Abstract
Most strains of group B streptococci (GBS) elaborate a cell surface-associated enzyme that rapidly inactivates the human complement-derived chemoattractants C5a and C5adesarg by cleaving the His-Lys bond at positions 67 and 68 in the C5a molecule. We have suggested that rapid inactivation of C5a and C5adesarg by this enzyme, called C5a-ase, can hinder the inflammatory response at sites of GBS infection. We tested the ability of GBS C5a-ase to inactivate C5a preparations from various animal species to determine the proper species for studying the role of GBS C5a-ase in the pathogenesis of GBS infections. Exposure of C5a preparations from humans, monkeys, and cows to GBS caused inhibition of C5a functional activity as measured by the ability of C5a to stimulate human polymorphonuclear leukocyte (PMN) adherence and human PMN chemotaxis. Bovine PMN chemotaxis to bovine C5a was also abolished after exposure of bovine C5a to GBS. In contrast, mouse, rat, guinea pig, rabbit, pig, and sheep C5a preparations retained full functional activity after exposure to GBS as measured by chemotaxis of human PMNs, PMNs from the same animal species, or both. These data suggest that there are structural differences between C5a proteins from different species which alter their susceptibility to GBS C5a-ase and indicate that most commonly used animal models of human GBS infection are inadequate for detection of a contribution of GBS C5a-ase to GBS virulence.
Full text
PDF

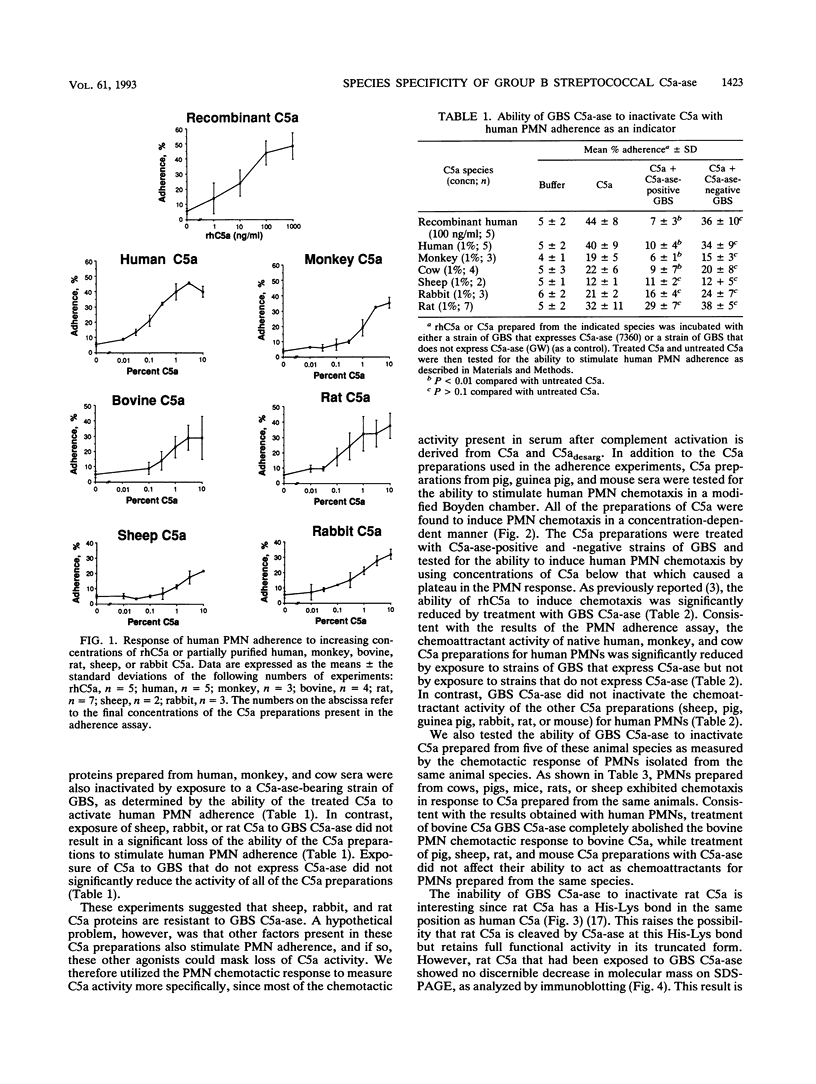
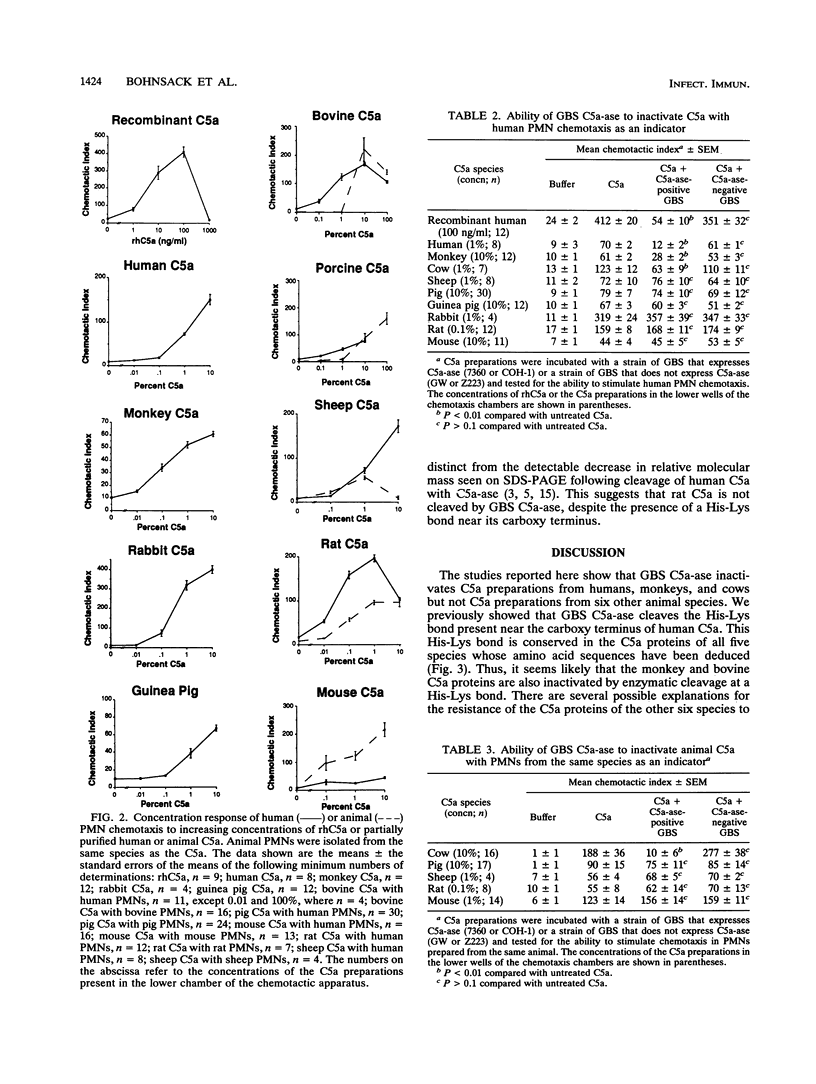
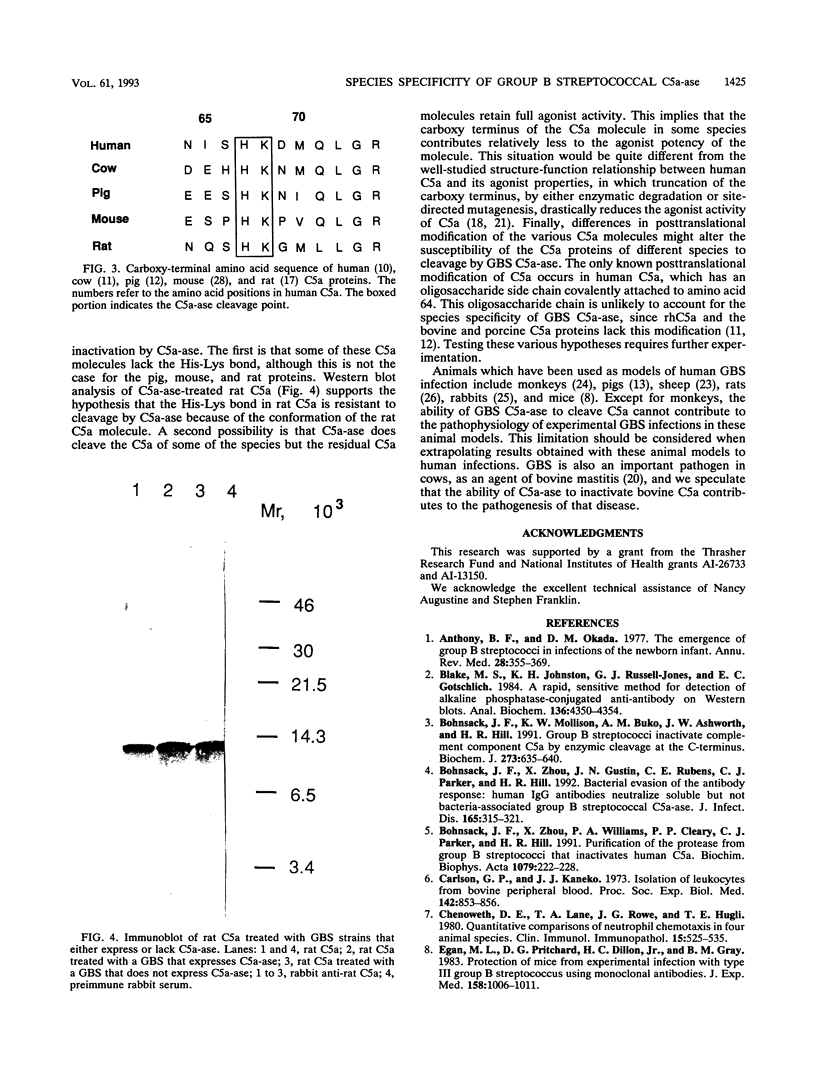

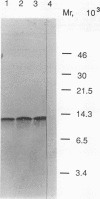
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Okada D. M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Mollison K. W., Buko A. M., Ashworth J. C., Hill H. R. Group B streptococci inactivate complement component C5a by enzymic cleavage at the C-terminus. Biochem J. 1991 Feb 1;273(Pt 3):635–640. doi: 10.1042/bj2730635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., Zhou X. N., Gustin J. N., Rubens C. E., Parker C. J., Hill H. R. Bacterial evasion of the antibody response: human IgG antibodies neutralize soluble but not bacteria-associated group B streptococcal C5a-ase. J Infect Dis. 1992 Feb;165(2):315–321. doi: 10.1093/infdis/165.2.315. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Zhou X. N., Williams P. A., Cleary P. P., Parker C. J., Hill H. R. Purification of the proteinase from group B streptococci that inactivates human C5a. Biochim Biophys Acta. 1991 Aug 30;1079(2):222–228. doi: 10.1016/0167-4838(91)90129-n. [DOI] [PubMed] [Google Scholar]
- Carlson G. P., Kaneko J. J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973 Mar;142(3):853–856. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Lane T. A., Rowe J. G., Hugli T. E. Quantitative comparisons of neutrophil chemotaxis in four animal species. Clin Immunol Immunopathol. 1980 Mar;15(3):525–535. doi: 10.1016/0090-1229(80)90064-1. [DOI] [PubMed] [Google Scholar]
- Egan M. L., Pritchard D. G., Dillon H. C., Jr, Gray B. M. Protection of mice from experimental infection with type III group B Streptococcus using monoclonal antibodies. J Exp Med. 1983 Sep 1;158(3):1006–1011. doi: 10.1084/jem.158.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Gennaro R., Simonic T., Negri A., Mottola C., Secchi C., Ronchi S., Romeo D. C5a fragment of bovine complement. Purification, bioassays, amino-acid sequence and other structural studies. Eur J Biochem. 1986 Feb 17;155(1):77–86. doi: 10.1111/j.1432-1033.1986.tb09460.x. [DOI] [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Anaphylatoxin from the fifth component of porcine complement. Purification and partial chemical characterization. J Biol Chem. 1979 Jul 25;254(14):6346–6351. [PubMed] [Google Scholar]
- Gibson R. L., Redding G. J., Henderson W. R., Truog W. E. Group B streptococcus induces tumor necrosis factor in neonatal piglets. Effect of the tumor necrosis factor inhibitor pentoxifylline on hemodynamics and gas exchange. Am Rev Respir Dis. 1991 Mar;143(3):598–604. doi: 10.1164/ajrccm/143.3.598. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., McCloskey D. W., Hill H. R. Pneumonia in the neonate associated with group B streptococcal septicemia. Am J Dis Child. 1976 Nov;130(11):1231–1233. doi: 10.1001/archpedi.1976.02120120065011. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Bohnsack J. F., Morris E. Z., Augustine N. H., Parker C. J., Cleary P. P., Wu J. T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988 Nov 15;141(10):3551–3556. [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Marques M. B., Kasper D. L., Pangburn M. K., Wessels M. R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun. 1992 Oct;60(10):3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. S. Streptococcal and staphylococcal mastitis. J Am Vet Med Assoc. 1977 May 15;170(10 Pt 2):1157–1159. [PubMed] [Google Scholar]
- Mollison K. W., Mandecki W., Zuiderweg E. R., Fayer L., Fey T. A., Krause R. A., Conway R. G., Miller L., Edalji R. P., Shallcross M. A. Identification of receptor-binding residues in the inflammatory complement protein C5a by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):292–296. doi: 10.1073/pnas.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkowski A. A., Gooneratne S. R., Christensen D. A. Effects of diets of high sulphur content and varied concentrations of copper, molybdenum and thiamine on in vitro phagocytic and candidacidal activity of neutrophils in sheep. Res Vet Sci. 1990 Jan;48(1):82–86. [PubMed] [Google Scholar]
- Rojas J., Green R. S., Hellerqvist C. G., Olegard R., Brigham K. L., Stahlman M. T. Studies on group B beta-hemolytic Streptococcus. II. Effects on pulmonary hemodynamics and vascular permeability in unanesthetized sheep. Pediatr Res. 1981 Jun;15(6):899–904. doi: 10.1203/00006450-198106000-00003. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Raff H. V., Jackson J. C., Chi E. Y., Bielitzki J. T., Hillier S. L. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis. 1991 Aug;164(2):320–330. doi: 10.1093/infdis/164.2.320. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Johnson J. T., Rothlein R., Hughes B. J., Smith C. W., Anderson D. C. Role of pulmonary phagocytes in host defense against group B streptococci in preterm versus term rabbit lung. J Infect Dis. 1992 Oct;166(4):818–826. doi: 10.1093/infdis/166.4.818. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Pincus S. H., Rote N. S., Hill H. R. Protective efficacy of hybridoma type-specific antibody against experimental infection with group-B Streptococcus. J Infect Dis. 1984 Mar;149(3):363–372. doi: 10.1093/infdis/149.3.363. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel R. A., Ogata R. T., Tack B. F. Primary structure of the fifth component of murine complement. Biochemistry. 1987 Feb 10;26(3):737–743. doi: 10.1021/bi00377a013. [DOI] [PubMed] [Google Scholar]