Abstract
Background/Aims
Insulin-like growth factor-I (IGF-I) regulates human intestinal smooth muscle growth by stimulating proliferation and inhibiting apoptosis. IGF-I-stimulated growth is augmented when αvβ3 integrin is occupied by its ligands, fibronectin and vitronectin. Increased IGF-I expression and muscle cell hyperplasia are features of stricturing Crohn's disease, however, the role of IGF-I in stricture formation is unknown. The aim was to identify the functional role of endogenous IGF-I and αvβ3 integrin ligands in regulating muscle cell hyperplasia in stricturing Crohn's disease.
Methods
Smooth muscle cells were isolated from muscularis propria of stricturing Crohn's disease or normal margins. Quantitative PCR, immunoblot analysis and ELISA were used to measure fibronectin, vitronectin, αVβ3 integrin, and IGF-I levels. Activation of the IGF-I receptor, Erk1/2, p70S6 kinase and GSK-3β was measured by immunoblot. Proliferation was quantified by Ki67 immunostaining and [3H]thymidine incorporation. Apoptosis was measured from caspase-3 cleavage and nucleosome accumulation.
Results
IGF-I, vitronectin and fibronectin RNA and protein levels were increased 1.8 – 3.4 fold in muscle cells from strictures over normal margins. Basal IGF-I receptor phosphorylation was increased 320% in strictured over normal muscle and basal Erk1/2, p70S6 kinase and GSK-3β phosphorylation was increased 205 - 292% in strictures. In muscle cells from strictures, Ki67 immunoreactivity and [3H]thymidine incorporation were increased and apoptosis was decreased compared to normal margins. Antagonists of the IGF-I receptor or αVβ3 integrin reversed these changes.
Conclusion
Smooth muscle cell hyperplasia in stricturing Crohn's disease is regulated by increased endogenous IGF-I and αVβ3 integrin ligands that regulate augmented proliferation and diminished apoptosis.
INTRODUCTION
Crohn's Disease is complicated by stricture formation in ~30% of patients 1, 2. Three features are characteristic of smooth muscle cells in the muscularis propria of stricturing Crohn's disease: increased muscle cell proliferation (hyperplasia), increased muscle cell hypertrophy, and increased net extracellular matrix production 3, 4. Insulin-like growth factor-I (IGF-I) produced in the liver acts in an endocrine fashion, whereas locally produced IGF-I, e.g. by smooth muscle cells acts, in an autocrine fashion to regulate the growth of smooth muscle cells 5, 6. Two lines of evidence demonstrate the importance of endogenous IGF-I in regulating the growth of intestinal smooth muscle cells: (i) in mice with a CreLox/P-mediated hepatic deletion of IGF-I, intestinal muscle develops normally7, and (ii) smooth muscle hyperplasia in the muscularis propria develops in mice over-expressing IGF-I8, 9.
In human intestinal smooth muscle cells IGF-I and αvβ3 integrin share a unique relationship. Occupancy of αvβ3 integrin (vitronectin receptor) by its ligands, vitronectin and fibronectin, augments the intensity and duration of IGF-I-stimulated IGF-I receptor activation, and muscle growth 10-12. Interplay between IGF-I and αVβ3 is thought to play a role in pathophysiologic responses of other smooth muscle types: atheroma formation in vascular muscle and fibroid formation in uterine muscle 8, 13, 14. Activation of the IGF-I receptor tyrosine kinase in human intestinal smooth muscle is augmented by αVβ3 ligands and is coupled to Erk1/2 and p70S6 kinase activation, which jointly mediate IGF-I-stimulated proliferation, and to GSK-3β activation, which mediates IGF-I-stimulated inhibition of apoptosis 15-17.
The IGF-I gene is alternatively spliced with the main isoform of IGF-I encoded by the IGF-IEa isoform. IGF-IEa expression is increased in the muscularis propria of active and stricturing Crohn's disease over that in normal intestinal margin at the time of resection18. Expression was increased in muscle cells, and fibroblasts but IGF-IEa expression was not observed in the inflammatory cells infiltrating the muscular layer18. While endogenous IGF-I has been shown to regulate growth of normal intestinal smooth muscle cells, neither the functional significance of increased IGF-I expression in Crohn's disease nor the mechanisms that regulate increased muscle cell hyperplasia of stricturing Crohn's disease have been identified.
This paper shows that the expression of IGF-I, and the αVβ3 integrin ligands, fibronectin and vitronectin, are increased in smooth muscle cells isolated from the muscularis propria of stricturing Crohn's disease over that in normal muscle. Basal IGF-I receptor activity and that of its signaling intermediates coupled to stimulation of proliferation and inhibition of apoptosis are also increased in muscle cells of stricturing Crohn's disease. The results indicate that the increased proliferation and decreased apoptosis in intestinal smooth muscle cells in stricturing Crohn's disease, compared to normal intestine, are regulated by endogenous IGF-I and αVβ3 integrin ligands. The results also suggest that the long term sequelae of these two complementary processes that regulate growth may be smooth muscle cell hyperplasia of the muscularis propria, one characteristic of stricturing Crohn's disease.
MATERIALS AND METHODS
Isolation of Intestinal Muscle Cells from Human Intestine
Segments of intestine were obtained from patients undergoing ileal or ileo-cecal resection for stricturing Crohn's Disease according to a protocol approved by the VCU Institutional Review Board. Muscle cells were isolated from the ileal circular muscle layer using previously reported techniques from regions of stricturing Crohn's Disease and from the normal proximal ileal resection margin 6, 10, 19, 20. Demographic data on patients consenting to provide tissue for this study are presented in Table 1. Muscle cells isolated by enzymatic digestion were used to prepare RNA, and whole cell lysates or placed into cell culture. Epithelial cells, endothelial cells, neurons and interstitial cells of Cajal are not detected in cells isolated in this fashion21. These cells possess a smooth muscle phenotype: immunostaining for smooth muscle markers but not fibroblast markers, expression of γ-enteric actin, and the physiologic characteristics of contractile intestinal smooth muscle. Each characteristic is retained by the muscle cells in culture21.
Table 1.
Demographics of patients with stricturing Crohn's disease.
| Patient No. (% of total) | |
|---|---|
| Age (years) | |
| under 20 | 1 (6.7) |
| 20-29 | 1 (6.7) |
| 30-39 | 3 (20.0) |
| 40-49 | 4 (26.7) |
| 50-59 | 5 (33.3) |
| over 60 | 1 (6.7) |
| Sex | |
| Male | 3 (20.0) |
| Female | 12 (80.0) |
| Race | |
| White | 9 (60.0) |
| Black or African | 5 (33.3) |
| Other/unknown | 1 (6.7) |
Preparation of RNA and quantitative real-time PCR (qRT-PCR)
The expression of fibronectin (Hs.203717), vitronectin (Hs.2257), αV (Hs.436873) and β3 (Hs.218040) integrin subunits, and IGF-IEa (Hs.160562) were measured by qRT-PCR. Total RNA is prepared using RNAqueous™ kits (Ambion, Austin, TX). First stand cDNA synthesis is performed from two μg of total RNA using qScript™ kits (Quanta, Gaithersburg, MD). Quantitative RT-PCR reactions are performed in 25μl volume containing TAQman master mix, 2.5 pmol of forward and reverse primers (Applied Biosystems, Foster City, CA), and 2 μl of cDNA using a GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA). Standard curves for amplicons are generated from a dilution series of cDNA from normal cells and results quantified using the 2ΔCt method based on β-actin amplification22.
Preparation of whole cell lysates
Cell lysates were prepared as described previously in immunoprecipitation buffer (in mM): 50 Tris-HCl (pH 7.5), 150 NaCl, 50 NaF, 1 Na orthovanadate, 1 dithiothreitol, 1 phenylmethylsulfonyl fluoride and 0.5% NP-40 to which was added 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 μg/ml aprotinin10, 20, 23. Lysates were centrifuged at 14,000g for 10 min at 4°C prior to use for immunoprecipitation or immunoblot analysis.
Immunoprecipitation of IGF-I receptor
IGF-I receptors were immunoprecipitated as previously described 10, 20, 23. Lysates containing 1 mg protein were incubated with 2 μg of rabbit anti-IGF-IR β-subunit overnight at 4°C with Protein A-agarose beads, washed three times, resuspended in 25 μl sample buffer and boiled for 5 min prior to immunoblotting for phosphorylated IGF-IR identified in phosphotyrosine immunoblots as described below.
Immunoblot Analysis
Analysis of integrin proteins and phosphorylated signaling proteins was performed as previously described10, 16. Cell lysates containing equal amounts of total protein or immunoprecipitated from samples containing equal amounts of protein (BioRad, Hercules, CA) were separated with SDS-PAGE under denaturing conditions and electrotransferred to nitrocellulose. Membranes were incubated overnight with antibodies: αV, β3, fibronectin, vitronectin, phosphotyrosine, Erk1/2, phospho-Erk1/2(Thr202/Tyr204), p70S6 kinase or phospho-p70S6 kinase(Ser389), GSK-3β, GSK-3β(Ser9), caspase-3, cleaved caspase-3 or β-actin (Cell Signaling Technologies, Beverly, MA; Upstate Biotechnology, Lake Placid, NY; Biosource International, Camarillo, CA; Becton-Dickinson, Franklin Lakes, NJ). Membranes were reblotted to measure total protein or β-actin. Bands were visualized with ECL using a FluoChem 8800; digital images were quantified using AlphaEaseFC version 3.1.2 software (Alpha Innotech, San Leandro, CA). Densitometric values were reported in arbitrary units after normalization to total protein levels or β-actin.
Immunohistochemical staining of Ki67
Proliferating muscle cells was measured by counting Ki67 immunoreactive cells in histologic sections by two blinded investigators in ten successive high power fields. Sections of formalin fixed, paraffin embedded tissue specimens from strictures or normal margins were mounted on charged slides, deparaffinized and dehydrated. Heat-citrate pretreatment was used for antigen retrieval. Sections were incubated with a 1:50 dilution of anti-human mouse monoclonal Ki67 antigen (DAKO, Carpinteria, CA), a secondary biotinylated goat anti-mouse antibody, and a biotin-streptavidin detection kit was used with DAB as substrate.
[3H]Thymidine Incorporation Assay
Proliferation of smooth muscle cells in culture was measured by the incorporation of [3H]thymidine as described previously6, 10, 20. Quiescent cells were incubated for 24 h in serum-free DMEM in the presence or absence of echistatin or AG1024 (Sigma Chemical, St Louis, MO). During the final 4 h of incubation, 1 μCi/ml [3H]thymidine was added to the medium. [3H]Thymidine incorporation into the perchloric acid extractable pool was used as a measure of DNA synthesis.
Nucleosome Apoptosis Assay
Apoptosis in cultured muscle cells was quantified by measurement of cytoplasmic histone-associated DNA fragments (nucleosomes) using a quantitative sandwich-enzyme-immunoassay ELISA (Roche Applied Science, Indianapolis, IN)19. Quiescent muscle cells were incubated for 24 h in serum-free DMEM in the presence and absence of test agents and lysed. 20 μl of lysate containing cytoplasmic DNA fragment were incubated on streptavidin-coated ELISA plates an antibody to histone-biotin (clone H11-4) and antibody to anti-DNA-POD (clone MCA-33). ABTS solution was added for visualization using a Victor2 1420 multichannel counter (Perkin Elmer, Boston, MA) and reported as ΔA405
Statistical analysis
Values given represent the mean ± SE of n experiments where n represents the number of experiments on specimens derived from separate intestinal specimens or primary cultures. Statistical significance was tested by Student's t-test for either paired or unpaired data as was appropriate with P < 0.05.
RESULTS
IGF-I expression is increased in intestinal smooth muscle cells of stricturing Crohn's disease
The IGF-I gene is alternatively spliced. The main circulating isoform of IGF-I is encoded by the IGF-IEa splice variant. Quantitative-PCR analysis confirmed that the IGF-IEa splice variant is expressed in smooth muscle. Levels of IGF-IEa mRNA expression are increased 3.4 ± 0.3 fold in muscle cells isolated from strictured intestine compared to muscle cells from the normal resection margin (Fig. 1A).
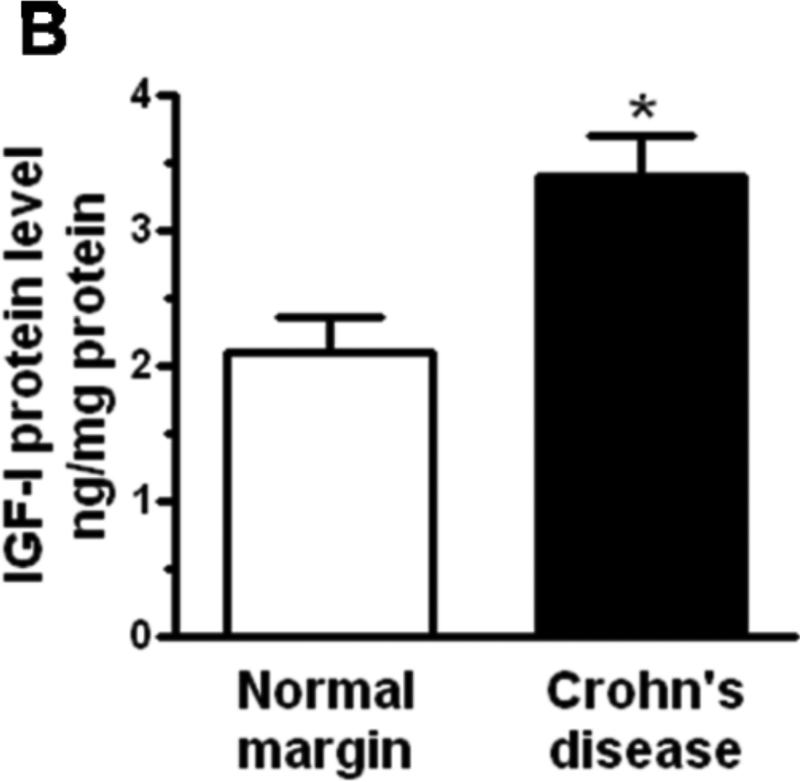
Figure 1. IGF-I expression is increased in smooth muscle cells of stricturing Crohn's disease.
Panel A: IGF-IEa RNA transcript levels are increased in muscle cells isolated from stricturing Crohn's disease compared to normal margins. Q-PCR results are expressed as fold increase over normal using the 2ΔCt method with β-actin as control. Panel B: IGF-I protein levels are increased in muscle cells isolated from stricturing Crohn's disease compared to normal margins. Results from ELISA are expressed in pg/mg protein. Values represent the mean ± SEM of 4-5 separate experiments. * denotes P < 0.05 vs normal margin.
IGF-I is produced by smooth muscle cells of human intestine6. Levels of IGF-I protein in muscle cells isolated from normal margins (2.1 ± 0.3 ng/mg protein) are increased 62 ± 9% in muscle cells isolated from regions of stricturing Crohn's disease (Fig. 1B).
Vitronectin and Fibronectin Production is Increased in Intestinal Smooth Muscle Cells in Stricturing Crohn's Disease
Vitronectin and fibronectin, two ligands of αVβ3 integrin (vitronectin receptor), are expressed in intestinal smooth muscle cells10, 24-26. The levels of vitronectin mRNA were increased 1.8 ± 0.3 fold (P < 0.05 vs normal margin) in muscle cells isolated from stricturing Crohn's disease, and levels of fibronectin mRNA increased 2.3 ± 0.4 fold (P < 0.05 vs normal margin) (Fig. 2A).
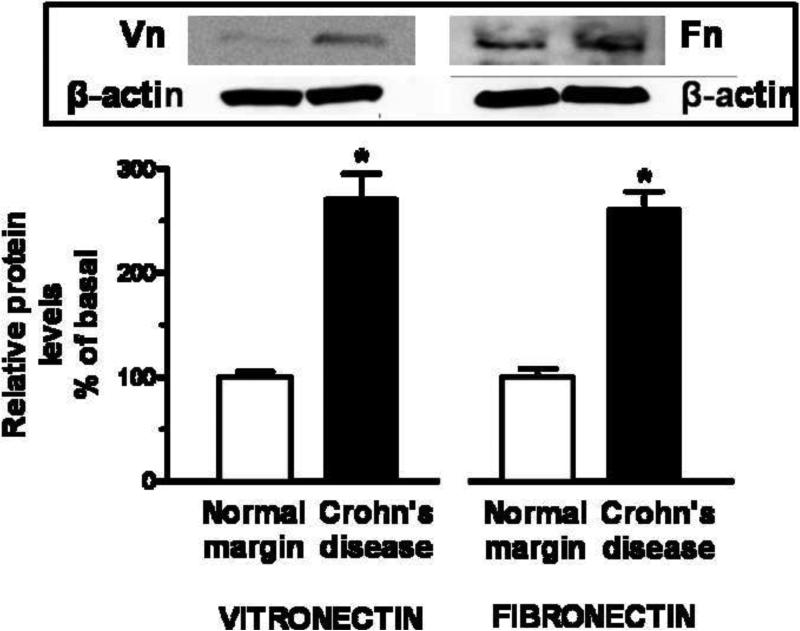
Figure 2. αVβ3 integrin ligands, vitronectin and fibronectin, are increased in smooth muscle cells of stricturing Crohn's disease.
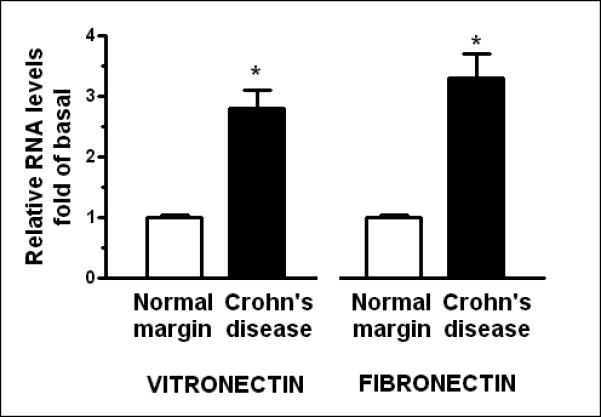
Panel A: Vitronectin and fibronectin RNA transcript levels are increased in muscle cells isolated from stricturing Crohn's disease compared to normal margins. Q-PCR results are expressed as fold increase over normal using the 2ΔCt method with β-actin as control. Panel B: Vitronectin and fibronectin levels are increased in muscle cells isolated from stricturing Crohn's disease compared to normal margins. Inset: Representative immunoblots of vitronectin and fibronectin levels and β-actin. Results are expressed in relative densitometric units normalized to β-actin levels. Values represent the mean ± SE of 5 separate experiments. * denotes P < 0.05 vs normal margin.
The levels of vitronectin protein in smooth muscle cells isolated from stricturing Crohn's disease were similarly increased by 170 ± 25% over the levels in muscle cells from normal margins. Levels of fibronectin protein were increased 160 ± 20% in muscle cells from stricturing regions compared to normal margins (Fig. 2).
In contrast to αVβ3 integrin ligand expression, no significant change in either αV or β3 integrin subunit protein levels were seen in regions of stricturing Crohn's disease (36 ± 25% and 7 ± 19% above normal margins, respectively).
Basal IGF-I Receptor Phosphorylation is Increased in Muscle Cells of Stricturing Crohn's Disease
Endogenous IGF-I regulates the growth of human intestinal smooth muscle cells by activation of the cognate IGF-I receptor17. Occupation of αVβ3 integrin by its ligands, i.e. vitronectin and fibronectin, augments the intensity and duration of IGF-I-stimulated, IGF-I receptor activity10. We hypothesized that the increased levels of vitronectin and fibronectin in regions of active Crohn's disease in conjunction with the increased expression of IGF-I would increase basal levels of IGF-I receptor tyrosine kinase activity in the intestinal muscle of stricturing Crohn's disease.
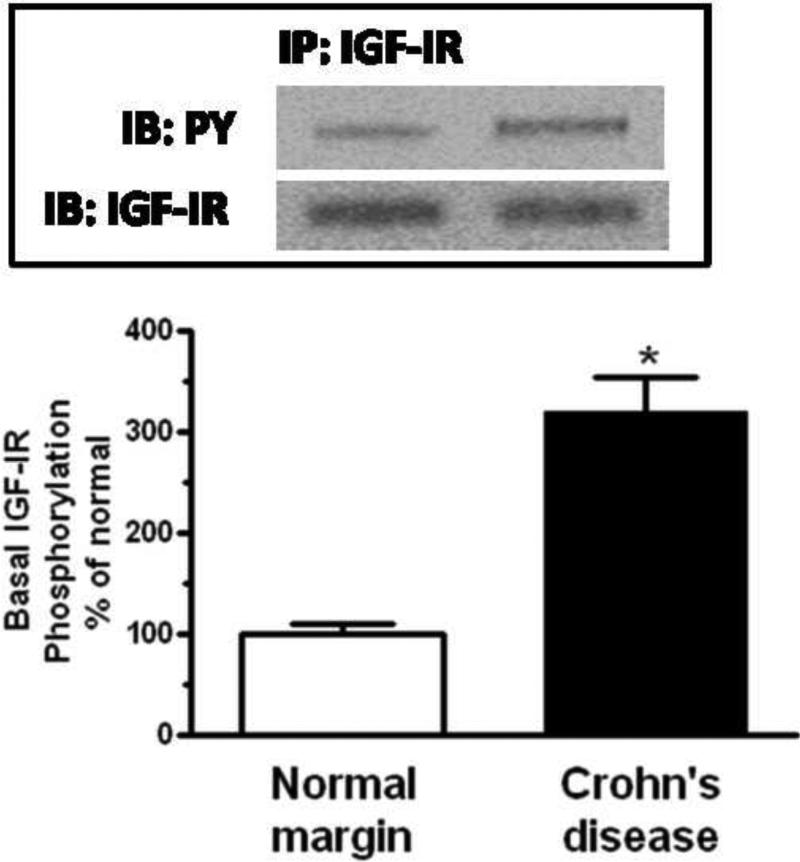
Phosphorylation of the IGF-I receptor was observed even under basal conditions in muscle cells isolated from normal resection margins as expected for an autocrine regulator of intestinal smooth muscle growth. Basal IGF-I receptor phosphorylation was an additional 320 ± 35% higher in muscle cells isolated from strictures (Fig 3).
Figure 3. Basal IGF-I receptor phosphorylation is increased in smooth muscle cells of stricturing Crohn's disease.
Densitometric analysis showing increased basal IGF-I receptor phosphorylation in smooth muscle cells isolated from stricturing Crohn's disease compared to normal margins. Inset: Representative immunoblots of IGF-I receptor phosphorylation (pY) and total IGF-I receptor levels. Results are expressed in relative densitometric units normalized to total IGF-I receptor levels. Values represent the mean ± SE of 4 separate experiments. * denotes P < 0.05 vs normal margin.
Basal Activity of IGF-I-stimulated Signaling Intermediates are Increased in Muscle Cells of Stricturing Crohn's Disease
Endogenous IGF-I regulates intestinal smooth muscle cell growth by jointly activating the Erk1/2 and the p70S6 kinase (via PI 3-kinase) signaling pathways, and inhibiting apoptosis via GSK-3β16, 17. We hypothesized that activity of these signaling intermediates would be increased in muscle cells isolated from strictures compared to normal margins. Phosphorylation of Erk1/2(Thr202/Tyr204) parallels Erk1/2 activity, GSK-3β(Ser9) parallels GSK-3β activity, and phosphorylation of p70S6 kinase(Ser389) most closely parallels p70S6 kinase activity. These phosphoproteins were therefore used in immunoblot analysis as measures of enzyme activity in lysates prepared from isolated muscle cells 16, 17, 19.
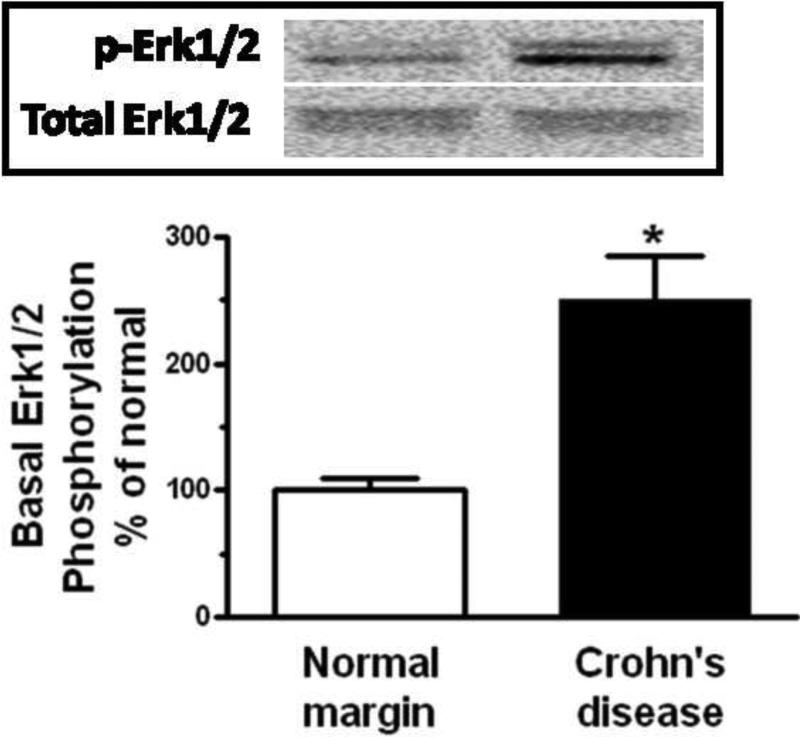
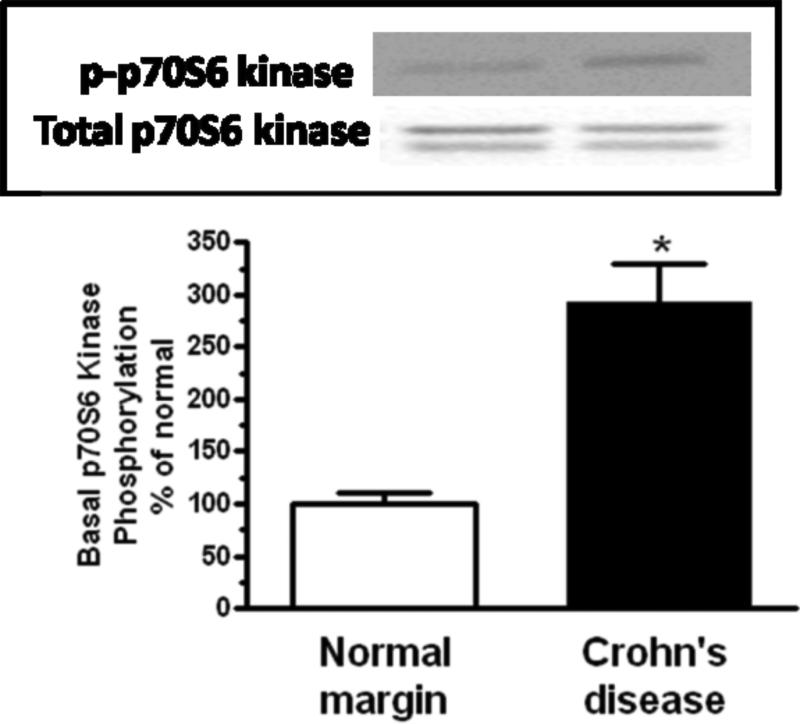
Erk1/2 was phosphorylated under basal conditions in muscle cells isolated from normal resection margins and increased an additional 251 ± 34% in the muscle cells isolated from strictures (Fig. 4A). Similarly, p70S6 kinase(Ser389) phosphorylation was observed with basal p70S6 kinase(Ser389) phosphorylation was present in the muscle cells isolated from normal margins and increased an additional 292 ± 37% in muscle cells isolated from strictures (Figure 4B).
Figure 4. Basal signaling intermediate phosphorylation is increased in smooth muscle cells of stricturing Crohn's disease.
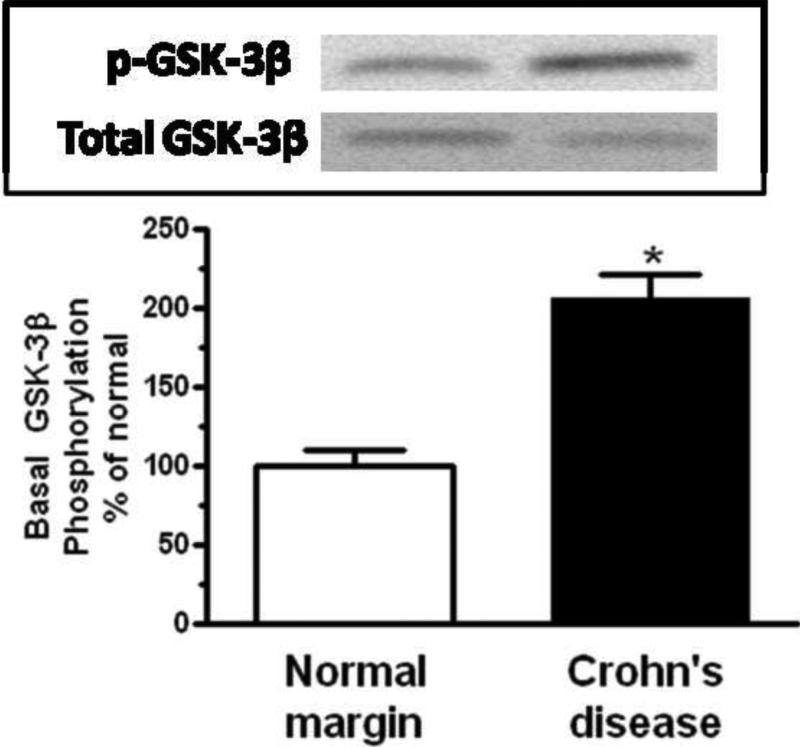
Panel A: Densitometric analysis showing increased basal Erk1/2 phosphorylation in smooth muscle cells isolated from stricturing Crohn's disease compared to normal margins. Inset: Representative immunoblots of phospho-Erk1/2(Thr202/Tyr204) and total Erk1/2 levels. Panel B: Densitometric analysis showing increased basal p70S6 kinase(Thr389) phosphorylation in smooth muscle cells isolated from stricturing Crohn's disease compared to normal margins. Inset: Representative immunoblots of phospho-p70S6 kinase(Thr389) and total p70 S6 kinase. Panel C: Densitometric analysis showing increased basal GSK-3β phosphorylation in smooth muscle cells isolated from stricturing Crohn's disease and normal margins. Inset: Representative immunoblots of phospho-GSK-3β(Ser9) and total GSK-3β. Results are expressed in relative densitometric units normalized to total signaling intermediate levels. Values represent the mean ± SE of 4 separate experiments. * denotes P < 0.05 vs normal margin.
GSK-3β(Ser9), coupled to IGF-I-stimulated inhibition of apoptosis, was phosphorylated under basal conditions in muscle cells isolated from normal resection margins. Phosphorylation was increased an additional 206 ± 16% in muscle cells isolated from strictures (Fig. 4C).
Smooth Muscle Cell Proliferation is Increased in Stricturing Crohn's Disease and Dependent on Endogenous IGF-I and αVβ3 Integrin Activation
We hypothesized that the concomitant increase in IGF-I, vitronectin and fibronectin expression; and increased activation of the IGF-I receptor and IGF-I-stimulated signaling intermediates coupled to proliferation would result in increased muscle cell proliferation in regions of stricturing Crohn's disease compared to normal muscle. This was examined in two ways: i) muscle cell proliferation was measured from the number of Ki67 positive cells in histologic sections of stricture and normal margin. Smooth muscle cells were identified morphologically by their characteristic spindle shaped appearance. ii) Increased proliferation specifically in muscle cells was confirmed by measurement [3H]thymidine incorporation in homogeneous cell cultures.
The number of proliferating muscle cells in histologic sections from normal margins showed a mean of 1.2 ± 0.3 Ki67 positive smooth muscle cells per HPF (Fig. 5A). The number of Ki67 positive muscle cells increased to 4.3 ± 0.2 per HPF in regions of stricturing Crohn's Disease (Fig. 5B).
Figure 5. Ki67 immunoreactive smooth muscle cells are increased in stricturing Crohn's disease over normal margins.
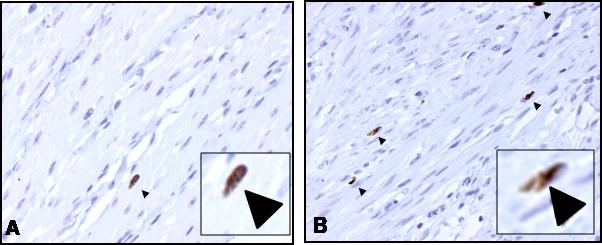
Representative micrographs of muscularis propria of human intestine showing the increased numbers of Ki67 positive smooth muscle cells in stricturing Crohn's disease (Panel B) compared to normal margins (Panel A). Arrows denote Ki67 immunoreactive muscle cells stained with DAB on background hematoxylin/eosin staining identifiable by their characteristic spindle shape appearance. Magnification=200x. Insets: Expanded view of representative Ki67 positive smooth muscle cells.
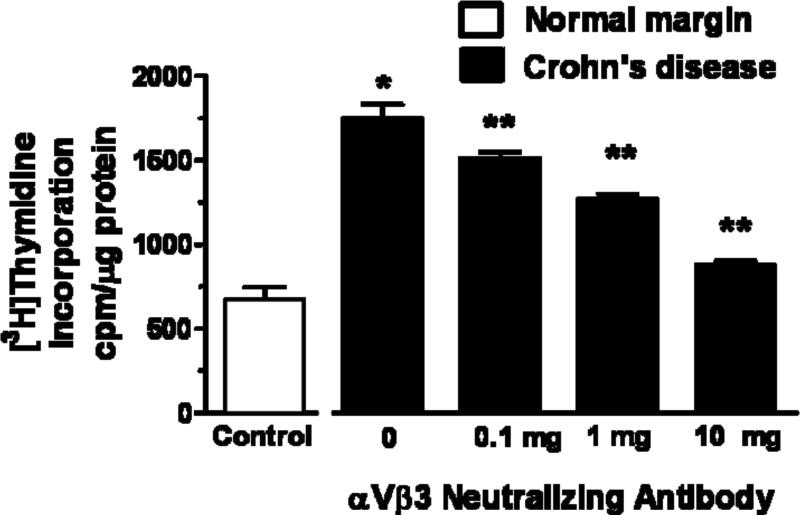
Basal [3H]thymidine incorporation in muscle cells isolated and cultured from normal margins was 675 ± 72 cpm/mg protein. Proliferation was inhibited 51 ± 4% by the IGF-I receptor tyrosine kinase inhibitor, AG1024 (1 μM) and 61 ± 3% by the αVβ3 integrin antagonist, echistatin (10 nM) (Fig. 6A)27, 28. Basal [3H]thymidine incorporation in muscle cells from stricturing Crohn's disease increased to 1750 ± 80 cpm/mg protein, 260 ± 30% above that in cells from normal margins (Fig. 6A) and was inhibited 54 ± 4% by AG1024 and 67 ± 8% by echistatin (Fig. 6A). Similarly, incubation of muscle cells isolated from strictures with a neutralizing antibody to αVβ3 integrin caused concentration-dependent inhibition of proliferation (Fig. 6B). These results indicate that endogenous IGF-I and αVβ3 integrin ligands regulate intestinal smooth muscle proliferation and mediate muscle cell hyperplasia in regions of stricturing Crohn's disease.
Figure 6. Smooth muscle cell proliferation is increased in stricturing Crohn's disease and dependent on endogenous IGF-I and αVβ3 integrin ligands.

Panel A: Basal [3H]thymidine incorporation is increased in muscle cells isolated from stricturing Crohn's disease compared to normal margins. Proliferation is inhibited by the αVβ3 integrin antagonist, echistatin (100 nM), or by the IGF-I receptor tyrosine kinase inhibitor, AG1024 (10 μM). Panel B: [3H]thymidine incorporation in muscle cells isolated from stricturing Crohn's disease is inhibited in a concentration-dependent fashion by an αVβ3 integrin neutralizing antibody. Results are expressed in cpm/μg total cell protein. Values represent the mean ± SEM of 5 separate experiments. * denotes P < 0.05 vs control muscle cells from normal margins, ** denotes P < 0.05 vs control muscle cells from stricturing Crohn's disease, *** denotes P < 0.05 vs control muscle cells from normal margins.
Smooth Muscle Cell Apoptosis is Decreased in Stricturing Crohn's disease and Dependent on Endogenous IGF-I and αVβ3 Integrin Activation
Endogenous IGF-I also regulates intestinal muscle cell growth by inhibiting apoptosis19. We hypothesized that the events that stimulate proliferation would also decrease apoptosis. This notion was tested in two ways: i) by direct measurement of the relative levels of cleaved and total caspase-3 in freshly isolated smooth muscle cells, and ii) by measurement of nucleosome accumulation in cultured smooth muscle cells.
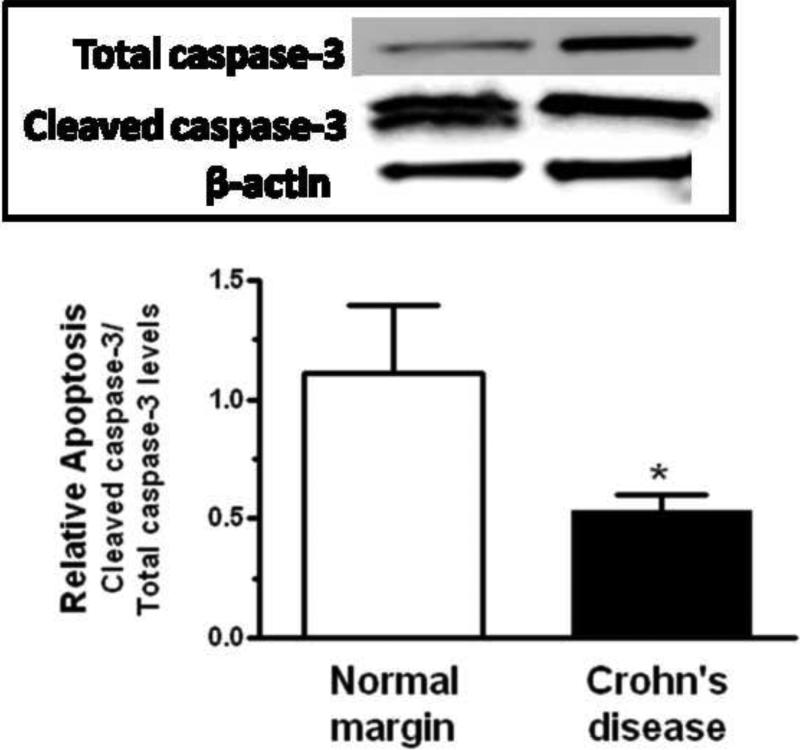
In lysates derived from muscle cells isolated from normal margins, the fraction of cleaved caspase-3/total caspase-3 was 1.36 ± 0.29. This fraction decreased to 0.53 ± 0.07 in muscle cells isolated from stricturing Crohn's disease reflecting a decrease in the level of apoptosis in these cells (Fig 7A).
Figure 7. Rates of smooth muscle cell apoptosis in stricturing Crohn's disease are decreased and dependent on endogenous IGF-I and αVβ3 integrin ligands.
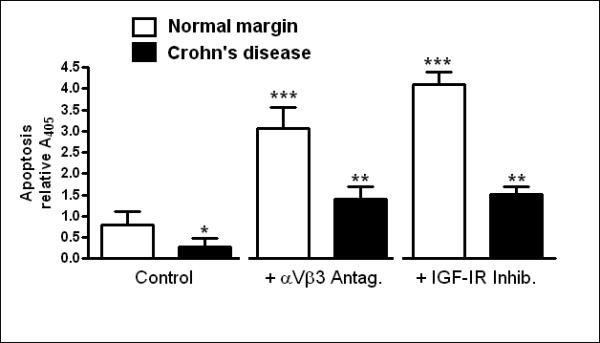
Panel A: Apoptosis, measured as cleaved caspase-3/total caspase-3, is lower in muscle cells isolated from stricturing Crohn's disease compared to normal margin. Inset: Representative immunoblots of total caspase-3, cleaved caspase-3 and β-actin. Results were expressed as cleaved caspase-3/total caspase-3. Panel B: Apoptosis, measured as nucleosome levels, is decreased in muscle cells cultured from stricturing Crohn's disease compared to normal margin. Apoptosis was augmented by the αVβ3 integrin antagonist, echistatin (100 nM), or by the IGF-I receptor tyrosine kinase inhibitor, AG1024 (10 μM) in both. Results from nucleosome-specific ELISA are expressed as ΔA405. Values represent the mean ± SEM of 5 separate experiments. * denotes P < 0.05 vs control muscle cells from normal margins, ** denotes P < 0.05 vs control muscle cells from stricturing Crohn's disease, *** denotes P < 0.05 vs control muscle cells from normal margins.
Apoptosis was also measured from the level of nucleosomes in muscle cells isolated and cultured from normal resection margins and from strictures. Basal levels of nucleosomes in normal cells increased 66 ± 15% in the presence of AG1204, and increased 119 ± 18% in the presence of echistatin (Fig. 8B). In smooth muscle cells isolated from regions of stricturing Crohn's Disease, nucleosome levels were only 90 ± 4% of that measured in muscle cells from normal margin indicating a lower basal level of apotosis. In the presence of echistatin, nucleosome levels in cells isolated from strictures increased 63 ± 8%, and in the presence of AG1024 increased by 107 ± 16% (Fig 8B).
DISCUSSION
Three characteristic features are hallmarks of the muscularis propria in stricturing Crohn's disease: smooth muscle cell hyperplasia, smooth muscle cell hypertrophy and excess net extracellular matrix production. This paper shows that in patients with stricturing Crohn's disease, growth of intestinal smooth muscle in strictures is increased compared to regions of normal muscle. Increased IGF-IEa mRNA and IGF-I protein production by the smooth muscle cells of the muscularis propria of stricturing Crohn's disease is associated with increased smooth muscle cell growth18. This is in contrast to the ~46% decrease in serum levels of hepatic-derived IGF-I in patients with active Crohn's disease that is thought to arise from the effects of the disease on nutrition and inflammation29. We have previously shown that endogenous IGF-I regulates smooth muscle cell hyperplasia in vitro by concomitantly stimulating proliferation and inhibiting apoptosis6, 19. However, the functional role of IGF-I in mediating the increased growth of smooth muscle of the muscularis propria in stricturing Crohn's disease and the mechanism involved have not previously been examined. This paper shows that basal rates of muscle cell proliferation are increased in smooth muscle of strictures, and are accompanied by decreased rates of apoptosis; both are regulated by the increased levels of endogenous IGF-I.
This paper also provides evidence that along with increased IGF-I production in muscle cells of stricturing Crohn's disease, there is increased production of the αVβ3 ligands, fibronectin and vitronectin. Our recent work provides insight on how these events are linked and contribute to increased muscle growth: occupancy of the αVβ3 integrin by its endogenous ligands, vitronectin and fibronectin, in intestinal smooth muscle increases the intensity and duration of IGF-I-stimulated IGF-I receptor tyrosine kinase activity, signaling and growth10. These results suggest that regulation of muscle growth by IGF-I and augmentation by occupancy of αVβ3 integrin contributes further to the hyperplasia of smooth muscle cells of the muscularis propria in stricturing Crohn's disease.
The evidence supporting the participation of both αVβ3 integrin and IGF-I in the increased smooth muscle growth in strictured intestine of Crohn's disease can be summarized as follows: i. αVβ3 integrin is expressed by intestinal smooth muscle cells and the expression of vitronectin and fibronectin, ligands of αVβ3 integrin, was increased in regions of stricturing; ii. basal IGF-I receptor tyrosine kinase phosphorylation in intestinal smooth muscle is increased in regions of stricturing; iii. basal phosphorylation of Erk1/2 and p70S6 kinase, both coupled to stimulation of proliferation, is increased in muscle cells in regions of stricturing; iv. basal phosphorylation of GSK-3β, coupled to inhibition of apoptosis, is increased in muscle cells in regions of stricturing; v. basal rates of smooth muscle cell proliferation are increased in regions of stricturing and are dependent on endogenous IGF-I and αVβ3 integrin27, 28; vi. basal levels of smooth muscle cell apoptosis are decreased in regions of stricturing and are dependent on endogenous IGF-I and αVβ3 integrin.
These results corroborate those of Zimmermann et al18 showing that the levels of IGF-IEa RNA were increased in regions of active Crohn's Disease when compared to uninflamed intestine but further show that IGF-I expression and production is upregulated specifically in intestinal smooth muscles cells. Increased IGF-IEa expression is also observed in animal models of colitis including TNBS-induced, peptidoglycan-polysaccharide-induced, and DSS-induced colitis18, 30, 31. The mechanisms responsible for increased IGF-I expression in stricturing Crohn's disease are unknown. Normally growth hormone (GH) is a main regulator of IGF-I expression. GH-dependent induction of suppressor of cytokine signaling (SOCS)-2 and SOCS-3 inhibits GH-dependent expression of IGF-I through a negative feedback mechanism32. However, a GH-resistant state exists in patients with Crohn's disease and in animal models of colitis where there is normal and stimulated GH secretion yet decreased circulating (hepatic-derived) IGF-I levels. SOCS-2 and SOCS-3 are also negative modulators of the effects of inflammatory cytokines. While GH did not increase SOCS-3 expression in vitro in cultured murine myofibroblasts from full thickness intestine, the Th1 cytokines, TNF-α or IL-6, in combination with GH did33. Recent (unpublished) data from our lab suggests that increased production of IGF-I by strictured muscle cells is accompanied by altered levels of SOCS-2 and SOCS-3 protein compared to muscle cells from normal margin. This apparent dysregulation of SOCS expression may contribute to the increased IGF-IEa expression in muscularis propria muscle cells of stricturing Crohn's disease.
IGF-I also regulates expression of one component of the extracellular matrix, collagen. The upregulation of IGF-I is accompanied by upregulation of IGF binding protein-5 (IGFBP-5) expression and collagens I, III, and V18, 34, 35. Our previous work has shown how these events are linked in human intestinal muscle where IGF-I increases IGFBP-5 expression and production, and both IGF-I and IGFBP-5 increase each others’ production in addition to increasing collagen production18, 20. Our recent work has also shown that IGFBP-3 and TGF-β1 expression is increased in muscle cells isolated from stricturing Crohn's disease; both regulate collagen I expression and production by activating the TGF-βRI/II receptor complex36, 37. While these results suggest that the processes that culminate in increased collagen production play a role in the development of stricture formation in Crohn's disease by increasing collagen production and net extracellular matrix formation, this has not been directly demonstrated.
Production of other extracellular matrix proteins, i.e. vitronectin and fibronectin, are also increased in muscle cells of stricturing Crohn's disease and play a regulatory role in the development of smooth muscle cell hyperplasia by activating αVβ3 integrin and augmenting IGF-I-stimulated smooth muscle cell hyperplasia10. Recently, αVβ3 integrin-dependent mechanisms mediating angiogenesis in the inflammatory response of the intestinal mucosa and submucosa in Crohn's disease, ulcerative colitis, and murine DSS-induced colitis have been elucidated38-40. Endothelial αVβ3 integrin expression is increased by inflammation in vivo or by experimental application of TNF-α, vascular endothelial growth factor or basic fibroblast growth factor41. In contrast to the response of intestinal endothelial cells to inflammation, αVβ3 integrin levels in intestinal smooth muscle cells are not altered in stricturing Crohn's disease.
The clinical significance of the findings of this paper is in the setting of active and stricturing Crohn's disease. Levels of the αVβ3 integrin ligands vitronectin and fibronectin are increased and act in concert with increased IGF-I expression to further increase IGF-I-stimulated proliferation and inhibition of apoptosis of intestinal smooth muscle cells. The long term sequelae of these two complementary processes regulating muscle cell growth may be muscle cell hyperplasia in the muscularis propria that is one characteristic of stricturing Crohn's disease.
Acknowledgments
This work was supported by Grant DK49691 from the National Institutes of Diabetes and Digestive and Kidney Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have relevant financial arrangements to disclose.
BIBLIOGRAPHY
- 1.Ruthruff B. Clinical review of Crohn's disease. J Am Acad Nurse Pract. 2007;19:392–7. doi: 10.1111/j.1745-7599.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45:210–7. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EY, Stenson WF, DeSchryver-Kecskemeti K. Thickening of muscularis mucosae in Crohn's disease. Mod Pathol. 1991;4:87–90. [PubMed] [Google Scholar]
- 5.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875–85. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- 6.Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology. 1997;113:817–24. doi: 10.1016/s0016-5085(97)70176-8. [DOI] [PubMed] [Google Scholar]
- 7.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–92. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425–39. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohneda K, Ulshen MH, Fuller CR, D'Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology. 1997;112:444–54. doi: 10.1053/gast.1997.v112.pm9024298. [DOI] [PubMed] [Google Scholar]
- 10.Kuemmerle JF. Occupation of alphavbeta3-integrin by endogenous ligands modulates IGF-I receptor activation and proliferation of human intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1194–202. doi: 10.1152/ajpgi.00345.2005. [DOI] [PubMed] [Google Scholar]
- 11.Clemmons DR, Horvitz G, Engleman W, Nichols T, Moralez A, Nickols GA. Synthetic alphaVbeta3 antagonists inhibit insulin-like growth factor-I-stimulated smooth muscle cell migration and replication. Endocrinology. 1999;140:4616–21. doi: 10.1210/endo.140.10.7027. [DOI] [PubMed] [Google Scholar]
- 12.Maile LA, Clemmons DR. The alphaVbeta3 integrin regulates insulin-like growth factor I (IGF-I) receptor phosphorylation by altering the rate of recruitment of the Src-homology 2-containing phosphotyrosine phosphatase-2 to the activated IGF-I receptor. Endocrinology. 2002;143:4259–64. doi: 10.1210/en.2002-220395. [DOI] [PubMed] [Google Scholar]
- 13.Nichols TC, du Laney T, Zheng B, Bellinger DA, Nickols GA, Engleman W, Clemmons DR. Reduction in atherosclerotic lesion size in pigs by alphaVbeta3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circ Res. 1999;85:1040–5. doi: 10.1161/01.res.85.11.1040. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CV, Letarte M, Lye SJ. The expression of integrins and cadherins in normal human uterus and uterine leiomyomas. Am J Obstet Gynecol. 1996;175:411–9. doi: 10.1016/s0002-9378(96)70155-2. [DOI] [PubMed] [Google Scholar]
- 15.Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G101–10. doi: 10.1152/ajpgi.00032.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kuemmerle JF. IGF-I elicits growth of human intestinal smooth muscle cells by activation of PI3K, PDK-1, and p70S6 kinase. Am J Physiol Gastrointest Liver Physiol. 2003;284:G411–22. doi: 10.1152/ajpgi.00310.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kuemmerle JF, Bushman TL. IGF-I stimulates intestinal muscle cell growth by activating distinct PI 3-kinase and MAP kinase pathways. Am J Physiol. 1998;275:G151–8. doi: 10.1152/ajpgi.1998.275.1.G151. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1022–9. doi: 10.1152/ajpgi.2001.280.5.G1022. [DOI] [PubMed] [Google Scholar]
- 19.Kuemmerle JF. Endogenous IGF-I promotes survival of human intestinal muscle cells by Akt-dependent inhibition of GSK-3β activity. Mol. Biol. Cell. 2002;13:165a. [Google Scholar]
- 20.Kuemmerle JF, Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J Biol Chem. 2002;277:20563–71. doi: 10.1074/jbc.M200885200. [DOI] [PubMed] [Google Scholar]
- 21.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–51. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 22.Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil. 2008 doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gbetagamma - dependent mitogen-activated protein kinase activation and growth. J Biol Chem. 2001;276:7187–94. doi: 10.1074/jbc.M011145200. [DOI] [PubMed] [Google Scholar]
- 24.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology. 2003;144:1664–70. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 25.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 26.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–86. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 27.Jones JI, Prevette T, Gockerman A, Clemmons DR. Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci U S A. 1996;93:2482–7. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–33. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- 29.Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Horm IGF Res. 2001;11:364–7. doi: 10.1054/ghir.2001.0248. [DOI] [PubMed] [Google Scholar]
- 30.Zeeh JM, Riley NE, Hoffmann P, Reinshagen M, Goebell H, Gerken G. Expression of insulin-like growth factor binding proteins and collagen in experimental colitis in rats. Eur J Gastroenterol Hepatol. 2001;13:851–8. doi: 10.1097/00042737-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann EM, Sartor RB, McCall RD, Pardo M, Bender D, Lund PK. Insulinlike growth factor I and interleukin 1 beta messenger RNA in a rat model of granulomatous enterocolitis and hepatitis. Gastroenterology. 1993;105:399–409. doi: 10.1016/0016-5085(93)90713-m. [DOI] [PubMed] [Google Scholar]
- 32.Rico-Bautista E, Flores-Morales A, Fernandez-Perez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–9. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Theiss AL, Fuller CR, Simmons JG, Liu B, Sartor RB, Lund PK. Growth hormone reduces the severity of fibrosis associated with chronic intestinal inflammation. Gastroenterology. 2005;129:204–19. doi: 10.1053/j.gastro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Graham MF, Diegelmann RF, Elson CO, Lindblad WJ, Gotschalk N, Gay S, Gay R. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–65. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 35.Kuemmerle JF. Endogenous IGF-I regulates IGF binding protein production in human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G710–717. doi: 10.1152/ajpgi.2000.278.5.G710. [DOI] [PubMed] [Google Scholar]
- 36.Flynn RaK JF. Endogenous IGFBP-3 activates TGF-beta receptors and regulates type I collagen expression and production in human intestinal smooth muscle cells in Crohn's Disease. Gastroenterology. 2008:132. [Google Scholar]
- 37.Graham MF, Bryson GR, Diegelmann RF. Transforming growth factor beta 1 selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology. 1990;99:447–53. doi: 10.1016/0016-5085(90)91028-5. [DOI] [PubMed] [Google Scholar]
- 38.Chidlow JH, Jr., Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–30. doi: 10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chidlow JH, Jr., Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 40.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–73. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 41.Danese S, Sans M, Spencer DM, Beck I, Donate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855–62. doi: 10.1136/gut.2006.114314. [DOI] [PMC free article] [PubMed] [Google Scholar]