Abstract
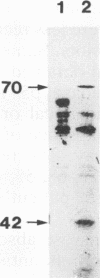
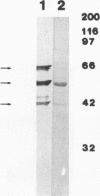
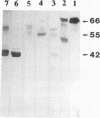
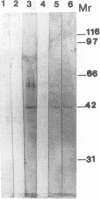
In an effort to identify the protein structure on Candida albicans, pseudohyphal forms which had been shown earlier to bind human iC3b, a protein of about 42 kDa (p42), were obtained from lysates of pseudohyphal forms by absorption with C3(H2O)-Sepharose. An antiserum raised in rabbits against this protein effectively inhibited adherence of sheep erythrocytes carrying iC3b (EAC3bi) to pseudohyphal forms. p42 cross-reacted with OKM-1, a monoclonal antibody directed against the human complement receptor type 3 (CR3, CD11b). This protein, p42, was designated p42-CR3. The antiserum against p42-CR3 was used for further purification of lysates by affinity chromatography. Three proteins of 66, 55, and 42 kDa were isolated. All were recognized by OKM-1 in immunoblots (p66-, p55-, and p42-CR3). The different proteins were separated and treated with neuraminidase and endoglycosidase F. Almost complete deglycosylation of the p66-CR3 protein was obtained after treatment with neuraminidase, indicating a high degree of glycosylation. Neuraminidase also had an effect on p55-CR3, but not on p42-CR3. Endoglycosidase F did not alter any of the three proteins. In ligand blots, p42-CR3 bound C3(H2O), C3b, and iC3b but not C3d; p55-CR3 clearly reacted with C3(H2O) and weakly reacted with C3b and iC3b. p66-CR3 never showed reactivity. It is suggested that p55 and p66 represent glycosylated forms of p42-CR3. Although C. albicans CR3 and human CR3 cross-react and bind identical ligands, the two receptors differ in structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Dierich M. P., Mūller-Eberhard H. J. Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1989–1993. doi: 10.1073/pnas.72.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Eigentler A., Schulz T. F., Larcher C., Breitwieser E. M., Myones B. L., Petzer A. L., Dierich M. P. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989 Feb;57(2):616–622. doi: 10.1128/iai.57.2.616-622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D., Rosen F. S., Lachmann P. J. The mechanism of action of the C3b inactivator (conglutinogen-activating factor) on its naturally occurring substrate, the major fragment of the third component of complement (C3b). J Exp Med. 1975 May 1;141(5):1221–1226. doi: 10.1084/jem.141.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. O., Morrison L., Kilpatrick W. S., Lappin D., Bensa J. C., Riches D. W., Whaley K. Role of C3 in the control of monocyte C2 production. Immunology. 1984 Jan;51(1):169–176. [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollert M. W., Wadsworth E., Calderone R. A. Reduced expression of the functionally active complement receptor for iC3b but not for C3d on an avirulent mutant of Candida albicans. Infect Immun. 1990 Apr;58(4):909–913. doi: 10.1128/iai.58.4.909-913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]