Abstract
Background & Aims
Colonic motor disturbances in chronic constipation (CC) are heterogeneous and incompletely understood; the relationship between colonic transit and motor activity is unclear. We sought to characterize the phenotypic variability in chronic constipation.
Methods
Fasting and postprandial colonic tone and phasic activity and pressure–volume relationships were assessed by a barostat manometric assembly in 35 healthy women and 111 women with CC who had normal colon transit (NTC, n=25), slow transit (STC, n=19), and defecatory disorders with normal (DD-normal, n=34) or slow transit (DD-slow, n=33). Logistic regression models assessed whether motor parameters could discriminate among these groups. Among CC, phenotypes were characterized by principal components analysis of these measurements.
Results
Compared to 10th percentile values in healthy subjects, fasting and/or postprandial colonic tone and/or compliance were reduced in 40% with NTC, 47% with STC, 53% with DD-normal, and 42% with DD-slow transit. Compared to healthy subjects, compliance was reduced (p ≤ 0.05) in isolated STC and DD but not in NTC. Four principal components accounted for 85% of the total variation among patients; factors 1 and 2 were predominantly weighted by fasting and postprandial colonic phasic activity and tone respectively; factor 3 by postprandial high-amplitude propagated contractions, and factor 4 by postprandial tonic response.
Conclusions
Fasting and/or postprandial colonic tone are reduced, reflecting motor dysfunctions, even in NTC. Colonic motor assessments allow chronic constipation to be characterized into phenotypes. Further studies are needed to evaluate the relationship between these phenotypes, enteric neuropathology and response to treatment in CC.
INTRODUCTION
The American Gastroenterological Association guidelines and expert reviews recommend that colonic transit and anorectal functions should be assessed in patients with chronic constipation, thereby permitting patients to be characterized into 3 groups, i.e., normal transit constipation (NTC), isolated slow transit constipation (STC), and functional defecatory disorders; colonic transit is either normal (DD-normal) or slow (DD-slow) in defecatory disorders.1, 2 Implicit in this classification is the concept that slow colonic transit reflects colonic motor dysfunction. Indeed, manometry reveals colonic motor dysfunction [i.e., fewer high amplitude propagated contractions (HAPCs), reduced phasic contractile responses to a meal and pharmacological stimuli (e.g., bisacodyl)] in some patients with STC. 3–8 However, the relationship between colonic transit and intraluminal motor activity in patients with chronic constipation is unclear for 3 reasons. First, these studies were limited to STC and did not include patients with NTC or defecatory disorders. Second, while there is evidence that reduced propagating phasic pressure activity may explain delayed colonic transit, 4 the relationship between colonic phasic pressure activity and transit is incompletely understood. Indeed, only 28% of colonic isotope movements in healthy subjects were associated with propagating sequences; the remainder were accompanied by either nonpropagating activity (32%) or no pressure events (40%). 9 Moreover, a subset of patients with chronic constipation have normal phasic pressure activity, including HAPCs, under fasting conditions, after a meal, and after bisacodyl. 8 Third, with one exception, 10 these studies did not assess colonic tone, which is essential for normal colonic motor functions, 11 or stratify patients into those who did or did not have disordered defecation. In an earlier study from Mayo Clinic, the postprandial tonic contractile response was normal in normal transit (n = 12) but reduced in STC (n = 15) and in functional defecatory disorders (n = 13). 10 However, individual values for postprandial colonic tone and other parameters (e.g., number of postprandial HAPCs) overlapped among groups, suggesting that colonic transit or motility measurements may not accurately discriminate among the different pathophysiological phenotypes presenting with chronic constipation. 12
In addition to tone and phasic pressure activity, pressure-volume relationships (“compliance curves”) can also characterize colonic motor activity, specifically its viscoelastic properties. At pressures in the low and intermediate-range, pressure-volume relationships primarily reflect active (i.e., contractile) properties while higher imposed pressures predominantly reflect passive elements (e.g., connective tissue elements). Thus, drugs which increase or decrease colonic contractility modulate colonic pressure-volume relationships. 13 While the rectum is stiffer (i.e., less compliant) in irritable bowel syndrome (IBS), 14–16 colonic compliance has not been systematically assessed in IBS or in patients with chronic constipation.
It is important to clarify the relationship between colonic motor activity and transit because in addition to clarifying the pathophysiology, the results of colonic transit tests also guide the management of chronic constipation. Thus, subtotal colectomy is recommended for patients with medically refractory STC but not NTC.1, 2 The major hypotheses of this study were: first, that compared to healthy subjects, colonic motor activity is preserved in NTC but significantly reduced in STC, with or without DD. A second hypothesis is that detailed assessments of colonic motor functions would reveal phenotypic variability in chronic constipation.
METHODS
Study Subjects
Between July 1997 and June 2007, colonic motor functions were assessed by a barostat-manometric assembly in 243 patients with refractory chronic constipation at our institution. Secondary causes of chronic constipation (e.g., medications and colon cancer) were excluded by a careful clinical assessment, blood counts and serum biochemistry, and lower gastrointestinal endoscopy. Anorectal functions, colonic transit, and, in some patients, gastrointestinal transit were also assessed. Since the objective of this study was to characterize the relationship between transit and left colonic motor dysfunctions in women without overt motility disorders, patients (n = 47) with neurological or other disorders associated with colonic dysmotility (e.g., spinal cord injury, autonomic, peripheral, or pelvic neuropathy, known upper gastrointestinal dysmotility, inflammatory bowel disease, diabetes mellitus, amyloidosis, and Parkinsons disease), chronic megacolon, or a left hemicolectomy were excluded from this study. (Figure 1) Twenty nine patients with an incomplete dataset and 2 patients who did not provide research authorization were also not included, providing 111 patients for this report. Permission to review these studies was obtained from the Institutional Review Board at Mayo Clinic.
Figure 1.
CONSORT Diagram.
Procedures
Gastrointestinal transit
In 12 patients, a radioopaque marker study performed elsewhere revealed either delayed (n = 11) or normal (n = 1) colonic transit. Colonic transit was assessed using established scintigraphic techniques in the remaining 99 patients; gastrointestinal transit was also assessed in 88 patients. 17 Gastric emptying was summarized at 4 hours while small intestinal transit was summarized by the proportion of colonic filling at 6 hours after the meal. Colonic transit was summarized as the colonic geometric center (GC), which is the weighted average of counts in the different colonic regions. A higher GC reflects a faster colonic transit. Based on the 10th percentile value for healthy subjects in our laboratory, delayed colonic transit was defined by a GC24 value less than 1.47.
Anorectal functions
Anorectal functions were assessed by manometry and a rectal balloon expulsion test in all patients. 18 When the results of anorectal manometry and rectal balloon expulsion tests were inconclusive, scintigraphic defecography was performed (32 patients). 19 Defecatory disorders were diagnosed by the presence of clinical features (i.e., history and digital rectal examination) and abnormal diagnostic tests [i.e., an abnormal rectal balloon expulsion test (≥ 200 gm external traction) or abnormal anorectal motion during simulated evacuation assessed by defecography [i.e., inadequate angle change (< 12°) or reduced (< 1 cm) perineal descent]. 18, 20
Colonic Motor Activity
Before the colonic motility study, all medications with potential effects on colonic motility were discontinued for at least 48 hours, or earlier where necessary for medications with a longer half-life. Patients fasted overnight and the colon was cleansed with 2–5 l of polyethylene glycol 3350 and electrolyte solution (Golytely, Abbott Laboratories, Chicago, IL). The next morning, a colonic manometric-barostat assembly was positioned in the left colon (i.e., descending colon in 88 patients and sigmoid colon in 23 patients) by colonoscopy without sedation. 21 This assembly comprised an infinitely compliant 10-cm long balloon (Hefty Baggies, Mobil Chemical, Pittsford, NY) linked to an electronic rigid piston barostat (Engineering Department, Mayo Clinic, Rochester, MN).
The study commenced after a 30 minute equilibration period. Colonic motor activity was measured as described previously. 13, 21 A conditioning distension, during which the balloon was inflated from 0–44 mmHg in 4 mmHg steps at 15 second intervals, was performed to minimize order effects during subsequent distentions. 22 Thereafter, colonic compliance, contractile responses to a meal (1000 Kcal with 35% carbohydrate, 53% fat, and 12% protein) and to neostigmine (1 mg i.v.) were assessed in that order.
Colonic motor activity was quantified using established approaches. 13, 23 High amplitude propagating contractions were ≥ 75mmHg and propagated caudally for ≥ 15 cm as. Phasic pressure activity was expressed as a motility index per hour (MI/hr) for the descending colon (sensors 1–3) and sigmoid colon (sensors 4–6). The MI/hr was calculated as ln [(Number of contractions * sum of amplitude of contractions) + 1]. Fasting and postprandial colonic tone were estimated by separating the baseline balloon volume, representing colonic tone, from phasic volume deflections, which were greater than 10 ml lower than the baseline volume.
Tonic and phasic colonic motor responses to meal ingestion were calculated in a log scale. The postprandial tonic response was calculated as 100*[log (30 minutes pre-meal average baseline balloon volume volume/60 minutes post-meal average baseline balloon volume)]. For phasic activity, the postprandial response was calculated as 100*[log (60 minute post-meal average MI/Hr /30 minute pre-meal average MI/Hr)]. Since increased postprandial tonic and phasic activity is characterized by reduced volume and increased phasic pressure activity, these formulae ensured that by convention, normal responses were positive.
Colonic Compliance
After the conditioning distention, balloon volumes and pressures were measured as the balloon was inflated from 0 to 44 mmHg (4-mmHg steps, 30 second intervals). Thereafter, colonic pressures corresponding to 50% of maximum volume (i.e., Pr50) and the maximum volume were estimated by linear interpolation. 24
Statistical Analysis
Colonic motor activity was summarized by the following responses: fasting colonic tone and phasic pressure activity, postprandial tonic and phasic responses, and colonic compliance (i.e., Pr50) before and after neostigmine. Normal values were obtained from 35 healthy women studied in our laboratory. The Kruskal-Wallis test assessed the univariate association of these responses with subject status. Multiple predictor variable logistic regression models analyzed whether combinations of colonic motor responses could discriminate between healthy subjects and subtypes of chronic constipation defined by colonic transit and anorectal functions after adjusting for relevant covariates. Three multiple logistic regression models analyzed barostat data [fasting colonic volume, colonic response to a meal, and colonic compliance (Pr50)], phasic pressure activity in the sigmoid colon, and separately in the descending colon. Phasic pressure activity models included fasting phasic pressure activity, and the postprandial phasic response. Age and balloon location (descending or sigmoid colon) were covariates in these models. Thereafter, a separate logistic regression model evaluated whether Pr50 could discriminate between normal and delayed colonic transit in patients only; DD status and an interaction term (DD*Pr50) were incorporated in this model to ascertain whether the relationship between colonic transit and compliance was influenced by anorectal dysfunctions. The odds ratios for specific patient subtypes (relative to healthy controls) and their 95% confidence intervals (CIs) were computed from the estimated coefficients and their corresponding standard errors.
A principal components analysis of the correlation matrix for the measured responses was used to identify potential (latent) dimensions (factors) that may be manifested in theses responses. Visual inspection of the distributions of the computed principle component scores for each patient (e.g. univariate and bivariate scatter-plots) were examined to assess whether patients could be clustered into subgroups by colonic motor responses. 25 This analysis incorporated 6 response variables (i.e., fasting tone and phasic activity, Pr50, postprandial tonic and phasic responses and HAPCs). In the first step, the analysis constructed a score (i.e., factor 1), which was the weighted linear combination of the 6 variables that accounted for the maximum between-subjects variance among all such linear combinations of these variables. A second linear combination was then constructed to account for the maximum possible remaining (between subjects) variation, as were the third and fourth linear combinations. These scores are subject to the constraint that they are uncorrelated with each other. For each patient, each factor was derived as a specific weighted linear combination of the 6 variables. The “loading” for a specific variable in a particular factor was the weight used for that variable in the given linear combination (factor). After these factors were computed, the (Pearson) correlation between original variables and factors were assessed.
RESULTS
Demographic Features and Clinical Characteristics
For the entire patient group, the age distribution was 38.3 ± 1.3 years (Mean ± SEM); age and BMI were comparable across groups and in controls (age 31.7 ± 1.7 years, BMI 24.3 ± 0.7 kg/m2). (Table 1) Consistent with the classification criteria, the GC24 value for colonic transit was lower, indicating slower colonic transit, in patients with STC with or without DD. (Table 1) While gastric emptying was normal in all groups, small intestinal transit was delayed (p = 0.01) in 8 patients (42%) with isolated STC and 17 (52%) patients with DD-slow.
Table 1.
Demographics and Patient Characteristics
| Normal Transit Constipation | Isolated Slow Transit Constipation | Defecatory Disorders | ||
|---|---|---|---|---|
| Normal Transit | Slow Transit | |||
| Number of subjects | 25 | 19 | 34 | 33 |
| Age | 41.0 ± 3.3 | 36.9 ± 2.5 | 38.8 ± 1.9 | 36.4 ± 2.4 |
| BMI, kg/m2 | 24.5 ± 1.1 | 23.9 ± 1.3 | 23.6 ± 0.7 | 22.4 ± 0.8 |
| Gastrointestinal transit | ||||
| Gastric emptying at 4h (%) | 89.3 ± 3.3 | 81.8 ± 5.9 | 89.4 ± 2.6 | 89.6 ± 2.2 |
| Small intestinal transit (% colonic filling at 6h) * | 53.5 ± 7.0 | 31.5 ± 8.4 | 57.6 ± 6.4 | 32 ± 6.1 |
| Colonic transit (GC24) † | 2.0 ± 0.1 | 1.2 ± 0.0 | 2.0 ± 0.1 | 1.2 ± 0.0 |
| Anorectal testing | ||||
| Anal resting pressure (mmHg) | 72.3 ± 4.6 | 67.2 ± 6.2 | 81.1 ± 6.0 | 78.4 ± 5.3 |
| Anal squeeze pressure (mmHg) | 140.6 ± 10.1 | 132.8 ± 16.0 | 134.4 ± 8.3 | 152.0 ± 12.4 |
| Rectal balloon expulsion (gm) | 34.2 ± 14.6 | 27.7 ± 13.4 | 387 ± 37.4 | 368.4 ± 36.6 |
All values are Mean ± SEM
p = 0.01, Kruskal-Wallis test
Colonic transit was assessed by scintigraphy in 99 patients. In 12 patients, (4 with STC, 1 with DD-NTC, and 7 with PFD-STC), colonic transit was evaluated by radioopaque markers.
The diagnosis of DD was based on clinical features confirmed by an abnormal rectal balloon expulsion test in 61 of 67 patients and by abnormal defecography in the remaining 6 patients.
Fasting Colonic Tone and Colonic Compliance
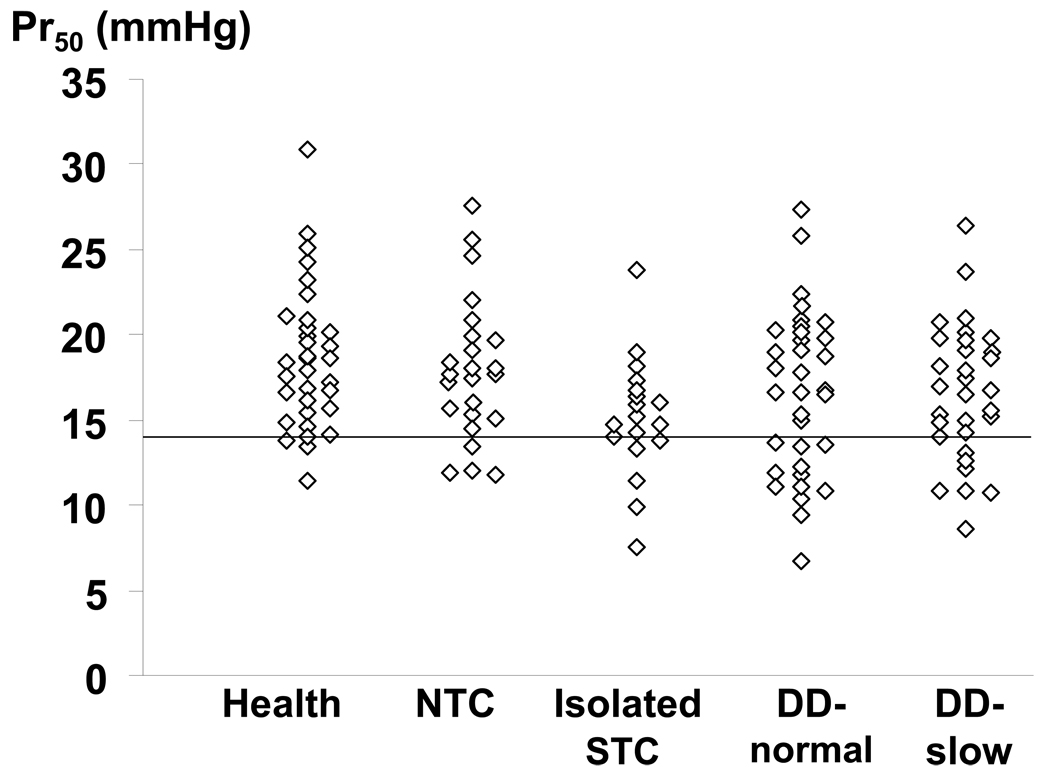
In 28/35 (80%) of controls and 88/111 (79%) of patients, the balloon was located in the descending colon. The intra-balloon operating pressure and fasting colonic volume at operating pressure were not significantly associated with patient versus control status. (Table 2) However, compared to healthy subjects, Pr50 was reduced (i.e., less than the 10th percentile value in healthy subjects), reflecting increased colonic compliance, in 4 patients (16%) with normal transit, 6 (32%) with isolated slow transit, 12 (35%) with DD-normal transit, and 10 (30%) with DD-slow colonic transit. Table 3 shows that reduced compliance was either an isolated phenomenon or associated with other features of colonic motor dysfunction. Increasing Pr50 values were associated with decreased odds for isolated STC (p=0.002 vs controls, multivariable analysis) and DD-normal (p=0.026) or DD-slow (p=0.01), reflecting the lower Pr50 values observed in these subgroups. (Table 4) A separate logistic regression model was used to ascertain whether colonic compliance could discriminate among patients with normal and delayed colonic transit. In this model, patients were collapsed into 2 categories [i.e., normal (n = 59) and delayed transit (n = 52)]. In this model also, a higher Pr50 (i.e., stiffer colon) was associated with a reduced risk (OR, 0.81; 95% CI, 0.67–0.98) for delayed colonic transit in subjects without DD. Moreover the interaction term (Pr50*DD status) was borderline significant (p = 0.06), suggesting that the ability of colonic compliance (Pr50) to discriminate between normal and delayed colonic transit was influenced by DD status. As Figure 2 suggests, among patients without DD, the Pr50 was lower in STC than in NTC. While Pr50 was lower in patients with DD than controls, it did not discriminate between DD-NTC and DD-STC. However, balloon location was not significant in this model. In contrast to the Pr50, the maximum volume during colonic pressure-volume relationships was not significantly associated with subgroup status by univariate analysis.
Table 2.
Distribution of Colonic Motor Parameters in Chronic Constipation
| Parameter | Healthy Controls | Normal Transit | Isolated Slow Transit | Defecatory Disorders | |
|---|---|---|---|---|---|
| Normal Transit | Slow Transit | ||||
| Number of subjects | 35 | 25 | 19 | 34 | 33 |
| Location of barostat balloon | |||||
| Descending colon | 28 | 24 | 15 | 24 | 25 |
| Sigmoid colon | 7 | 1 | 4 | 10 | 8 |
| Operating pressure (mm Hg) | 12.5 ± 0.8 | 13.4 ± 0.6 | 12.1 ± 0.4 | 12.8 ± 0.5 | 12.5 ± 0.6 |
|
Colonic tone | |||||
| Fasting volume (mL) | 127.1 ± 5.6 | 119.2 ± 9.2 | 142.3 ± 16.4 | 143.9 ± 9.8 | 128.5 ± 8.4 |
| Postprandial volume change* | 43.2 ± 5.9 | 34.7 ± 8.7 | 33.6 ± 8.0 | 24.2 ± 4.6 | 31.0 ± 5.6 |
|
Colonic compliance | |||||
| Pre neostigmine (Pr50, mmHg) | 18.5 ± 0.7 | 17.8 ± 0.8 | 15 ± 0.8‡ | 16.6 ± 0.8† | 16.5 ± 0.7† |
| Pre neostigmine - Maximum volume (mL) | 278.4 ± 8.2 | 290 ± 15 | 327.9 ± 28.0 | 308.9 ± 14.4 | 317.8 ± 28.6 |
| Post neostigmine (Pr50, mmHg) | NA | 21.8 ± 0.9 | 20.9 ± 1.0 | 21.5 ± 0.9 | 21.1 ± 1.0 |
| Post neostigmine - Maximum volume (mL) | NA | 295 ± 15 | 339.7 ± 32.3 | 318.7 ± 15.7 | 343.5 ± 35.0 |
| Post-neostigmine change in Pr50, mmHg | NA | 4.2 ± 1.1 | 6.4 ± 0.6 | 5 ± 0.8 | 4.6 ± 0.9 |
|
Phasic pressure activity | |||||
| Fasting motility (MI/Hr) | |||||
| Descending | 7.9 ± 0.6 | 8.1 ± 0.8 | 7.8 ± 0.9 | 7 ± 0.7 | 7.7 ± 0.7 |
| Sigmoid | 9.0 ± 0.6 | 8.6 ± 0.7 | 7.4 ± 0.8† | 7.8 ± 0.6 | 7.1 ± 0.6‡ |
| Postprandial change+ (MI/Hr) | |||||
| Descending | 1.1 ± 0.4 | 1.5 ± 0.7 | 1.4 ± 0.6 | 1.6 ± 0.8 | 1.8 ± 0.4 |
| Sigmoid | 1.0 ± 0.4 | 0.7 ± 0.7 | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.0 ± 0.3 |
| Postprandial HAPCs§ | 0.7 ± 0.2 | 0.4 ± 0.2 | 0 ± 0 | 0.3 ± 0.2 | 0.2 ± 0.2 |
All values are Mean ± SEM except where stated
NA – not available
Postprandial volume change = 100*[ log (30 minutes pre-meal average/60 minutes post-meal average)]
Postprandial change in phasic motility = Postprandial MI – Fasting MI
p < 0.05
p < 0.005 vs controls;
p = 0.01, Kruskal-Wallis test
Table 3.
Distribution of Colonic Motor Disturbances in Chronic Constipation*
| Normal Transit | Isolated Slow Transit | Defecatory Disorders | ||
|---|---|---|---|---|
| Normal Transit | Slow Transit | |||
| Number of subjects | 25 | 19 | 34 | 33 |
| Fasting abnormalities only | ||||
| Reduced tone only | 0 | 4 (21%) | 3 (9%) | 1 (3%) |
| Increased compliance only | 3 (12%) | 4 (21%) | 8 (24%) | 8 (24%) |
| Reduced tone and increased compliance | 0 | 1 (5%) | 3 (9%) | 1 (3%) |
| Reduced postprandial tonic response only | 6 (24%) | 2 (10%) | 3 (9%) | 4 (12%) |
| Reduced fasting tone/increased compliance and reduced postprandial response† | 1 (4%) | 3 (15%) | 3 (9%) | 1 (3%) |
| At least one abnormal fasting or postprandial parameter‡ | 10 (40%) | 9 (47%) | 18 (53%) | 14 (42%) |
Number (percent) of subjects with values less than 10th percentile value for controls in that category.
Table 4.
Multiple Variable Analysis Predicting Constipation Subtype by Colonic Motor Assessments
| Parameter* | Normal Transit Constipation | Isolated Slow Transit Constipation | Defecatory Disorders | |
|---|---|---|---|---|
| Normal Transit | Slow Transit | |||
| Barostat Parameters | ||||
| Age | 1.08 (1.02, 1.13) | 1.06 (1.00, 1.12) | 1.06 (1.01, 1.11) | 1.05 (1.00, 1.10) |
| Balloon location | 6.20 (0.67, 56.94) | 1.06 (0.24, 4.80) | 0.66 (0.20, 2.20) | 0.85 (0.25, 2.92) |
| Fasting volume | 1.0 (0.98, 1.01) | 1.0 (0.99, 1.01) | 1.0(0.99, 1.02) | 1.0 (0.99, 1.01) |
| Postprandial volume change | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.01) | 0.98 (0.97, 1.0) | 0.99 (0.97, 1.0) |
| Fasting Compliance (Pr50) | 0.89 (0.77, 1.03) | 0.77 (0.65, 0.91) | 0.87 (0.76, 0.98) | 0.84 (0.74, 0.96) |
| Manometric Parameters – Sigmoid Colon | ||||
| Age | 1.08 (1.03, 1.13) | 1.05 (1.00, 1.11) | 1.06 (1.01, 1.11) | 1.05 (1.01, 1.10) |
| Balloon location | 6.18 (0.66, 57.49) | 0.89 (0.20, 3.99) | 0.71 (0.21, 2.44) | 1.14 (0.31, 4.17) |
| Sigmoid fasting MI | 0.83 (0.66, 1.06) | 0.78 (0.61, 0.99) | 0.85 (0.68, 1.04) | 0.73 (0.59, 0.9) |
| Sigmoid absolute postprandial MI change | 0.79 (0.57, 1.10) | 0.84 (0.59, 1.20) | 0.94 (0.69, 1.28) | 0.77 (0.57, 1.04) |
| Manometric Parameters – Descending Colon | ||||
| Age | 1.07 (1.02, 1.13) | 1.05 (0.99, 1.12) | 1.06 (1.01, 1.12) | 1.04 (0.99, 1.10) |
| Balloon location | NA | NA | NA | NA |
| Descending fasting MI | 1.03 (0.79, 1.34) | 0.96 (0.71, 1.30) | 0.86 (0.67, 1.11) | 1.04 (0.81, 1.33) |
| Descending absolute postprandial MI change | 1.10 (0.80, 1.52) | 1.02 (0.71, 1.46) | 0.97 (0.72, 1.30) | 1.15 (0.85, 1.57) |
All values are OR (95% C.I.) per unit for subtypes compared to the index group which is healthy subjects for all parameters. Balloon location was a covariate for models incorporating manometric parameters in the sigmoid colon and barostat parameters.
Figure 2.
Comparison of colonic compliance (Pr50) among categories. Among patients without DD, Pr50 was lower in STC than NTC. However, among patients with DD, Pr50 was comparable in DD-NTC and DD-STC. The horizontal line is the 10th percentile value in healthy subjects.
Contractile Responses to a Meal
The postprandial tonic contractile response was reduced (i.e., less than the 10th percentile value for health) in 7 (28%) patients with normal transit, 5 (36%) with isolated slow transit, 6 (18%) with DD-normal transit, and 5 (15%) with DD-slow transit. (Table 3, Figure 3) In the univariate (data not shown) and multiple variable analyses (Table 4), the postprandial response was comparable amongst groups. Similar findings were observed when the analysis was based on the first 30 minutes rather than the entire 60 minute postprandial period (data not shown).
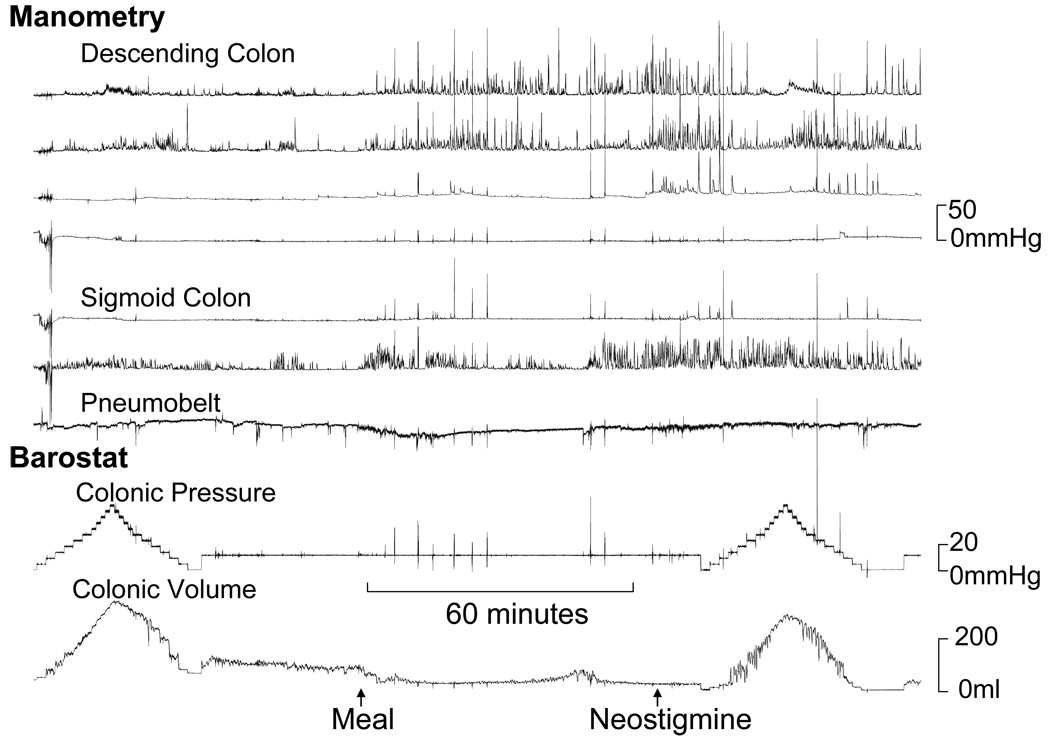
Figure 3.
Normal fasting, postprandial and post-neostigmine tonic and phasic colonic motor activity in a patient with STC. Observe reduced balloon volume, reflecting increased colonic tone, after a meal and after neostigmine. Manometry showed increased phasic pressure activity in the sigmoid colon after a meal and also in the descending colon after neostigmine. The principal component analysis revealed a high score for factor 2.
Effect of Neostigmine on Colonic Compliance
After neostigmine, the colon was less compliant (i.e., stiffer), as evidenced by a higher Pr50, in all subtypes. However, this effect was not significantly associated with subtypes (Table 2) and was not correlated (rs = 0.05; p=0.66) with the tonic contractile response to a meal.
Fasting and Postprandial Phasic Motility
Compared to controls, fasting sigmoid but not descending colonic phasic motility was reduced in isolated STC and also in DD-slow transit. (Table 2 and Table 4) However, the postprandial phasic contractile response in the sigmoid and descending colon was not significantly associated with subgroup status. Healthy subjects (0.7 ± 0.2 HAPCs) had more frequent (p=0.01) postprandial HAPCs than patients with normal transit (0.4 ± 0.2), isolated STC (0 ± 0), DD-normal transit (0.3 ± 0.2), and DD-slow transit (0.2 ± 0.2 HAPCs).
Can Colonic Motor Parameters Identify Clusters in Patients?
A principal components analysis incorporating selected response variables examined whether patients could be clustered into subtypes by objective (latent) criteria. Table 5 provides the loadings on each factor and Pearson correlation coefficients for the 6 variables used for constructing these factors.
Table 5.
Variable Loading and Correlation Coefficients for Principal Component Scores
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Loading | Correlation Coefficient | Loading | Correlation Coefficient | Loading | Correlation Coefficient | Loading | Correlation Coefficient | |
| Fasting volume | −0.19 | −0.25† | −0.62 | −0.74** | −0.30 | −0.30* | 0.17 | 0.15 |
| Pr50 | 0.31 | 0.41** | 0.57 | 0.68** | 0.01 | 0.01 | −0.34 | −0.31* |
| Postprandial tonic response | −0.21 | −0.29* | 0.47 | 0.57** | −0.15 | −0.15 | 0.83 | 0.75** |
| Fasting sigmoid phasic activity | 0.65 | 0.87** | −0.06 | −0.08 | −0.13 | −0.14 | 0.27 | 0.25† |
| Postprandial sigmoid phasic response | −0.62 | −0.83** | 0.19 | 0.22† | 0.24 | 0.24† | −0.15 | −0.13 |
| Postprandial HAPCs | 0.16 | 0.22† | −0.19 | −0.23† | 0.90 | 0.90** | 0.27 | 0.25† |
p < 0.05;
p < 0.01;
p < 0.001;
p ≤ 0.0001
The factor scores for each patient were computed by summing the products of (Loading *value for that variable) in each patient.
This analysis revealed four composite scores, which accounted for 30%, 24%, 17%, and 14% respectively (i.e., total of 85%) of the total variance in the six response variables among subjects. The first composite score defined a latent dimension which was most strongly correlated with fasting sigmoid phasic motility and was inversely correlated to the postprandial change in phasic motility. (Table 5, Figure 4) The second composite score was most strongly associated with increased fasting tone (i.e., reduced fasting volume), a stiffer (i.e., less compliant) colon, and a more prominent tonic response to a meal. (Figure 3) The third composite score was most strongly correlated with postprandial HAPCs. The fourth score was most strongly correlated with a more prominent tonic response to a meal.
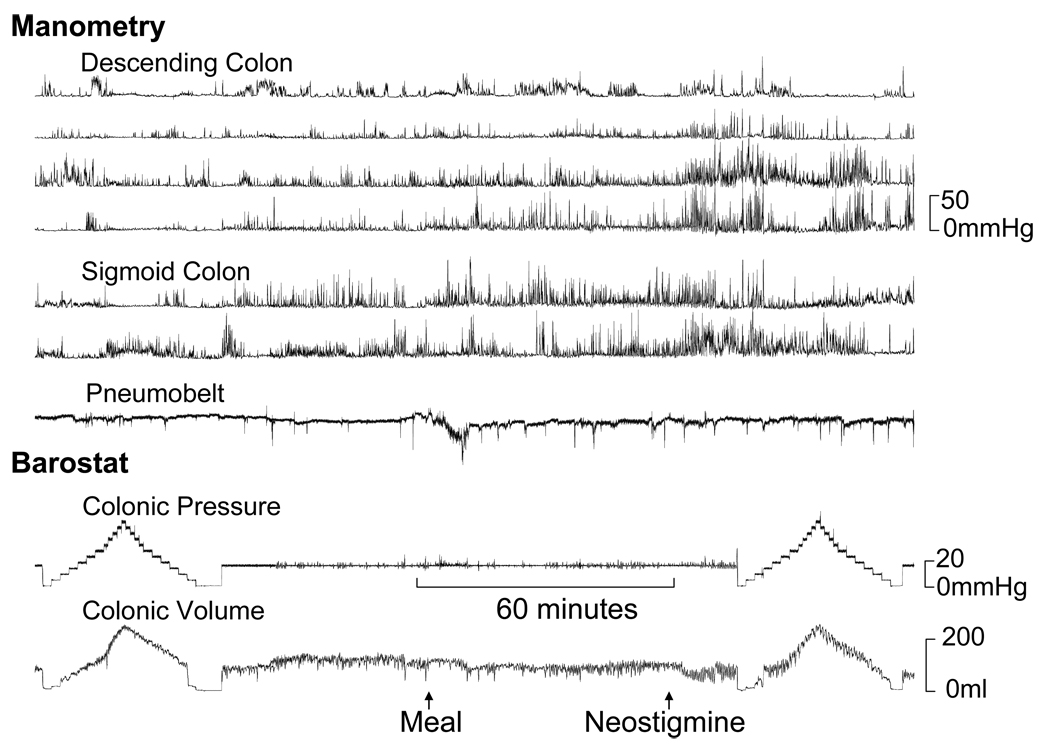
Figure 4.
Impaired colonic tonic contractile responses to a meal and neostigmine in a patient with STC. In contrast, manometry revealed increased fasting and postprandial phasic pressure activity. The principal component analysis revealed a high score for factor 1.
DISCUSSION
Assessments of colonic transit and anorectal functions are useful for classifying and facilitating a rational therapeutic approach to chronic constipation refractory to medical therapy but provide a limited understanding of the motor dysfunctions responsible for the symptom. This large series evaluated colonic tone, pressure-volume relationships, and phasic pressure activity under fasting conditions and contractile responses to a meal and neostigmine in patients with chronic constipation. As observed previously, patients with STC had fewer colonic HAPCs. 3, 8 In addition, there are several original and important findings. A substantial proportion of patients in all 4 categories had reduced fasting and/or postprandial colonic tone and/or increased compliance. Consequently, with the exception of colonic compliance, fasting and postprandial motor parameters were not useful for discriminating among groups.
Forty percent of patients with NTC had reduced colonic fasting and/or postprandial colonic tone. Colonic tone is responsible for mixing contents and for the contractile response to a meal; 11 indeed increased postprandial colonic tone is associated with accelerated colonic transit in carcinoid diarrhea. 26 Thus, it is conceivable that reduced tone may explain motor dysfunctions in chronic constipation. At the other end of the spectrum, even some patients with STC had normal fasting and postprandial tonic and phasic responses, extending previous observations by colonic manometry. 8, 10 Since the colonic transit is an imperfect surrogate marker of intraluminal motor activity, intraluminal assessments may be useful in selected patients with chronic slow transit constipation who do not respond to medical therapy. 27 For example, normal and abnormal colonic motility by intraluminal testing may prompt reconsideration or reinforce respectively the decision to pursue subtotal colectomy. However, to emphasize, the impact of intraluminal testing on the decision to pursue subtotal colectomy and outcomes after surgery needs to be studied.
The current system for classifying functional gastrointestinal disorders (FGIDs) is primarily symptom based, supplemented (e.g., for chronic constipation) by selected tests. Similar to other diseases (e.g., schizophrenia), it has been suggested that dissecting FGIDs into intermediate or endophenotypes, characterized by system dysfunction (e.g., visceral hypersensitivity, motor dysfunctions), will increase our understanding of pathophysiology of these disorders. 28 In this study, the principal components analysis revealed four factors (or latent dimensions) which best explained the total phenotypic variance amongst patients, providing a conceptual framework for a pathophysiological classification of colonic motor disturbances in chronic constipation. Factors 1 and 3 were most strongly weighted by phasic parameters while Factors 2 and 4 were primarily weighted by colonic tone. This underscores the utility of measuring colonic tone, particularly in constipated patients with normal fasting and postprandial phasic pressure activity. 8 Factor 1 was positively correlated with fasting phasic motility and inversely correlated with the posptrandial phasic contractile response. Indeed, increased fasting sigmoid phasic pressure activity (“spastic colon”) has been implicated to retard colonic transit in chronic constipation. 29, 30 Factor 3 was highly correlated with HAPCs. This factor was less strongly correlated with fasting tone and not correlated with colonic compliance, suggesting that similar to propagated contractions initiated by colonic distention in mice, HAPCs in humans do not require normal colonic tone. 31 In contrast, distention-induced propagated contractions in the guinea pig distal colon are critically dependent upon muscle tone, 32 probably because stretch-induced firing in myenteric AH (after-hyperpolarizing) sensory neurons is dependent upon smooth muscle tone. 33 The observed clustering of parameters (i.e., normal fasting tone and postprandial colonic tonic responses but not HAPCs) in the second factor is also consistent with the hypothesis that different mechanisms mediate colonic tone and HAPCs in humans. The fourth factor was strongly associated with the postprandial tonic response but not fasting tone. This may suggest that in some patients, the neurohormonal mechanisms responsible for increasing postprandial tone may be sufficient to overcome reduced fasting colonic tone. Further studies are required to assess whether these phenotypes can predict neuropathological disturbances (e.g., loss of interstitial cells of Cajal).
Patients with STC and DD, but not NTC, had increased colonic compliance, which suggests that patients with NTC have less severe colonic motor dysfunction. In isolated STC, the Pr50 for colonic compliance was 3.5 mmHg units lower than in healthy people. This difference is significant, since it is only slightly smaller than the effect of clonidine, an α2 adrenergic agonist, on colonic compliance in healthy subjects. 13 In contrast to the Pr50, which summarizes the entire curve, the maximum volume was not significantly different among constipation subtypes. While the Pr50 reflects “active” (i.e., contractile element) and passive (e.g., connective tissue) mechanical properties, the maximum volume predominantly reflects passive properties. Taken together, these observations suggest that increased compliance in chronic constipation predominantly reflects reduced colonic contractility, rather than altered connective tissue properties.
Perhaps, the limited correlation between colonic transit and intraluminal parameters is not surprising since transit is influenced not only by colonic motor activity but also by other variables (e.g., diet and physical activity). Another potential explanation for the limited correlation between transit and intraluminal motor activity is that intra-individual measurements of colonic transit are reproducible in chronic idiopathic constipation but not in patients with DD or STC. 34 Lastly, the analysis summarized colonic transit as a categorical variable, but motor parameters as continuous variables. Although the colon was cleansed before intraluminal testing but not prior to transit assessments, colonic cleansing does not have major effects on colonic motility. 35
Patients with DD also had increased colonic compliance, which may reflect either reduced colonic dysmotility in association with disordered defecation 36, 37 and/or altered connective tissue viscoleastic properties. Bladder outlet obstruction is associated with altered bladder contractility and compliance in humans 38 and experimental animal models, wherein the effects on bladder functions vary depending on the experimental model (i.e., partial or complete bladder obstruction), the duration after obstruction, and methods for assessing bladder function. Taken together, these studies suggest that detrusor contractility is initially preserved and sometimes increased in an attempt to overcome obstruction. 39, 40 However, contractility declines over time, resulting in an overcompliant bladder. Similarly, patients with DD had increased colonic compliance. In guinea pigs with partial urethral obstruction, recovery of bladder function was more likely when obstruction was reversed sooner rather than later. 39 Further studies are necessary to determine the natural history of altered colonic compliance and the effects of pelvic floor retraining in DD.
Consistent with previous studies, many patients (33/67 or 49%) with DD had delayed colonic transit. 41 Among DD, intraluminal colonic motor assessments, including colonic compliance, were not significantly different in patients with normal and slow colonic transit, perhaps suggesting that slow colonic transit is not a marker for more severe colonic motor dysfunction in these patients. However, small intestinal transit, which was assessed in 88 of 111 patients, was delayed in patients with isolated STC and in DD-slow, but not DD-normal transit. Delayed small intestinal transit may be secondary to obstruction by stool in the right colon, to viscerovisceral (i.e., colointestinal) inhibitory reflexes, or to small intestinal dysmotility as part of a primary, more generalized, motor disorder. 42
The extent to which these observations, which were obtained in patients with refractory symptoms at a tertiary center, are applicable to patients in other settings is unclear. We did not assess colonic sensation or colonic secretion, which may be abnormal, and also contribute to the pathophysiology of chronic constipation. 43, 44
In summary, intraluminal assessments with a barostat-manometric assembly revealed fasting and postprandial motor disturbances in a majority of patients with chronic constipation. Even patients with normal colonic transit, which is considered a surrogate marker for normal colonic functions, frequently had reduced fasting and/or postprandial colonic tone. Colonic compliance was normal in NTC but reduced in isolated STC and in DD. Together, these motor assessments allow chronic constipation to be characterized into phenotypes. Further studies are needed to confirm these phenotypes, and to ascertain whether these phenotypes can predict neuropathological disturbances (e.g., loss of interstitial cells of Cajal) and guide management in chronic constipation.
Acknowledgments
This study was supported in part by USPHS NIH Grants P01 DK068055 and RO1 DK 78924.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 2008 Annual Meeting of the American Gastroenterology Association, San Diego, CA.
None of the authors have conflicts of interests to disclose.
REFERENCES
- 1.Locke GR, 3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 2.Lembo A, Camilleri M. Current concepts: Chronic constipation. New England Journal of Medicine. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 3.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. American Journal of Physiology. 1988;255:G660–G664. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 4.Bazzocchi G, Ellis J, Villanueva-Meyer J, Jing J, Reddy SN, Mena I, Snape WJ., Jr Postprandial colonic transit and motor activity in chronic constipation. Gastroenterology. 1990;98:686–693. doi: 10.1016/0016-5085(90)90289-d. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, Sadeghi P, Batterson K, Beaty J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterology & Motility. 2001;13:591–598. doi: 10.1046/j.1365-2982.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 6.Hagger R, Kumar D, Benson M, Grundy A. Colonic motor activity in slow-transit idiopathic constipation as identified by 24-h pancolonic ambulatory manometry. Neurogastroenterology & Motility. 2003;15:515–522. doi: 10.1046/j.1365-2982.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 7.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Digestive Diseases & Sciences. 2003;48:1206–1212. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 8.Herve S, Savoye G, Behbahani A, Leroi AM, Denis P, Ducrotte P. Results of 24-h manometric recording of colonic motor activity with endoluminal instillation of bisacodyl in patients with severe chronic slow transit constipation.[see comment] Neurogastroenterology & Motility. 2004;16:397–402. doi: 10.1111/j.1365-2982.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook I, Furukawa Y, Panagopoulos V, Collins P, Dent J. Relationships between spatial patterns of colonic pressure and individual movements of content. American Journal of Physiology. 2000;278:G329–G341. doi: 10.1152/ajpgi.2000.278.2.G329. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien MD, Camilleri M, von der Ohe MR, Phillips SF, Pemberton JH, Prather CM, Wiste JA, Hanson RB. Motility and tone of the left colon in constipation: a role in clinical practice? American Journal of Gastroenterology. 1996;91:2532–2538. [PubMed] [Google Scholar]
- 11.Christensen J. The Motility of the Colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Third ed. New York: Raven Press; 1994. pp. 991–1024. [Google Scholar]
- 12.Wald A. Chronic constipation: advances in management. Neurogastroenterology & Motility. 2007;19:4–10. doi: 10.1111/j.1365-2982.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. American Journal of Physiology. 1997;273:G997–G1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 14.Steens J, Van Der Schaar PJ, Penning C, Brussee J, Masclee AAM. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterology & Motility. 2002;14:241–247. doi: 10.1046/j.1365-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwan CL, Davis KD, Mikula K, Diamant NE. Abnormal rectal motor physiology in patients with irritable bowel syndrome. 2004:251–263. doi: 10.1111/j.1365-2982.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 16.Basilisco G, De Marco E, Tomba C, Cesana BM. Bowel urgency in patients with irritable bowel syndrome. Gastroenterology. 2007;132:38–44. doi: 10.1053/j.gastro.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. Journal of Nuclear Medicine. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 18.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology. 2005 doi: 10.1053/j.gastro.2005.03.021. in press. [DOI] [PubMed] [Google Scholar]
- 19.Barkel DC, Pemberton JH, Pezim ME, Phillips SF, Kelly KA, Brown ML. Scintigraphic assessment of the anorectal angle in health and after ileal pouchanal anastomosis. Annals of Surgery. 1988;208:42–49. doi: 10.1097/00000658-198807000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000;47:667–674. doi: 10.1136/gut.47.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharucha AE, Hubmayr RD, Ferber IJ, Zinsmeister AR. Viscoelastic properties of the human colon. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281:G459–G466. doi: 10.1152/ajpgi.2001.281.2.G459. [DOI] [PubMed] [Google Scholar]
- 23.Ford MJ, Camilleri M, Wiste JA, Hanson RB. Differences in colonic tone and phasic response to a meal in the transverse and sigmoid human colon. Gut. 1995;37:264–269. doi: 10.1136/gut.37.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floyd BNI, Camilleri M, Andresen V, Esfandyari T, Busciglio I, Zinsmeister AR. Comparison of mathematical methods for calculating colonic compliance in humans: power exponential, computer-based and manual linear interpolation models. Neurogastroenterology & Motility. 2008;20:330–335. doi: 10.1111/j.1365-2982.2007.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison DF. The structure of multivariate observations: I. Principal Components. In: Blackwell D, Solomon H, editors. Multivariate statistical methods. 2nd ed. New York: McGraw-Hill Book Company; 1976. pp. 266–301. [Google Scholar]
- 26.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. [erratum appears in N Engl J Med 1993 Nov 18;329(21):1592] New England Journal of Medicine. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Bharucha AE, di Lorenzo C, Hasler WL, Prather CM, Rao SS, Wald A. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterology & Motility. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 28.Mayer EA. The challenge of studying the biology of complex, symptom-based GI disorders. Gastroenterology. 2008;134:1826–1827. doi: 10.1053/j.gastro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary NA, Truelove SC. Human colonic motility: a comparative study of normal subjects, patients with ulcerative colitis, and patients with the irritable colon syndrome I. Resting patterns of motility. Gastroenterology. 1961;40:1–17. [PubMed] [Google Scholar]
- 30.Connell AM. The motility of the pelvic colon. Part II. Paradoxical motility in diarrhea and constipation. Gut. 1962;3:342–348. doi: 10.1136/gut.3.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell AK, O'Brien SD, Fida R, Bywater RAR. Neural integrity is essential for the propagation of colonic migrating motor complexes in the mouse. Neurogastroenterology & Motility. 2002;14:495–504. doi: 10.1046/j.1365-2982.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith TK, Oliver GR, Hennig GW, O'Shea DM, Vanden Berghe P, Kang SH, Spencer NJ. A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. Journal of Physiology. 2003;551:955–969. doi: 10.1113/jphysiol.2003.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annual Review of Physiology. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 34.Nam YS, Pikarsky AJ, Wexner SD, Singh JJ, Weiss EG, Nogueras JJ, Choi JS, Hwang YH. Reproducibility of colonic transit study in patients with chronic constipation. Diseases of the Colon & Rectum. 2001;44:86–92. doi: 10.1007/BF02234827. [DOI] [PubMed] [Google Scholar]
- 35.Lemann M, Flourie B, Picon L, Coffin B, Jian R, Rambaud JC. Motor activity recorded in the unprepared colon of healthy humans [see comments] Gut. 1995;37:649–653. doi: 10.1136/gut.37.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mollen RM, Salvioli B, Camilleri M, Burton D, Kost LJ, Phillips SF, Pemberton JH. The effects of biofeedback on rectal sensation and distal colonic motility in patients with disorders of rectal evacuation: evidence of an inhibitory rectocolonic reflex in humans? American Journal of Gastroenterology. 1999;94:751–756. doi: 10.1111/j.1572-0241.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 37.Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, Cook IJ. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004;127:49–56. doi: 10.1053/j.gastro.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Madersbacher S, Pycha A, Klingler CH, Mian C, Djavan B, Stulnig T, Marberger M. Interrelationships of bladder compliance with age, detrusor instability, and obstruction in elderly men with lower urinary tract symptoms.[comment] Neurourology & Urodynamics. 1999;18:3–15. doi: 10.1002/(sici)1520-6777(1999)18:1<3::aid-nau2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Wolffenbuttel KP, Kok DJ, Minekus JP, van Koeveringe GA, van Mastrigt R, Nijman JM. Urodynamic follow-up of experimental urethral obstruction in individual guinea pigs. Neurourology & Urodynamics. 2001;20:699–713. doi: 10.1002/nau.1021. [DOI] [PubMed] [Google Scholar]
- 40.Thiruchelvam N, Wu C, David A, Woolf AS, Cuckow PM, Fry CH. Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2003;284:R1296–R1305. doi: 10.1152/ajpregu.00688.2002. [DOI] [PubMed] [Google Scholar]
- 41.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. American Journal of Gastroenterology. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 42.Law N-M, Bharucha A. Phasic Rectal Distention Induces Colonic Realxation in Humans. Gastroenterology. 1998;114:G3233. [Google Scholar]
- 43.Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. American Journal of Gastroenterology. 1999;94:131–138. doi: 10.1111/j.1572-0241.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 44.Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. American Journal of Gastroenterology. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]