Abstract
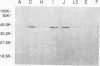
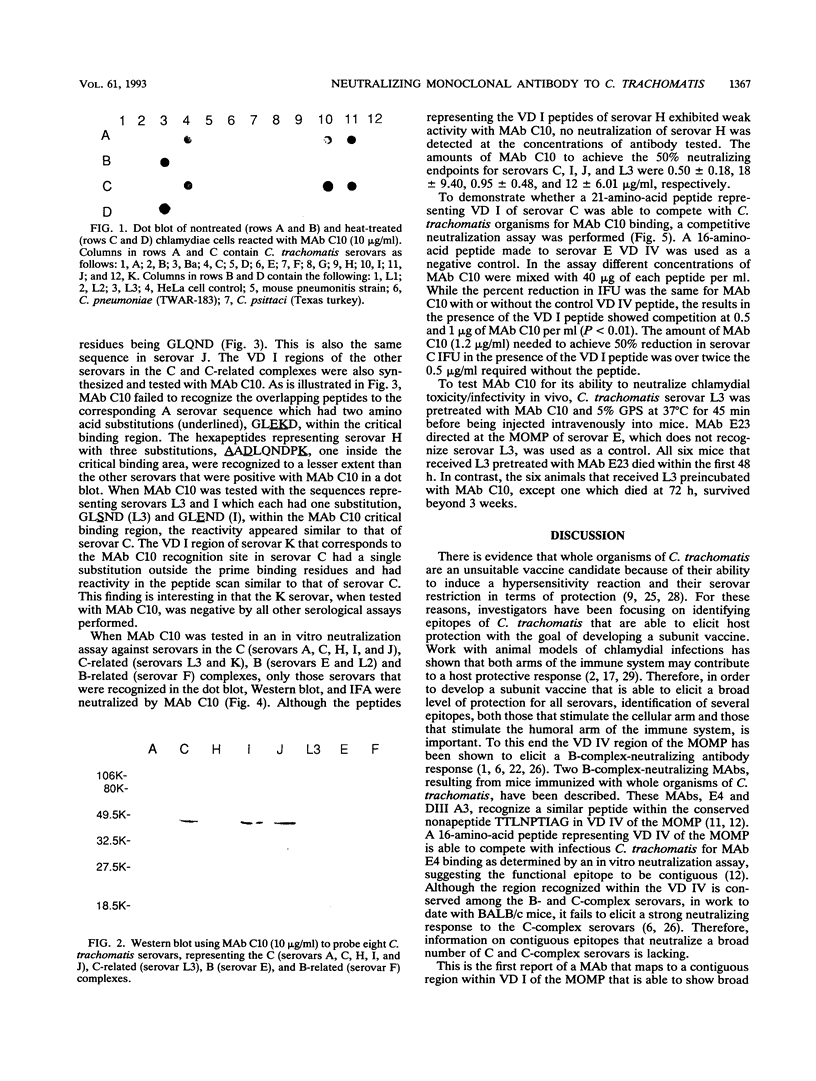
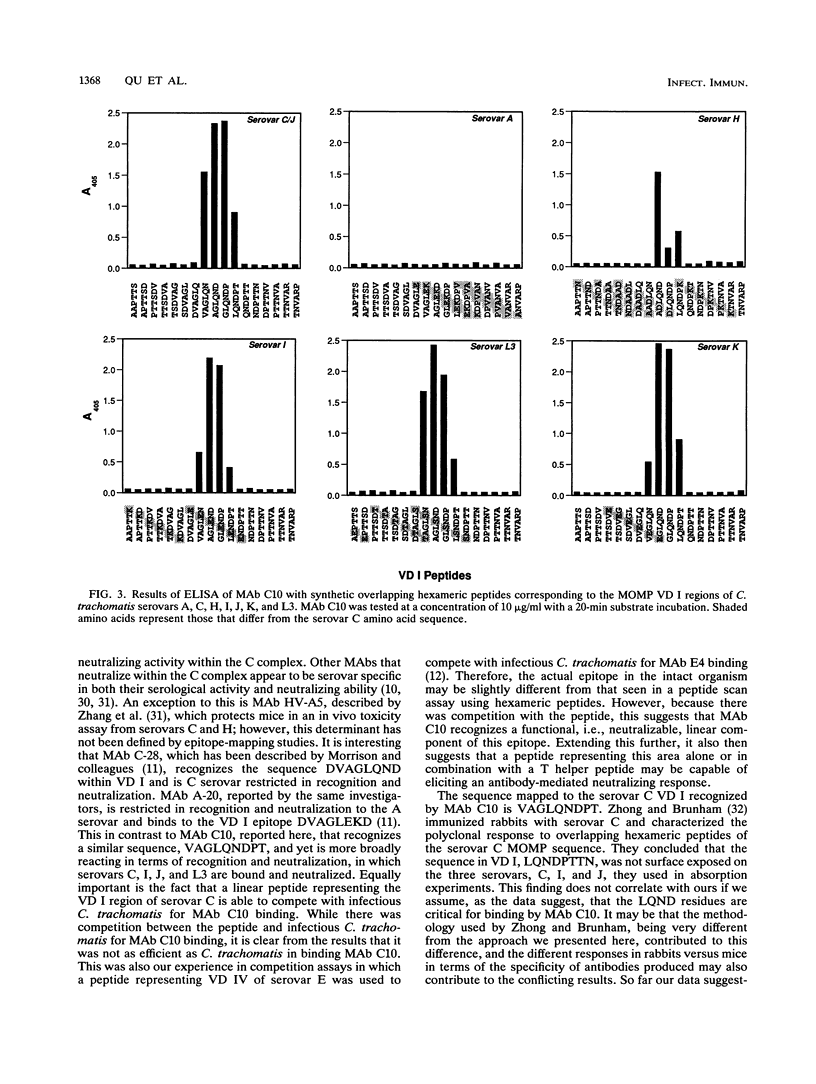
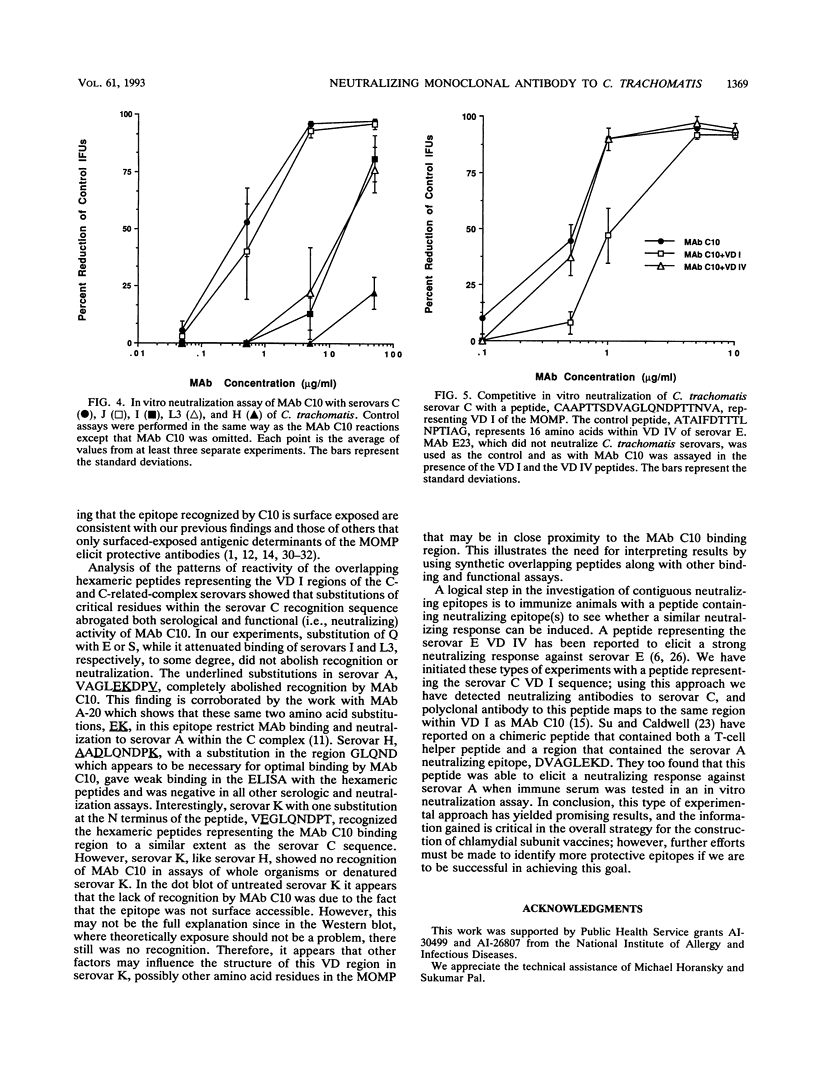
A monoclonal antibody (MAb), C10, that neutralized in vitro the infectivity of serovars C, I, J, and L3 (members of the C and C-related complexes) of Chlamydia trachomatis was identified. Of the 15 major serovars and the mouse pneumonitis strain of C. trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae, which were used as nontreated and heat-treated (56 degrees C, 30 min) antigens in a dot blot assay, only serovars C, I, J, and L3 were recognized with both the native and treated antigens. Western blot (immunoblot) results showed that MAb C10 recognized the major outer membrane protein of these four serovars. Overlapping hexameric peptides corresponding to variable domains (VDs) I, II, III, and IV of the major outer membrane protein of C. trachomatis serovar C were synthesized, and peptide screening showed that MAb C10 mapped to the VD I amino acid sequence VAGLQNDPT. Results of an in vitro neutralization assay correlated with those of the indirect immunofluorescence assay, Western blot, and dot blot assay in that only serovars C, I, J, and L3 were neutralized by MAb C10. In vitro competitive neutralization experiments, using a peptide representing VD I of serovar C to compete with C. trachomatis serovar C for MAb C10 binding, revealed that both serological and neutralizing activities of MAb C10 were inhibited by the VD I peptide. In an in vivo toxicity/infectivity assay using serovar L3 pretreated with MAb C10, there was 100% survival of mice infected with a lethal dose at 48 h. In contrast, the control group, consisting of mice injected with the same dose of L3 pretreated with a MAb that does not recognize L3, had no survivors during a 48-h observation period. In summary, since the surface-exposed contiguous epitope recognized by MAb C10 binds neutralizing antibodies that are subspecies specific for the C and C-related complexes, it should be considered for inclusion in the development of a chlamydial vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect Immun. 1987 Aug;55(8):1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E. J., Peterson E. M., de la Maza L. M. Cloning and characterization of a Chlamydia trachomatis L3 DNA fragment that codes for an antigenic region of the major outer membrane protein and specifically hybridizes to the C- and C-related-complex serovars. Infect Immun. 1989 Feb;57(2):487–494. doi: 10.1128/iai.57.2.487-494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Pal S., de la Maza L. M., Peterson E. M. Characterization of the humoral response induced by a peptide corresponding to variable domain IV of the major outer membrane protein of Chlamydia trachomatis serovar E. Infect Immun. 1992 Aug;60(8):3428–3432. doi: 10.1128/iai.60.8.3428-3432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Mason T. J., Rodda S. J. Cognitive features of continuous antigenic determinants. J Mol Recognit. 1988 Feb;1(1):32–41. doi: 10.1002/jmr.300010107. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Lucero M. E., Kuo C. C. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect Immun. 1985 Nov;50(2):595–597. doi: 10.1128/iai.50.2.595-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Cheng X., Markoff B. A., Fielder T. J., de la Maza L. M. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect Immun. 1991 Nov;59(11):4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Tse P., Gastaldi C., Stone S. C., de la Maza L. M. Comparison of a single-antigen microimmunofluorescence assay and inclusion fluorescent-antibody assay for detecting chlamydial antibodies and correlation of the results with neutralizing ability. J Clin Microbiol. 1989 Feb;27(2):350–352. doi: 10.1128/jcm.27.2.350-352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989 Jan;57(1):299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992 Jan 1;175(1):227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye B., Zhong G. M., Peeling R., Brunham R. C. Immunologic characterization of a cloned fragment containing the species-specific epitope from the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1990 Dec;58(12):3909–3913. doi: 10.1128/iai.58.12.3909-3913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967 May;63(5 Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- Zhong G. M., Brunham R. C. Immunoaccessible peptide sequences of the major outer membrane protein from Chlamydia trachomatis serovar C. Infect Immun. 1990 Oct;58(10):3438–3441. doi: 10.1128/iai.58.10.3438-3441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]