Abstract
Phagocytosis of microorganisms represents a stress not only for the phagocytosed agent but also for the host cell. We have investigated the stress response induced in human monocytes-macrophages (M phi) phagocytosing inactivated Staphylococcus aureus. Exposure of human M phi to S. aureus induced in these cells (i) a threefold increase in superoxide dismutase activity, (ii) a selective and differentiation-dependent induction of host heat shock protein synthesis (HSP70 but not HSP65), and (iii) de novo synthesis of heme oxygenase, but only when exogenous iron was added to the cultures. The coordinate upregulation of two scavenging enzymes and of HSP70 suggests that all three are part of cellular protective mechanisms against phagocytosis-related oxidative injury to host cells.
Full text
PDF





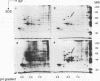
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bucker E. R., Martin S. E. Superoxide dismutase activity in thermally stressed Staphylococcus aureus. Appl Environ Microbiol. 1981 Feb;41(2):449–454. doi: 10.1128/aem.41.2.449-454.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Mesler D. E., Snipes R. G., Gray T. K. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986 Feb 1;136(3):1049–1053. [PubMed] [Google Scholar]
- Cosgrove J. W., Brown I. R. Heat shock protein in mammalian brain and other organs after a physiologically relevant increase in body temperature induced by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1983 Jan;80(2):569–573. doi: 10.1073/pnas.80.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Sundström L., Polla B. S., Junod A. F. Cultured human alveolar macrophages from smokers with lung cancer: resolution of factors that stimulate fibroblast proliferation, production of collagenase, or prostaglandin E2. J Leukoc Biol. 1985 May;37(5):641–649. doi: 10.1002/jlb.37.5.641. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Norton P., Ivanyi J. Distribution in tissue sections of the human groEL stress-protein homologue. APMIS. 1990 May;98(5):437–441. doi: 10.1111/j.1699-0463.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Fincato G., Polentarutti N., Sica A., Mantovani A., Colotta F. Expression of a heat-inducible gene of the HSP70 family in human myelomonocytic cells: regulation by bacterial products and cytokines. Blood. 1991 Feb 1;77(3):579–586. [PubMed] [Google Scholar]
- Forsdyke D. R. Heat shock proteins defend against intracellular pathogens: a non-immunological basis for self/non-self discrimination? J Theor Biol. 1985 Aug 7;115(3):471–473. doi: 10.1016/s0022-5193(85)80205-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Measurement of O2- secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Jättelä M., Saksela K., Saksela E. Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol. 1989 Aug;19(8):1413–1417. doi: 10.1002/eji.1830190810. [DOI] [PubMed] [Google Scholar]
- Kantengwa S., Polla B. S. Flavonoids, but not protein kinase C inhibitors, prevent stress protein synthesis during erythrophagocytosis. Biochem Biophys Res Commun. 1991 Oct 15;180(1):308–314. doi: 10.1016/s0006-291x(05)81293-8. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. T cell recognition of stress proteins. A link between infectious and autoimmune disease. Mol Biol Med. 1990 Aug;7(4):311–321. [PubMed] [Google Scholar]
- Langer T., Neupert W. Heat shock proteins hsp60 and hsp70: their roles in folding, assembly and membrane translocation of proteins. Curr Top Microbiol Immunol. 1991;167:3–30. doi: 10.1007/978-3-642-75875-1_1. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry Y., Young D. B., Mukherjee R. hsp70 synthesis in Schwann cells in response to heat shock and infection with Mycobacterium leprae. Infect Immun. 1992 Aug;60(8):3105–3110. doi: 10.1128/iai.60.8.3105-3110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak T. S., Jr Synthesis of a stress protein following transient ischemia in the gerbil. J Neurochem. 1985 Nov;45(5):1635–1641. doi: 10.1111/j.1471-4159.1985.tb07236.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Sauer R. T. Induction of a heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 1989 Aug;3(8):1226–1232. doi: 10.1101/gad.3.8.1226. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Bonventre J. V., Krane S. M. 1,25-Dihydroxyvitamin D3 increases the toxicity of hydrogen peroxide in the human monocytic line U937: the role of calcium and heat shock. J Cell Biol. 1988 Jul;107(1):373–380. doi: 10.1083/jcb.107.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Amento E. P., Krane S. M. 1,25-Dihydroxyvitamin D3 maintains adherence of human monocytes and protects them from thermal injury. J Clin Invest. 1986 Apr;77(4):1332–1339. doi: 10.1172/JCI112438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Wojno W. C., Krane S. M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am J Physiol. 1987 Jun;252(6 Pt 1):C640–C649. doi: 10.1152/ajpcell.1987.252.6.C640. [DOI] [PubMed] [Google Scholar]
- Polla B. S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991 Mar;12(3):A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Kantengwa S. Heat shock proteins and inflammation. Curr Top Microbiol Immunol. 1991;167:93–105. doi: 10.1007/978-3-642-75875-1_6. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Werlen G., Clerget M., Pittet D., Rossier M. F., Capponi A. M. 1,25-dihydroxyvitamin D3 induces responsiveness to the chemotactic peptide f-Met-Leu-Phe in the human monocytic line U937: dissociation between calcium and oxidative metabolic responses. J Leukoc Biol. 1989 May;45(5):381–388. doi: 10.1002/jlb.45.5.381. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc Natl Acad Sci U S A. 1987 May;84(9):2723–2726. doi: 10.1073/pnas.84.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Yoshinaga T., Tokunaga R. Induction of heme oxygenase in rat hepatoma cells by exposure to heavy metals and hyperthermia. Biochem Int. 1988 Oct;17(4):665–672. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukas C. D., Provvedini D. M., Manolagas S. C. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984 Jun 29;224(4656):1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Mizzen L. A. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol. 1988 Apr;106(4):1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Hogervorst E. J., van der Zee R., van Embden J. D., Hensen E. J., Cohen I. R. The mycobacterial 65 kD heat-shock protein and autoimmune arthritis. Rheumatol Int. 1989;9(3-5):187–191. doi: 10.1007/BF00271878. [DOI] [PubMed] [Google Scholar]