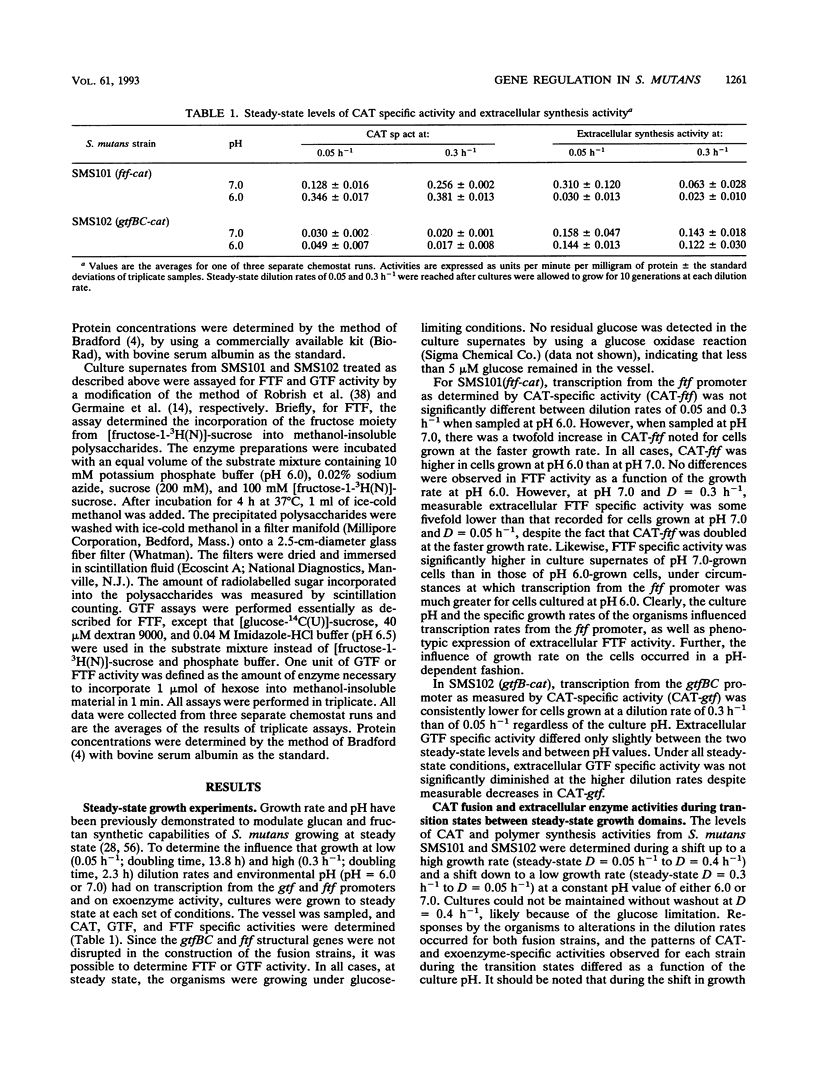
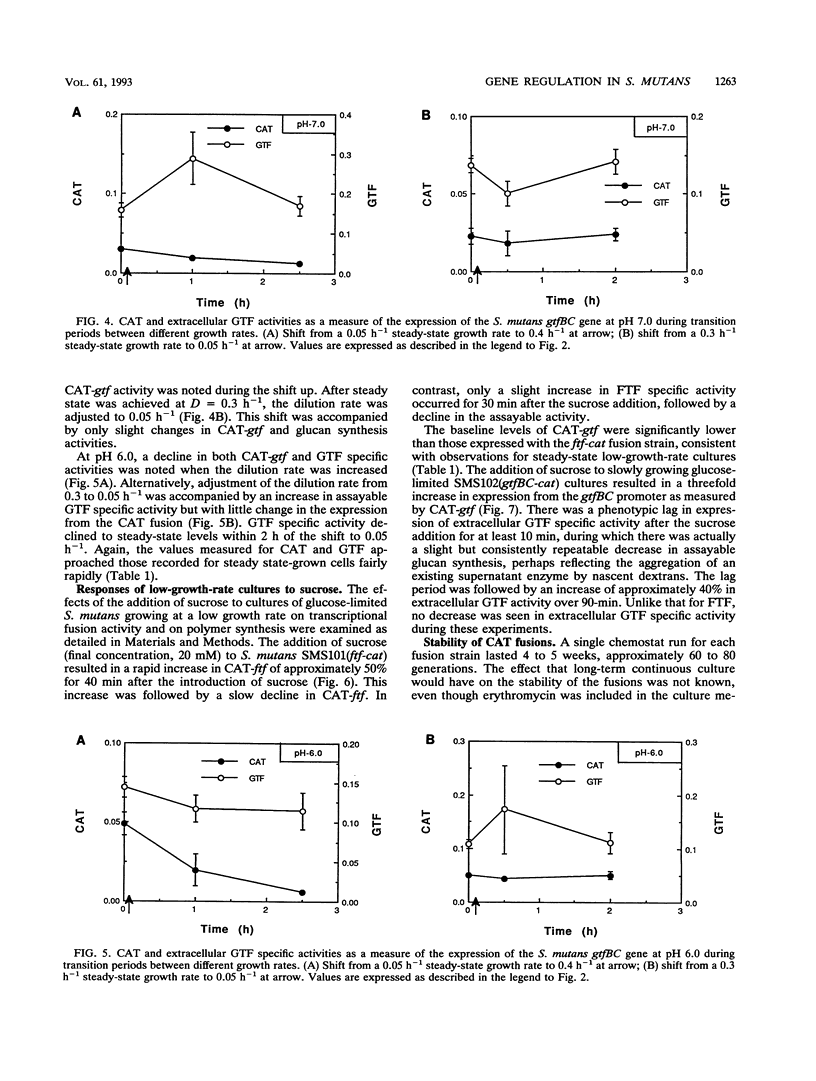
Abstract
Three glucosyltransferases (GTFs), which catalyze the formation of water-insoluble adherent glucans, and fructosyltransferase (FTF), which synthesizes fructans, are believed to contribute to the pathogenic potential of Streptococcus mutans. Study of the regulation of expression of GTF and FTF has been difficult because of the complexity and number of exoenzymes produced by this bacterium. By using continuous chemostat culture to control environmental conditions, chloramphenicol acetyltransferase (CAT) operon fusions were utilized to measure transcriptional activity of the ftf and gtfBC gene promoters. Expression of these operon fusions was differentially regulated in response to culture pH and growth rate and during transition states between growth domains. Furthermore, the addition of sucrose to steady-state cultures resulted in significant increases in CAT specific activities for both fusions. In a few cases, GTF and FTF enzyme specific activities did not parallel those of the corresponding CAT fusion activities; this lack of correspondence was likely due to posttranscriptional events controlling enzyme secretion and enzyme activity, as well as to the differential expression of dextranase(s) and fructan hydrolase by S. mutans. These results clearly demonstrate that the extracellular polymer synthesis machinery of S. mutans is regulated in a complex manner. The use of operon fusions in combination with chemostat culture is a viable approach to analyzing gene expression in S. mutans and will be helpful in defining the molecular mechanisms underlying regulation of expression of virulence attributes under conditions that may more closely mimic those in dental plaque.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli W. A., Marquis R. E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991 Apr;57(4):1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Schilling K., Bowen W. H., Yasbin R. E. Expression, purification, and characterization of an exo-beta-D-fructosidase of Streptococcus mutans. J Bacteriol. 1987 Oct;169(10):4507–4517. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Sundström B. Variations in composition of early dental plaque following ingestion of sucruse and glucose. Odontol Revy. 1968;19(2):161–169. [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M. Effect of sucrose in culture media on the location of glucosyltransferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun. 1978 Jun;20(3):592–599. doi: 10.1128/iai.20.3.592-599.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Buckley N. D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991 Apr;6(2):65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. C., Curtiss R., 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 1990 Feb;58(2):464–470. doi: 10.1128/iai.58.2.464-470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Wittenberger C. L. Inactivation of cell-associated fructosyltransferase in Streptococcus salivarius. J Bacteriol. 1981 Dec;148(3):912–918. doi: 10.1128/jb.148.3.912-918.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. J., Morrey-Jones J. G., Walker G. J. Inducible and constitutive formation of fructanase in batch and continuous cultures of Streptococcus mutans. J Gen Microbiol. 1985 Jul;131(7):1625–1633. doi: 10.1099/00221287-131-7-1625. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Marsh P. D., Ellwood D. C. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J Bacteriol. 1984 Feb;157(2):560–567. doi: 10.1128/jb.157.2.560-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Utilization of a mini-mu transposon to construct defined mutants in Streptococcus mutans. Mol Microbiol. 1987 Sep;1(2):229–232. doi: 10.1111/j.1365-2958.1987.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrimore S., Murchison H., Shiota T., Michalek S. M., Curtiss R., 3rd In vitro and in vivo complementation of Streptococcus mutans mutants defective in adherence. Infect Immun. 1983 Nov;42(2):558–566. doi: 10.1128/iai.42.2.558-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Milward C. P., Jacques N. A. Secretion of fructosyltransferase by Streptococcus salivarius involves the sucrose-dependent release of the cell-bound form. J Gen Microbiol. 1990 Jan;136(1):165–169. doi: 10.1099/00221287-136-1-165. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Tsumori H., Shimamura A. Isolation and characterization of an extracellular glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. Infect Immun. 1985 Sep;49(3):790–796. doi: 10.1128/iai.49.3.790-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro C., Michalek S. M., Macrina F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991 Jul;59(7):2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Linkage of sucrose-metabolizing genes in Streptococcus mutans. Infect Immun. 1990 Oct;58(10):3462–3464. doi: 10.1128/iai.58.10.3462-3464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Campbell L. K., Knox K. W., Wicken A. J. Chemostat studies of the effect of environmental control on Streptococcus sanguis adherence to hydroxyapatite. Infect Immun. 1982 Jan;35(1):64–70. doi: 10.1128/iai.35.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schilling K. M., Bowen W. H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992 Jan;60(1):284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder V. A., Michalek S. M., Macrina F. L. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun. 1989 Nov;57(11):3560–3569. doi: 10.1128/iai.57.11.3560-3569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Antigenic relatedness of glucosyltransferase enzymes from streptococcus mutans. Infect Immun. 1977 Jan;15(1):91–103. doi: 10.1128/iai.15.1.91-103.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori K., Mizuno F., Yamamoto A., Etoh Y., Takahashi M., Takahashi N. Preliminary studies of fructan-hydrolyzing bacteria from human dental plaque. Microbiol Immunol. 1985;29(4):359–363. doi: 10.1111/j.1348-0421.1985.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Townsend-Lawman P., Bleiweis A. S. Multilevel control of extracellular sucrose metabolism in Streptococcus salivarius by sucrose. J Gen Microbiol. 1991 Jan;137(1):5–13. doi: 10.1099/00221287-137-1-5. [DOI] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Thibault L., Neron S., Halvorson H., Hamilton I. R. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J Bacteriol. 1987 Dec;169(12):5686–5691. doi: 10.1128/jb.169.12.5686-5691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Jacques V. L., Fiala-Beer E., Slodki M. E. Influence of the culture medium on the synthesis of alpha-D-glucans by Streptococcus cricetus AHT. Carbohydr Res. 1992 Apr 6;227:1–17. doi: 10.1016/0008-6215(92)85057-7. [DOI] [PubMed] [Google Scholar]
- Wexler D. L., Penders J. E., Bowen W. H., Burne R. A. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutants strain. Infect Immun. 1992 Sep;60(9):3673–3681. doi: 10.1128/iai.60.9.3673-3681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. The amount, distribution and metabolism of soluble polysaccharides in human dental plaque. Arch Oral Biol. 1967 Jul;12(7):849–858. doi: 10.1016/0003-9969(67)90107-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]


