pro-inflammatory cytokines [37]. This HMGB1-RAGE interaction might significantly contribute to faster progression of atherosclerosis in diabetic patients. Several studies have demonstrated the strong relationship existing between RAGE and diabetic neuropathy [38,39]; thus, it could be plausible that the HMGB1-RAGE axis might be involved also in autonomic dysfunction as HRR impairment is observed in diabetic patients.
Given the recognized role of HMGB1 proteins in the healing process after MI and the beneficial effects of cardiac rehabilitation on both LV remodeling [40] and autonomic function [11,13], the results of a 6-month exercise-based cardiac rehabilitation trial currently ran in our unit would be helpful in determining whether exercise training modulates HMGB1 protein expression pattern and its relationship with some powerful indicators of cardiovascular function, such as cardiovascular capacity, autonomic dysfunction and LV remodeling after MI [41].
In conclusion, this study provides the first evidence of a significant association between inflammation (as expressed by increased HMGB1 levels) and autonomic dysfunction (as expressed by post-exercise lower HRR) in patients after MI. The prognostic implication of such association needs to be explored. Studies aimed at clarifying whether HMGB1 could represent a valid marker for risk stratification either during the acute phase or the follow-up phases in postinfarction patients are also strongly encouraged.
Acknowledgments
This research was supported by University of Naples “Federico II” and in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling: concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 3.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high mobility group chromosomal proteins. Mol Cell Biol. 1999;19:5237–46. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 5.Pepper GS, Lee RW. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch Intern Med. 1999;159(3):225–34. doi: 10.1001/archinte.159.3.225. [DOI] [PubMed] [Google Scholar]
- 6.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 7.Nishime EO, Cole CR, Blackstone EH, et al. Heart-rate recovery and treadmill exercise score as a predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe J, Thamilarasan M, Blackstone EH, et al. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality. Circulation. 2001;104:1911–6. [PubMed] [Google Scholar]
- 9.Vivekananthan DP, Blackstone EH, Pothier CE, et al. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–8. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction] J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Giallauria F, De Lorenzo A, Pilerci F, et al. Long-term effects of cardiac rehabilitation on end-exercise heart rate recovery after myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2006;13:544–50. doi: 10.1097/01.hjr.0000216547.07432.fb. [DOI] [PubMed] [Google Scholar]
- 12.Giallauria F, Lucci R, Pietrosante M, et al. Exercise-based cardiac rehabilitation improves heart rate recovery in elderly patients after acute myocardial infarction. J Gerontol A Biol Sci Med Sci. 2006;61:713–7. doi: 10.1093/gerona/61.7.713. [DOI] [PubMed] [Google Scholar]
- 13.Giallauria F, Galizia G, Lucci R, D'Agostino M, Vitelli A, Maresca L, et al. Favourable effects of exercise-based cardiac rehabilitation after acute myocardial infarction on left atrial remodelling. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.05.026. in press. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo P, Giallauria F, Pacileo M, et al. Increased High Mobility Group Box-1 Protein levels are associated with impaired cardiopulmonary and echocardiographic findings after acute myocardial infarction. J Card Fail. 2009;15(4):362–7. doi: 10.1016/j.cardfail.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Sun M, Dawood F, Wen WH, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–6. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 17.Maekawa Y, Anzai T, Yoshikawa T, et al. Effect of granulocyte–macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–20. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 18.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 19.Kohno T, Anzai T, Naito K, et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodeling. Cardiovasc Res. 2009;81:565–73. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 20.Kitahara T, Takeishi Y, Harada M, et al. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res. 2008;80:40–4. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- 21.Limana F, Germani A, Zacheo A, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 22.Chavakis E, Hain A, Vinci M, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–12. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng HP, Fan J, Vallejo JG, et al. Negative inotropic effects of high-mobility group box 1 protein in isolated contracting cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H1490–6. doi: 10.1152/ajpheart.00910.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagiwara S, Iwasaka H, Uchino T, Noguchi T. High Mobility Group Box 1 Induces a Negative Inotropic Effect on the Left Ventricle in an Isolated Rat Heart Model of Septic Shock. Circ J. 2008;72:1012–7. doi: 10.1253/circj.72.1012. [DOI] [PubMed] [Google Scholar]
- 25.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M. High-mobility group box 1 protein (HMGB1) in ischemic heart disease: beneficial or deleterious? Cardiovasc Res. 2008;80:5–6. doi: 10.1093/cvr/cvn212. [DOI] [PubMed] [Google Scholar]
- 27.Lewis ME, Al-Khalidi AH, Bonser RS, et al. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J Physiol. 2001;534:547–52. doi: 10.1111/j.1469-7793.2001.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casado MA, Sevilla MA, Alonso MJ, et al. Muscarinic receptors involved in modulation of norepinephrine release and vasodilatation in guinea pig carotid arteries. J Pharmacol Exp Ther. 1994;271:1638–46. [PubMed] [Google Scholar]
- 29.Watanabe AM, McConnaughey MM, Strawbridge RA, et al. Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem. 1978;253:4833–6. [PubMed] [Google Scholar]
- 30.Mann DL, Kent RL, Parsons B, et al. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 31.Communal C, Singh K, Sawyer DB, et al. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 32.Sajadieh A, Wendelboe NO, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–70. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 34.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 35.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–7. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 36.Basta G. Receptor for advanced glycation end products and atherosclerosis: from basic mechanism to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth C, Rong LL, Yang C, et al. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002–17. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953–67. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 40.Giallauria F, Cirillo P, Lucci R, et al. Left ventricular remodelling in patients with moderate systolic dysfunction after myocardial infarction: favourable effects of exercise training and predictive role of N-terminal pro-Brain Natriuretic Peptide. Eur J Cardiovasc Prev Rehabil. 2008;15:113–8. doi: 10.1097/HJR.0b013e3282f00990. [DOI] [PubMed] [Google Scholar]
- 41.Giallauria F, Cirillo P, Lucci R, et al. Effects of Cardiac Rehabilitation on High Mobility Group Box-1 Levels after Acute Myocardial Infarction: Rationale and Design. J Cardiovasc Med (Hagerstown) 2009 doi: 10.2459/JCM.0b013e32832d4979. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


