Abstract
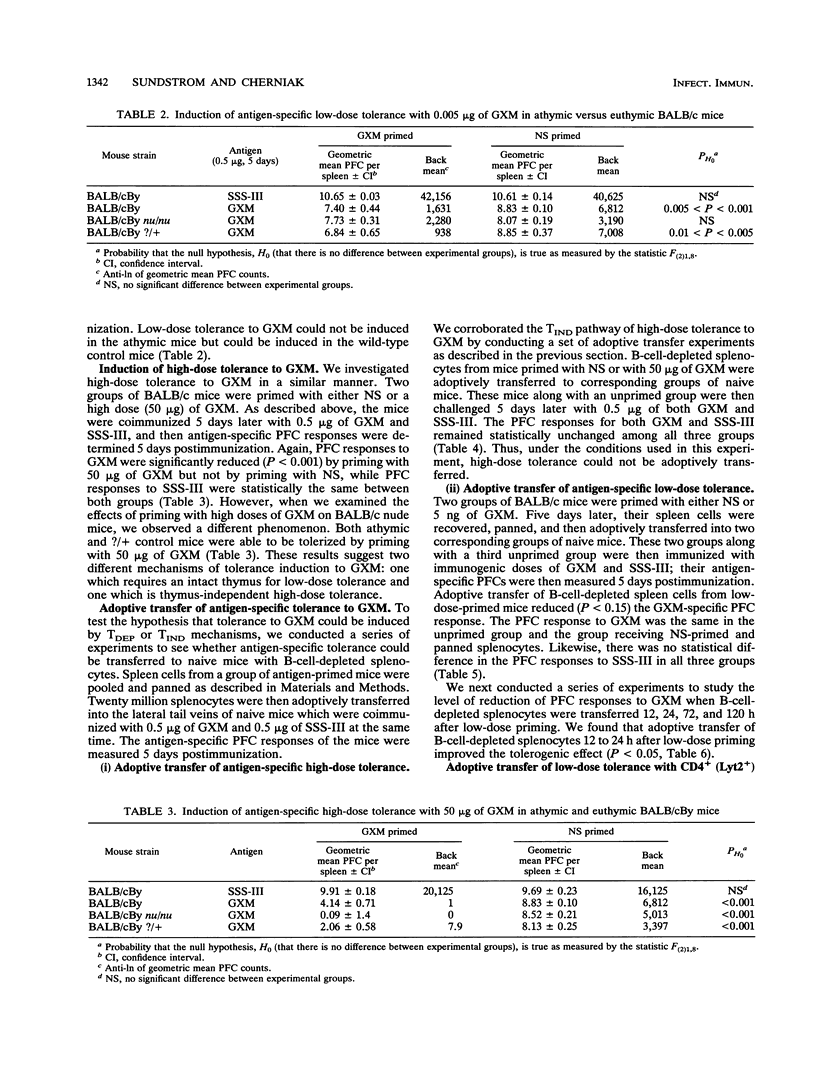
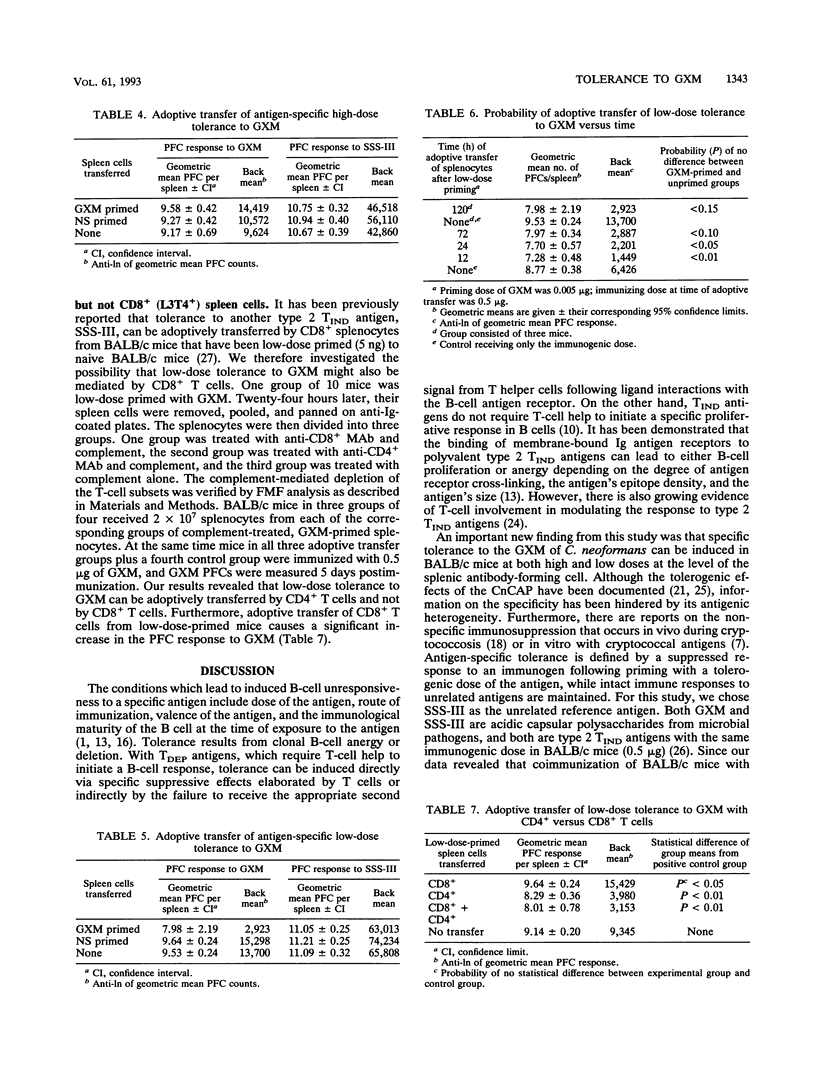
Glucuronoxylomannan (GXM), a type 2 T-independent antigen, is the major component of the capsular polysaccharide (CnCAP) of Cryptococcus neoformans. Previous studies have described the tolerogenic effects of high doses of CnCAP on the specific humoral response. In this investigation, evidence for both high-dose and low-dose tolerance to GXM is presented. BALB/cBy female mice, primed with either 5 ng or 50 micrograms of GXM, then coimmunized 3 days later with immunogenic doses of both GXM and type 3 pneumococcal polysaccharide (SSS-III), showed an antigen-specific inhibition in their splenic plaque-forming cell (PFC) responses to GXM compared with control groups primed with normal saline. SSS-III PFCs remained unchanged between GXM-primed and normal saline-primed groups. Low-dose tolerance appeared to be T dependent, whereas high-dose tolerance appeared to be T independent. Low-dose tolerance to GXM could not be induced in athymic BALB/c nu/nu mice, whereas high-dose tolerance in the same mice could be induced. Furthermore, low-dose tolerance was adoptively transferred with B-cell-depleted splenocytes to naive BALB/c mice, while high-dose tolerance was not. Complement-mediated depletion of CD4+ but not CD8+ splenocytes from low-dose-primed mice abrogated the transfer of low-dose tolerance. These findings indicate T-dependent and T-independent mechanisms of antigen-specific B-cell tolerance to GXM in BALB/c mice at low and high antigen doses, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstock R., McCormack J. M., Hall N. K. Induction of a macrophage-suppressive lymphokine by soluble cryptococcal antigens and its association with models of immunologic tolerance. Infect Immun. 1987 Jan;55(1):233–239. doi: 10.1128/iai.55.1.233-239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Modlin R. L., Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- Breen J. F., Lee I. C., Vogel F. R., Friedman H. Cryptococcal capsular polysaccharide-induced modulation of murine immune responses. Infect Immun. 1982 Apr;36(1):47–51. doi: 10.1128/iai.36.1.47-51.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R., Morris L. C., Anderson B. C., Meyer S. A. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect Immun. 1991 Jan;59(1):59–64. doi: 10.1128/iai.59.1.59-64.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dintzis R. Z., Middleton M. H., Dintzis H. M. Studies on the immunogenicity and tolerogenicity of T-independent antigens. J Immunol. 1983 Nov;131(5):2196–2203. [PubMed] [Google Scholar]
- James P. G., Cherniak R. Galactoxylomannans of Cryptococcus neoformans. Infect Immun. 1992 Mar;60(3):1084–1088. doi: 10.1128/iai.60.3.1084-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. G., Jemmerson R. Tolerance induction in resting memory B cells specific for a protein antigen. J Immunol. 1992 May 1;148(9):2682–2689. [PubMed] [Google Scholar]
- Kozel T. R., Gulley W. F., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977 Dec;18(3):701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masih D. T., Sotomayor C. E., Rubinstein H. R., Riera C. M. Immunosuppression in experimental cryptococcosis in rats. Induction of efferent T suppressor cells to a non-related antigen. Mycopathologia. 1991 Jun;114(3):179–186. doi: 10.1007/BF00437212. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Brunswick M. A role for IFN-gamma and NK cells in immune responses to T cell-regulated antigens types 1 and 2. Immunol Rev. 1987 Oct;99:105–118. doi: 10.1111/j.1600-065x.1987.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peçanha L. M., Snapper C. M., Lees A., Mond J. J. Lymphokine control of type 2 antigen response. IL-10 inhibits IL-5- but not IL-2-induced Ig secretion by T cell-independent antigens. J Immunol. 1992 Jun 1;148(11):3427–3432. [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Sundstrom J. B., Cherniak R. The glucuronoxylomannan of Cryptococcus neoformans serotype A is a type 2 T-cell-independent antigen. Infect Immun. 1992 Oct;60(10):4080–4087. doi: 10.1128/iai.60.10.4080-4087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B., Baker P. J. Cell surface antigens and other characteristics of T cells regulating the antibody response to type III pneumococcal polysaccharide. J Immunol. 1983 Jan;130(1):19–23. [PubMed] [Google Scholar]
- Turner S. H., Cherniak R. Glucuronoxylomannan of Cryptococcus neoformans serotype B: structural analysis by gas-liquid chromatography-mass spectrometry and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res. 1991 Apr 2;211(1):103–116. doi: 10.1016/0008-6215(91)84149-9. [DOI] [PubMed] [Google Scholar]
- Turner S. H., Cherniak R., Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984 Feb 15;125(2):343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- van den Eertwegh A. J., Fasbender M. J., Schellekens M. M., van Oudenaren A., Boersma W. J., Claassen E. In vivo kinetics and characterization of IFN-gamma-producing cells during a thymus-independent immune response. J Immunol. 1991 Jul 15;147(2):439–446. [PubMed] [Google Scholar]


