Abstract
Endotoxemia plays an important role in the pathogenesis of sepsis and is accompanied by dysregulated apoptosis of immune and non-immune cells. Treatment with statins reduces mortality in rodent models of sepsis and endotoxemia. Inhibition of protein isoprenylation, including farnesylation, has been proposed as a mechanism to mediate the lipid lowering-independent effects of statins. Nonetheless, the effects of the inhibition of isoprenylation have not yet been studied. To investigate the role of farnesylation, we evaluated the effects of farnesyltransferase inhibitor and statin on survival following lipopolysaccharide (LPS) challenge in mice. Both simvastatin (2 mg/kg BW) and FTI-277 (20 mg/kg BW) treatment improved survival by two-fold after LPS injection, as compared with vehicle alone (p<0.01). LPS-induced cleavage (activation) of caspase-3, an indicator of apoptotic change, and increased protein expression of proapoptotic molecules, Bax and Bim, and activation of c-Jun NH2-terminal kinase (JNK/SAPK) in the liver and spleen were attenuated by both simvastatin and FTI-277. These results demonstrate that farnesyltransferase inhibitor as well as statin significantly reduced LPS-induced mortality in mice. Our findings also suggest that inhibition of protein farnesylation may contribute to the lipid lowering-independent protective effects of statins in endotoxemia, and that protein farnesylation may play a role in LPS-induced stress response, including JNK/SAPK activation, and apoptotic change. Our data argue that farnesyltransferase may be a potential molecular target for treating patients with endotoxemia.
Keywords: farnesylation, statin, HMG-CoA reductase, lipopolysaccharide, caspase-3, c-Jun NH2-terminal kinase
INTRODUCTION
Sepsis is the leading cause of death amongst critically ill patients [1]. Despite extensive investigation over the past three decades, the incidence of sepsis and sepsis-related deaths appear to be increasing [2]. Lipopolysaccharide (LPS), the major structural component of the outer membrane of Gram-negative bacteria, has been implicated as the bacterial endotoxin responsible for the clinical syndrome of sepsis, including septic shock and multiple organ dysfunction syndrome.
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have had a major impact on healthcare by decreasing cardiovascular events. The efficacy of statins has been attributed primarily to their lipid-lowering properties. However, a growing body of evidence highlights statin effects that are independent of its lipid-lowering properties [3]. Observations, such as the rapid onset of clinical benefits and weak correlations between plasma cholesterol levels and coronary lumen change or cardiovascular events, argue that other non-lipid lowering actions must be involved [4, 5]. In fact, statins exert beneficial effects on increased flow-mediated vasodilation in normocholesterolemic subjects [6–8], as well. Recently, the National Cholesterol Education Project (NCEP) Adult Treatment Panel III guideline recommended that patients with diabetes and cardiovascular disease should initiate statin therapy regardless of baseline LDL cholesterol levels [9]. The lipid lowering-independent beneficial effects of statins also have been shown in animal models of atherosclerosis [10, 11].
Retrospective and prospective observational studies have shown that prior statin treatment reduces the incidence and mortality of sepsis in the intensive care unit [12–16], although controversial results also have been reported [17]. Prospective clinical trials are under way to evaluate the safety and efficacy of treatment with statins in septic patients [17]. Consistent with these observational studies in humans [12–16], other studies have demonstrated that statins reduce mortality in mouse models of sepsis and endotoxemia, along with preservation of cardiac function, amelioration of inflammatory alterations, or improved bacterial clearance [18–21]. In the latter studies, the beneficial effects of statins were attributed to the lipid-lowering-independent properties of the drug.
The non-lipid-lowering effects of statins presumably are accounted for by direct, pleiotropic actions, including anti-inflammatory and anti-oxidant effects. Nevertheless, the molecular mechanisms by which statins exert these pleiotropic actions remain to be determined. HMG-CoA reductase is the rate-limiting enzyme of cholesterol biosynthesis. Mevalonate, the immediate product of HMG-CoA reductase, is a precursor of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, as well as cholesterol. Hence, the inhibition of this enzyme results in decreased production not only of cholesterol, but also of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which, in turn, leads to reduced protein isoprenylation, namely, farnesylation and geranylgeranylation. Therefore, the inhibition of geranylgeranylation and/or farnesylation has been proposed as an appropriate mechanism to mediate the lipid lowering-independent protective effects of statins, although direct evidence is lacking.
Protein farnesylation, a lipid modification of cysteine residues, is catalyzed by protein farnesyltransferase and is essential for activity of the Ras family of small GTPases, such as p21ras. Previous studies have shown that farnesyltransferase inhibitors prevent apoptosis and ameliorate organ dysfunction in rodent models of trauma, such as ischemic/reperfusion and brain injury [22–24]. The activity of farnesyltransferase and protein farnesylation are increased by LPS, heat shock, and trauma [25–27]. The Ras family of small GTPases is a collection of key signaling molecules involved in the regulation of a variety of cellular processes, including apoptosis, survival, proliferation, and differentiation. Activation of Ras is required for the LPS-induced inflammatory response, including the induction of inducible nitric oxide synthase and oxidative stress [28], which suggests an important role of Ras activation in endotoxemia. We have shown previously that farnesyltransferase inhibitor prevents the development of atherosclerosis in apoE-deficient mice fed a high-fat diet, without altering serum cholesterol levels [29].
Nonetheless, the effects of farnesyltransferase inhibitor have not yet been investigated in endotoxemia or sepsis. In the present study, we investigate the effects of farnesyltransferase inhibitor in endotoxemia, by evaluating the actions of simvastatin and farnesyltransferase inhibitor, FTI-277, on survival following LPS challenge in mice.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice at 7 weeks of age, purchased from the Jackson Laboratory (Bar Harber, ME), were used for this study. The study was approved by the Institutional Animal Care Committee of Massachusetts General Hospital. The animal care facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The mice were housed in a pathogen-free animal facility maintained at 25°C and illuminated by 12: 12-h light-dark cycles. The mice were provided with standard rodent chow and water ad libitum. The animals received a bolus injection of LPS (27.5 mlg/kg BW, IP, Sigma, St. Louis, MO) or phosphate-buffered saline (PBS). At 2 h after the injection of LPS, treatment with simvastatin (2 mg/kg BW, IP, b.i.d., American Radiolabeled, St. Louis, MO), a specific inhibitior for protein farnesyltransferase, FTI-277 (20 mg/kg BW, IP, b.i.d., Sigma), or vehicle alone was initiated and continued up to 50 h after the LPS injection. The effects of the inhibitors on survival were observed up to 100 h after LPS injection.
Tissue homogenization
The liver and spleen were collected at 18 h after the LPS injection under anesthesia with pentobarbital sodium (50 mg/kg BW, IP). Tissue samples were homogenized in ice-cold homogenization buffer (50 mM HEPES-NaOH, pH 7.5, 150 mM NaC1, 2 mM EDTA, 1% lithium dodecylsulfate, 1% Nonidet P-40, 10% glycerol, 10 mM sodium fluoride, 2 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 µg/ml aprotinin, and 10 µg/ml leupeptin), as described previously [30].
Immunoblotting
Immunoblotting was performed as described previously [31]. Anti-cleaved caspase-3 (Cell Signaling, Danvers, MA), anti-Bax, anti-Bim, anti-JNK, anti-phosphorylated c-Jun, anti-c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphorylated JNK (Millipore, Billerica, MA), anti-β-actin (Sigma) were used as primary antibodies. Anti-rabbit or -mouse IgG antibody conjugated with horseradish peroxidase was used as secondary antibody. Western blotting chemiluminescence luminol reagent (Perkin-Elmer, Boston, MA) was used to visualize the blots. Bands of interest were scanned with the use of Power Look (UMAX Technologies, Dallas, TX) and quantified by NIH Image 1.62 software (NTIS, Springfield, VA).
Statistical analysis
The data were compared with one-way ANOVA followed by Newman-Keuls multiple comparison test. The effects of statin and farnesyltransferase inhibitor on survival were analyzed using Kaplan-Meier survival curve and log rank test. A value of P < 0.05 was considered statistically significant. All values are expressed as means ± SEM.
RESULTS
Statin and farnesyltransferase inhibitor improved survival following endotoxin challenge in mice
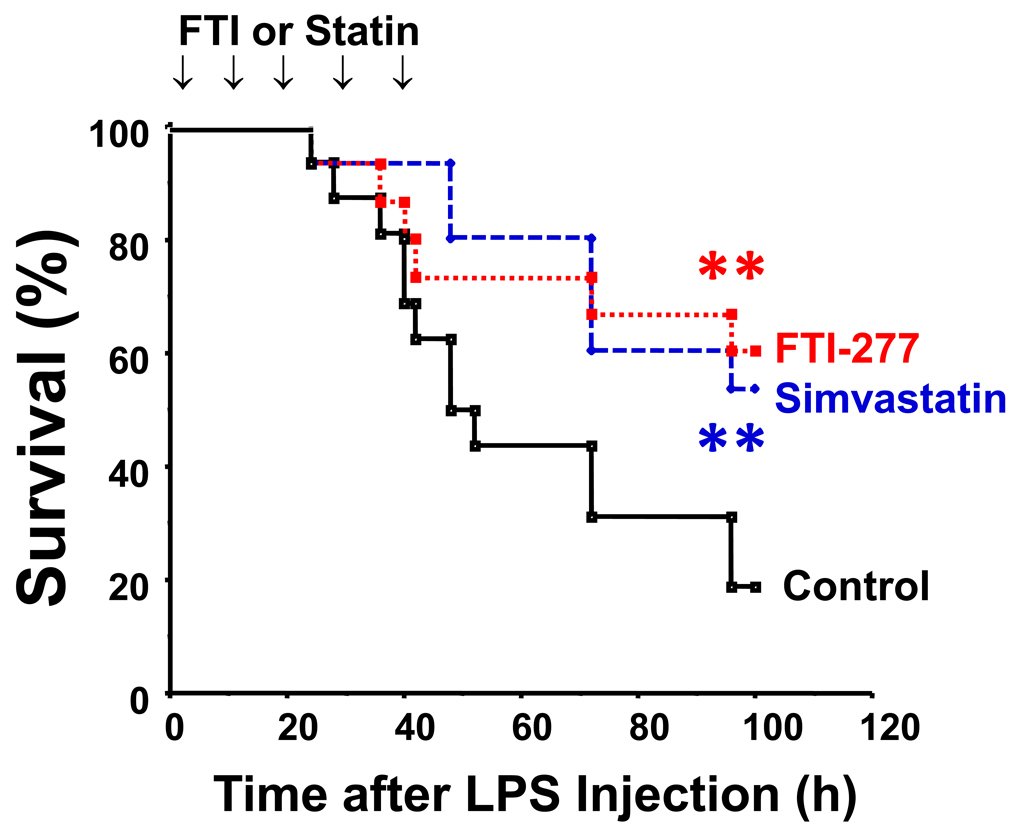
The majority of mice died within 100 h of LPS injection when treated with vehicle alone. Simvastatin and farnesyltransferase inhibitor, FTI-277, significantly prolonged survival following LPS challenge to a similar extent (p<0.01) compared with vehicle (Fig. 1). Both simvastatin and FTI-277 increased the median survival time more than two-fold (vehicle: 48 h; simvastatin: 96 h; FTI: > 96 h). Without LPS injection, however, none of the mice died by treatment with vehicle alone, simvastatin, or farnesyltransferase inhibitor (data not shown).
Fig. 1.
Farnesyltransferase inhibitor and statin improved survival following LPS challenge in mice. At 2 h after LPS injection, treatment with simvastatin (n=15), farnesyltransferase inhibitor, FTI-277 (n=20), or vehicle alone (control, n=16) was initiated, and the mice received injection of inhibitors or vehicle five times up to 50 h after LPS injection. **p<0.01 vs control.
Statin and farnesyltransferase inhibitor ameliorated apoptotic changes in the liver and spleen of LPS-administered mice
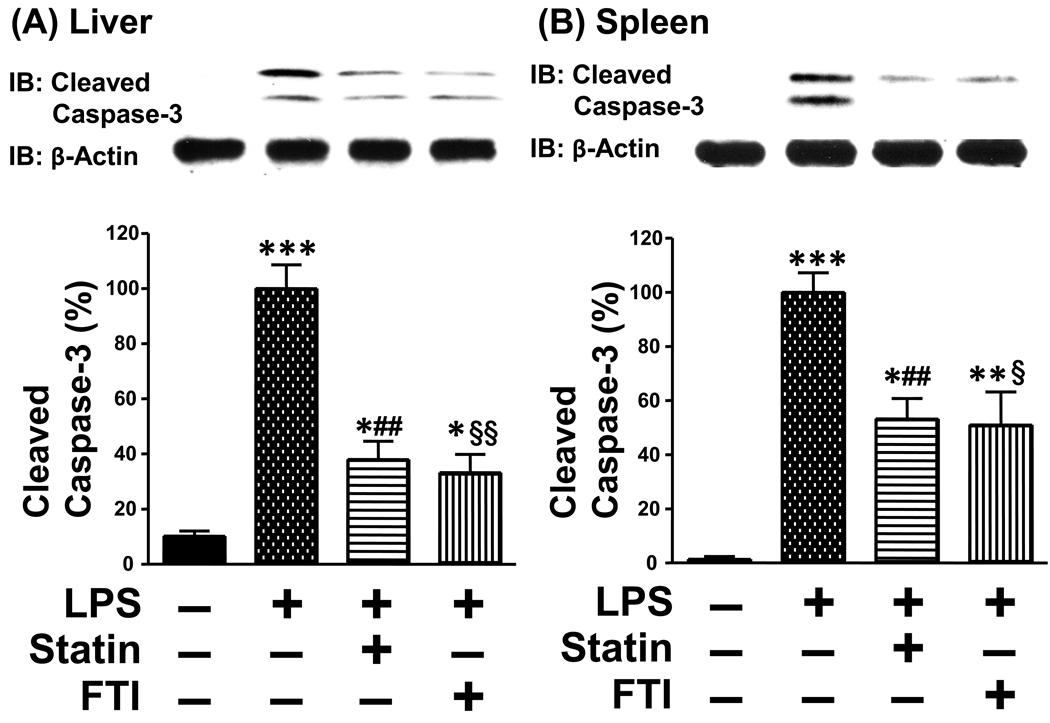
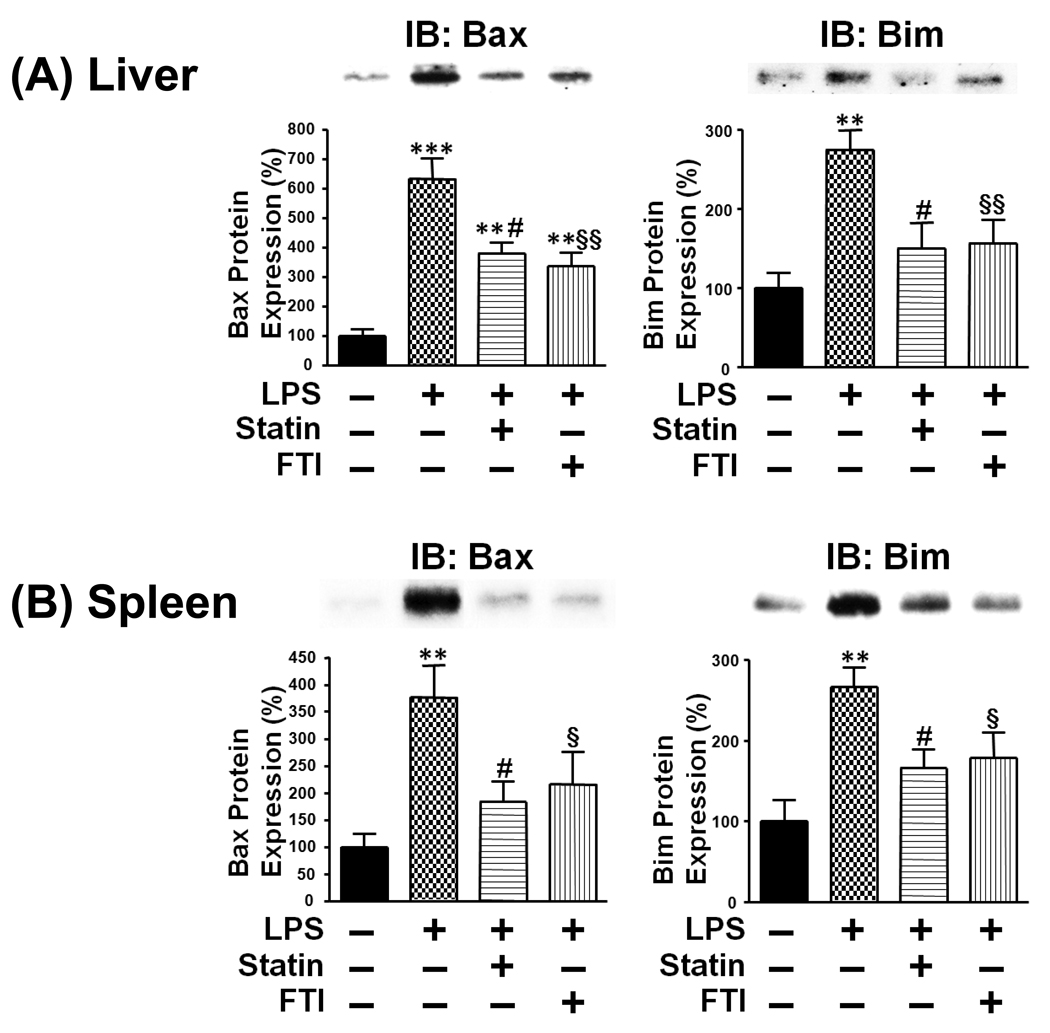
Previous studies indicate that apoptosis plays an important role in organ damage, dysfunction and mortality in rodent models of endotoxemia [32–34]. To characterize the beneficial effects of statin and farnesyltransferase inhibitor, we evaluated apoptotic change in the liver and spleen. LPS injection induced cleavage (activation) of caspase-3 in the liver and spleen, although without endotoxin challenge cleaved caspase-3 was undetectable. Consistent with the improved survival outcome, simvastatin and FTI-277 decreased LPS-induced cleavage of caspase-3 in the liver and spleen relative to vehicle (Fig. 2). However, the protein expression of β-actin was not affected by LPS or the inhibitors. In parallel with increased cleavage of caspase-3 by LPS, the expression of Bax and Bim, proapoptotic molecules, was increased in the liver and spleen of LPS-administered mice as compared with control exogenous endotoxin-naïve mice. Treatment with simvastatin and FTI-277 decreased the expression of Bax and Bim in LPS-administered mice, as compared with vehicle (Fig. 3). The inhibitors, however, failed to affect the protein expression of Bam and Bim in the liver and spleen of LPS-naïve mice (data not shown).
Fig. 2.
Farnesyltransferase inhibitor and statin prevented LPS-induced cleavage of caspase-3 in the liver (A) and spleen (B). At 18 h after LPS injection, the liver and spleen were excised under anesthesia. Immunoblot analysis (IB) revealed that LPS induced cleavage of caspase-3. Both simvastatin and farnesyltransferase inhibitor, FTI-277, decreased cleavage of caspase-3 in LPS-administered mice, as compared with vehicle. β-Actin expression was not altered by LPS or the inhibitors. *p<0.05, **p<0.01, ***p<0.001 vs without LPS, ##p<0.02 vs LPS + vehicle, §p<0.05, §§p<0.01 vs LPS + vehicle.
Fig. 3.
Farnesyltransferase inhibitor and statin attenuated LPS-induced increased expression of Bax and Bim in the liver (A) and spleen (B). At 18 h after LPS injection, the liver and spleen were excised under anesthesia. Immunoblot analysis (IB) revealed that LPS challenge increased the protein expression of Bax and Bim, proapoptotic molecules, in the liver and spleen. Both simvastatin and farnesyltransferase inhibitor, FTI-277, decreased expression of Bax and Bim in LPS-administered mice, as compared with vehicle. **p<0.01, ***p<0.001 vs without LPS, #p<0.05 vs LPS + vehicle, §p<0.05, §§p<0.01 vs LPS + vehicle.
Statin and farnesyltransferase inhibitor attenuated LPS-induced activation of c-Jun NH2-terminal kinase (JNK/SAPK)
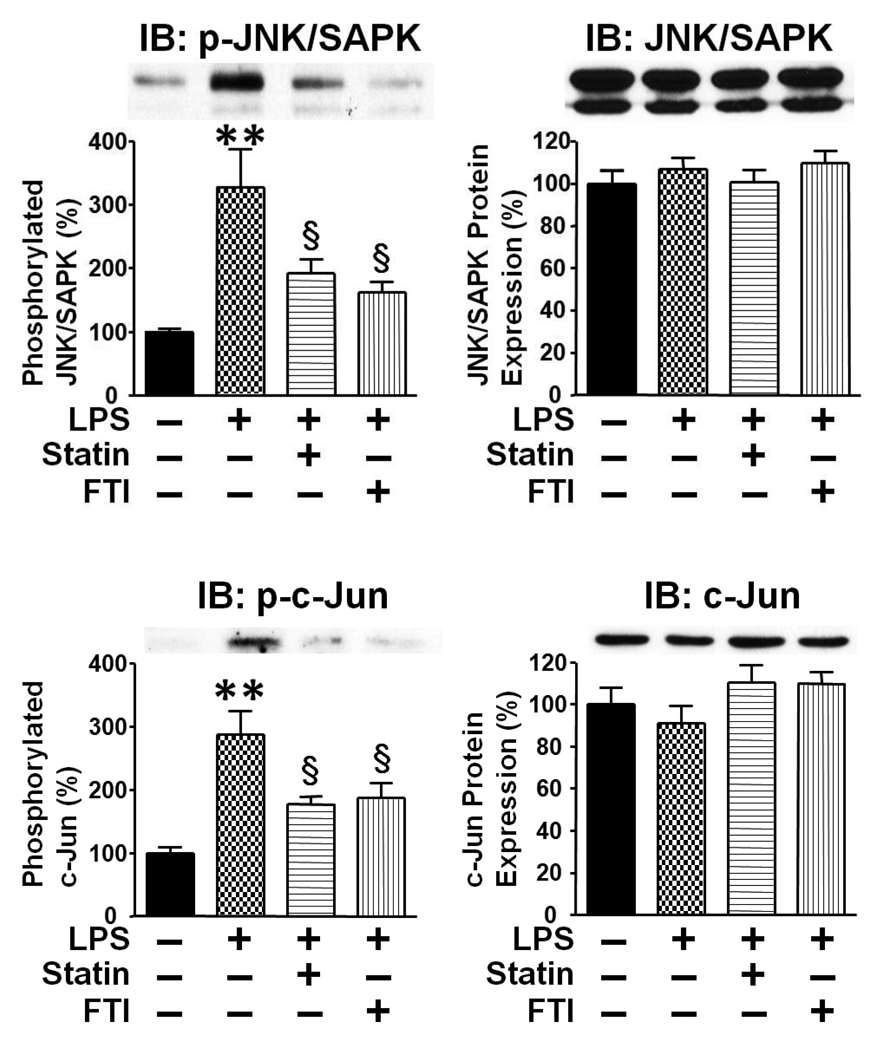
To further characterize the protective effects of the inhibitors, we assessed the phosphorylation (activation) status of c-Jun NH2-terminal kinase (JNK/SAPK, also known as stress-activated protein kinase), which plays a role in LPS-induced apoptosis in the liver and spleen [35, 36]. LPS injection resulted in increased phosphorylation of JNK/SAPK and c-Jun, an endogenous substrate of JNK/SAPK, in the liver and spleen. Both simvastatin and FTI-277 attenuated LPS-induced increased phosphorylation of JNK/SAPK and c-Jun in the liver (Fig. 4) and spleen (Supplementary Fig. S1). The protein expression of JNK/SAPK and c-Jun was not affected by LPS, simvastatin, or FTI- 277.
Fig. 4.
LPS-induced phosphorylation of JNK/SAPK and c-Jun were attenuated by statin and farnesyltransferase inhibitor in the liver. To assess the effects on JNK/SAPK activation, we examined the phosphorylation status of JNK/SAPK and c-Jun at 18 h after LPS injection. Immunoblot analysis (IB) revealed that LPS increased phosphorylation of JNK/SAPK and c-Jun. Both simvastatin and farnesyltransferase inhibitor, FTI-277, decreased phosphorylation of JNK/SAPK and c-Jun in LPS-administered mice, as compared with vehicle. Neither LPS nor the inhibitors significantly altered the protein expression of JNK/SAPK and c-Jun. **p<0.01 vs without LPS, §p<0.05 vs LPS + vehicle.
DISCUSSION
Here, we demonstrate that both simvastatin and farnesyltransferase inhibitor, FTI-277, prolonged survival following LPS challenge in mice (Fig. 1). The improved survival was accompanied by decreases in LPS-induced apoptotic changes in the liver and spleen (Fig. 2, Fig. 3). LPS injection led to cleavage (activation) of caspase-3 and increased protein expression of Bax and Bim, proapoptotic molecules, in the liver and spleen. The LPS-induced apoptotic changes observed in the liver and spleen are concordant with previous studies [34, 37–39]. Both simvastatin and farnesyltransferase inhibitor attenuated LPS-induced cleavage of caspase-3 and increased expression of Bax and Bim (Fig. 2, Fig. 3). The apoptotic changes induced by LPS and the attenuation of these changes by statin and farnesyltransferase inhibitor in LPS-administered mice correlated with LPS-induced activation of JNK/SAPK and decreased JNK/SAPK activation by the inhibitors as judged by the phosphorylation status of JNK/SAPK and its substrate, c-Jun (Figs. 4, S1). These findings are consistent with previous studies, which showed that JNK/SAPK plays an important role in endotoxin-induced apoptosis [35, 36].
Liver integrity and function are crucial to survival in patients with infection. During the course of bacterial infection, the liver clears the blood of endotoxins (e.g., LPS) and also bacteria. It has been proposed that apoptosis and caspase activation play important roles in endotoxin-associated liver damage. A number of previous studies have shown the beneficial effects of various treatments on LPS-induced liver damage, including insulin [37], erythropoietin [38], melatonin [40], and early growth response-1 deficiency [39]. In these studies, the mitigation of liver damage and dysfunction was associated with attenuation of LPS-induced activation of caspase-3. Combined with the previous studies, our data suggest that inhibition of LPS-induced apoptotic changes in the liver might contribute to the protective effects of statin and farnesyltransferase inhibitor in LPS-injected mice.
Endotoxin-mediated immune suppression is thought to contribute significantly to the development of sepsis and multiple organ dysfunction syndrome. Apoptosis of immune cells, including splenocytes and T and B lymphocytes [34, 41], plays a crucial role in immune suppression. The predominant hypothesis is that mortality in sepsis results from an unbridled hyper-inflammatory cytokine-mediated response [42]. However, more than 30 clinical trials have attempted to treat sepsis by controlling this pro-inflammatory cytokine response, including antagonists of TNF-α and IL-1β, and all have failed to improve survival in septic patients, or in some cases have exacerbated the condition [43, 44]. These failures led to reassessment of the presumption that mortality in sepsis is due to a hyper-inflammatory response [44]. Recent studies indicate that most septic deaths result from a substantially impaired immune response that manifests as an inability to eradicate the primary infection and/or the development of new secondary infection [45]. Autopsies of patients with sepsis have revealed extensive apoptosis of lymphocytes, in comparison with autopsies of ICU patients who died of non-septic etiology [46, 47]. Postmortem immunohistochemical analysis of septic spleens has revealed a striking decrease in B and T cells [48]. In aggregate, one can reasonably speculate that the decreased endotoxin-induced apoptotic changes that occur in spleen secondary to statin and farnesyltransferase inhibitor effects (Fig. 2, Fig. 3) may contribute to amelioration of immune suppression and multiple organ dysfunction in endotoxemia. Further studies will be required to clarify this point.
Inhibition of protein farnesylation prevents apoptosis in cultured (non-transformed) cells and in rodents in vivo. Although farnesyltransferase inhibitors promote apoptosis in cancer cells, farnesyltransferase inhibitors are non-toxic to normal (non-transformed) cells [49]. Farnesyltransferase inhibitors reduce apoptosis in excitotoxic insult-induced traumatic brain injury and post-ischemic renal injury by inhibiting the Ras pathway in rodents in vivo [22–24].
Our findings suggest that inhibition of farnesylation may contribute to the lipid lowering-independent protective effects of statins, at least in part, by preventing endotoxin-induced apoptosis of immune and non-immune cells. Statin-induced myopathy leading to rhabdomyolysis, a relevant clinical issue particularly in critically ill patients with renal insufficiency, could dampen the beneficial effects of statins. Statins, but not farnesyltransferase inhibitors, inhibit the biosynthesis of ubiquinone (coenzyme Q), an essential cofactor for mitochondrial electron transport, and protein geranylgeranylation. Statin-induced myopathy is thought to attributable, at least in part, to coenzyme Q deficiency [50] and/or insufficient geranylgeranylation, but not to inhibition of farnesylation [51]. Collectively, our results argue that protein farnesyltransferase may be a novel, potential molecular target that could be useful in treating patients with endotoxemia and preventing the development of severe sepsis. These findings also suggest that protein farnesylation may play an important role in stress response and apoptosis induced by endotoxin.
Supplementary Material
LPS-induced phosphorylation of JNK/SAPK and c-Jun were attenuated by statin and farnesyltransferase inhibitor in the spleen. To assess the effects on JNK/SAPK activation, we examined the phosphorylation status of JNK/SAPK and c-Jun at 18 h after LPS injection. Immunoblot analysis (IB) revealed that LPS increased phosphorylation of JNK/SAPK and c-Jun. Both simvastatin and farnesyltransferase inhibitor, FTI-277, decreased phosphorylation of JNK/SAPK and c-Jun in LPS-administered mice, as compared with vehicle. Neither LPS nor the inhibitors significantly altered the protein expression of JNK/SAPK and c-Jun. **p<0.01, ***p<0.001 vs without LPS, §p<0.05, §§ p<0.01 vs LPS + vehicle.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health, GM21700 (R. Tompkins) and DK58127 (M. Kaneki), and the Shriners Hospitals for Children (M. Kaneki, R. Tompkins, A. Fischman, Y. Ming-Yu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama. 1995;273:117–123. [PubMed] [Google Scholar]
- 2.Gao F, Linhartova L, Johnston AM, Thickett DR. Statins and sepsis. Br J Anaesth. 2008;100:288–298. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 4.Pierre-Paul D, Gahtan V. Noncholesterol-lowering effects of statins. Vasc Endovascular Surg. 2003;37:301–313. doi: 10.1177/153857440303700501. [DOI] [PubMed] [Google Scholar]
- 5.Kwak BR, Mulhaupt F, Mach F. Atherosclerosis: anti-inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2:332–338. doi: 10.1016/s1568-9972(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 7.Omori H, Nagashima H, Tsurumi Y, Takagi A, Ishizuka N, Hagiwara N, Kawana M, Kasanuki H. Direct in vivo evidence of a vascular statin: a single dose of cerivastatin rapidly increases vascular endothelial responsiveness in healthy normocholesterolaemic subjects. Br J Clin Pharmacol. 2002;54:395–399. doi: 10.1046/j.1365-2125.2002.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullen MJ, Wright D, Donald AE, Thorne S, Thomson H, Deanfield JE. Atorvastatin but not L-arginine improves endothelial function in type I diabetes mellitus: a double-blind study. J Am Coll Cardiol. 2000;36:410–416. doi: 10.1016/s0735-1097(00)00743-9. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 10.Kleemann R, Princen HM, Emeis JJ, Jukema JW, Fontijn RD, Horrevoets AJ, Kooistra T, Havekes LM. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108:1368–1374. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]
- 11.Sumi D, Hayashi T, Thakur NK, Jayachandran M, Asai Y, Kano H, Matsui H, Iguchi A. A HMG-CoA reductase inhibitor possesses a potent anti-atherosclerotic effect other than serum lipid lowering effects–the relevance of endothelial nitric oxide synthase and superoxide anion scavenging action. Atherosclerosis. 2001;155:347–357. doi: 10.1016/s0021-9150(00)00597-9. [DOI] [PubMed] [Google Scholar]
- 12.Martin CP, Talbert RL, Burgess DS, Peters JI. Effectiveness of statins in reducing the rate of severe sepsis: a retrospective evaluation. Pharmacotherapy. 2007;27:20–26. doi: 10.1592/phco.27.1.20. [DOI] [PubMed] [Google Scholar]
- 13.van de Garde EM, Hak E, Souverein PC, Hoes AW, van den Bosch JM, Leufkens HG. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax. 2006;61:957–961. doi: 10.1136/thx.2006.062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackam DG, Mamdani M, Li P, Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 16.Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Makris GC, Matthaiou DK, Rafailidis PI. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother. 2008;61:774–785. doi: 10.1093/jac/dkn019. [DOI] [PubMed] [Google Scholar]
- 18.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046. [PubMed] [Google Scholar]
- 19.Chaudhry MZ, Wang JH, Blankson S, Redmond HP. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surg Infect (Larchmt) 2008;9:183–194. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 20.Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 21.Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 22.Sabbatini M, Santillo M, Pisani A, Paterno R, Uccello F, Seru R, Matrone G, Spagnuolo G, Andreucci M, Serio V, Esposito P, Cianciaruso B, Fuiano G, Avvedimento EV. Inhibition of Ras/ERK1/2 signaling protects against postischemic renal injury. Am J Physiol Renal Physiol. 2006;290:F1408–F1415. doi: 10.1152/ajprenal.00304.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ruocco A, Santillo M, Cicale M, Seru R, Cuda G, Anrather J, Iadecola C, Postiglione A, Avvedimento EV, Paterno R. Farnesyl transferase inhibitors induce neuroprotection by inhibiting Ha-Ras signalling pathway. Eur J Neurosci. 2007;26:3261–3266. doi: 10.1111/j.1460-9568.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- 24.Shohami E, Yatsiv I, Alexandrovich A, Haklai R, Elad-Sfadia G, Grossman R, Biegon A, Kloog Y. The Ras inhibitor S-trans, trans-farnesylthiosalicylic acid exerts long-lasting neuroprotection in a mouse closed head injury model. J Cereb Blood Flow Metab. 2003;23:728–738. doi: 10.1097/01.WCB.0000067704.86573.83. [DOI] [PubMed] [Google Scholar]
- 25.Feingold KR, Hardardottir I, Memon R, Krul EJ, Moser AH, Taylor JM, Grunfeld C. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res. 1993;34:2147–2158. [PubMed] [Google Scholar]
- 26.Feingold KR, Pollock AS, Moser AH, Shigenaga JK, Grunfeld C. Discordant regulation of proteins of cholesterol metabolism during the acute phase response. J Lipid Res. 1995;36:1474–1482. [PubMed] [Google Scholar]
- 27.Memon RA, Shechter I, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin, tumor necrosis factor, and interleukin-1 decrease hepatic squalene synthase activity, protein, and mRNA levels in Syrian hamsters. J Lipid Res. 1997;38:1620–1629. [PubMed] [Google Scholar]
- 28.Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR. Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-kappaB in primary astrocytes. J Neurochem. 2000;74:2288–2295. doi: 10.1046/j.1471-4159.2000.0742288.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugita M, Sugita H, Kaneki M. Farnesyltransferase Inhibitor, Manumycin A, Prevents Atherosclerosis Development and Reduces Oxidative Stress in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2007;27:1390–1395. doi: 10.1161/ATVBAHA.107.140673. [DOI] [PubMed] [Google Scholar]
- 30.Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA, Kaneki M. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–14211. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 31.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 32.Liu LM, Zhang JX, Luo J, Guo HX, Deng H, Chen JY, Sun SL. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized rats. Dig Dis Sci. 2008;53:1316–1324. doi: 10.1007/s10620-007-9994-y. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- 34.Lamkanfi M, Moreira LO, Makena P, Spierings DC, Boyd K, Murray PJ, Green DR, Kanneganti TD. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood. 2009;113:2742–2745. doi: 10.1182/blood-2008-09-178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamura M, Matsuda Y, Yamagiwa S, Tamura Y, Honda Y, Suzuki K, Ichida T, Aoyagi Y. An inhibitor of c-Jun NH2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating BH3-only proteins. Life Sci. 2007;80:1335–1344. doi: 10.1016/j.lfs.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Liedtke C, Trautwein C. The role of JNK2 in toxic liver injury. J Hepatol. 2006;45:762–764. doi: 10.1016/j.jhep.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Jeschke MG, Rensing H, Klein D, Schubert T, Mautes AE, Bolder U, Croner RS. Insulin prevents liver damage and preserves liver function in lipopolysaccharide-induced endotoxemic rats. J Hepatol. 2005;42:870–879. doi: 10.1016/j.jhep.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Aoshiba K, Onizawa S, Tsuji T, Nagai A. Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit Care Med. 2009;37:889–898. doi: 10.1097/CCM.0b013e31819b8371. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard MT, Roychowdhury S, McMullen MR, Guo L, Arteel GE, Nagy LE. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1124–G1133. doi: 10.1152/ajpgi.00325.2007. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Xu DX, Lv JW, Ning H, Wei W. Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic liver damage in D-galactosamine-sensitized mice. Toxicology. 2007;237:49–57. doi: 10.1016/j.tox.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Efron PA, Tinsley K, Minnich DJ, Monterroso V, Wagner J, Lainee P, Lorre K, Swanson PE, Hotchkiss R, Moldawer LL. Increased lymphoid tissue apoptosis in baboons with bacteremic shock. Shock. 2004;21:566–571. doi: 10.1097/01.shk.0000126648.58732.8c. [DOI] [PubMed] [Google Scholar]
- 42.O'Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999;12:411–420. doi: 10.1097/00024382-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Fisher CJ, Jr., Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 44.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 46.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 49.Cox AD, Der CJ. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 50.Schaars CF, Stalenhoef AF. Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Curr Opin Lipidol. 2008;19:553–557. doi: 10.1097/MOL.0b013e3283168ecd. [DOI] [PubMed] [Google Scholar]
- 51.Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009;9:2844–2854. doi: 10.1096/fj.08-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LPS-induced phosphorylation of JNK/SAPK and c-Jun were attenuated by statin and farnesyltransferase inhibitor in the spleen. To assess the effects on JNK/SAPK activation, we examined the phosphorylation status of JNK/SAPK and c-Jun at 18 h after LPS injection. Immunoblot analysis (IB) revealed that LPS increased phosphorylation of JNK/SAPK and c-Jun. Both simvastatin and farnesyltransferase inhibitor, FTI-277, decreased phosphorylation of JNK/SAPK and c-Jun in LPS-administered mice, as compared with vehicle. Neither LPS nor the inhibitors significantly altered the protein expression of JNK/SAPK and c-Jun. **p<0.01, ***p<0.001 vs without LPS, §p<0.05, §§ p<0.01 vs LPS + vehicle.