Abstract
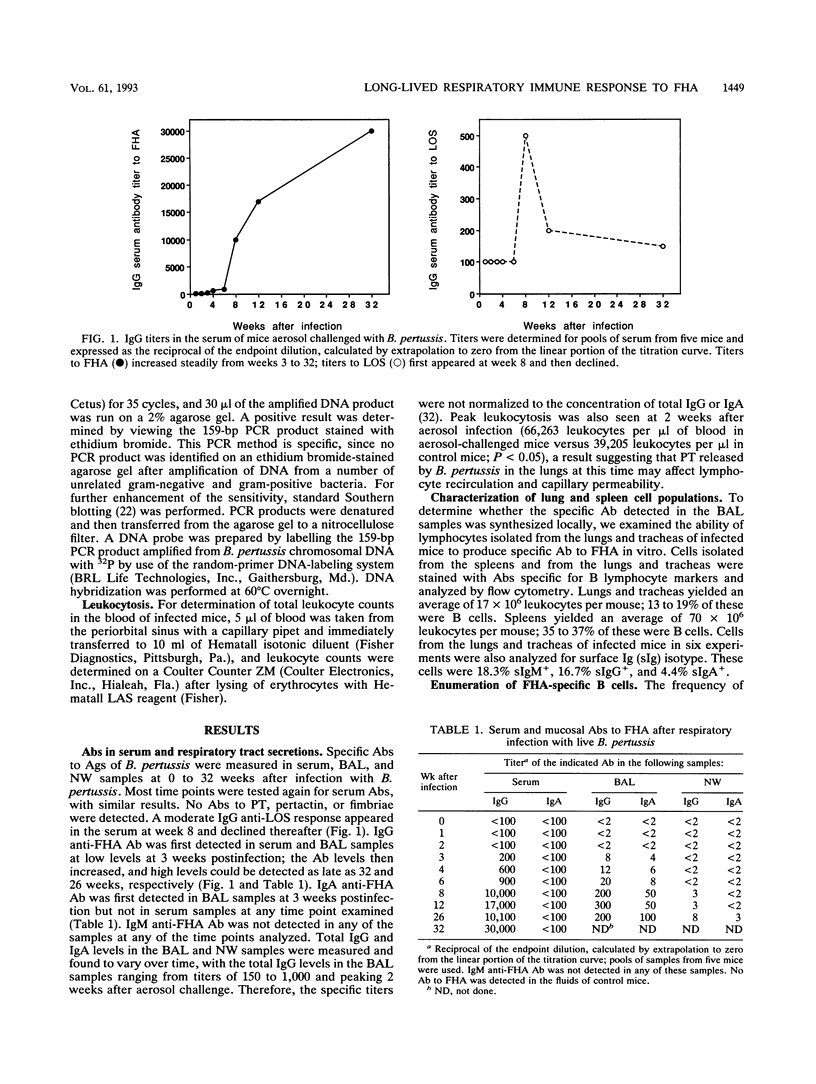
Systemic and mucosal B-cell-mediated immune responses to purified filamentous hemagglutinin (FHA) in mice were analyzed at different times following a single respiratory infection with Bordetella pertussis. Serum immunoglobulin G (IgG) anti-FHA and respiratory IgG and IgA anti-FHA antibodies were first detected at 3 weeks postinfection, reached high levels by 8 weeks postinfection, and remained at high levels 12 to 32 weeks postinfection. FHA-specific B lymphocytes isolated from the spleens or lungs of uninfected control mice or mice convalescing from B. pertussis respiratory infection were analyzed in limiting-dilution cultures. Analysis of culture supernatants for the production of antibodies to FHA revealed an increased frequency of FHA-specific B cells of both the IgG- and the IgA-secreting classes in the lungs and tracheas of aerosol-challenged mice; these levels remained high as late as 25 weeks postinfection, compared with those in uninfected controls. No corresponding increase in the frequency of FHA-specific B cells in the spleens of aerosol-infected mice was observed. This long-lasting response observed in cultured cells was radiation resistant, a result suggesting that this response was due to B cells already activated in vivo. Polymerase chain reaction analysis revealed low but detectable levels of B. pertussis chromosomal DNA in 75% of mice tested at 8 weeks postinfection and 37.5% of mice tested at 26 weeks postinfection, at which times high levels of anti-FHA antibody were detected. One explanation for these data may be that, in this animal model, a major adhesin of B. pertussis can persist and interact with components of the immune system to stimulate the production of specific antibody in the respiratory tract many weeks after a single B. pertussis infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman R. K., Munoz J. Action of the histamine sensitizing factor from Bordetella pertussis on inbred and random bred strains of mice. Int Arch Allergy Appl Immunol. 1968;34(4):331–338. doi: 10.1159/000230127. [DOI] [PubMed] [Google Scholar]
- Bromberg K., Tannis G., Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991 Dec;59(12):4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Gray D. F. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969 Dec;17(6):875–887. [PMC free article] [PubMed] [Google Scholar]
- Delisse-Gathoye A. M., Locht C., Jacob F., Raaschou-Nielsen M., Heron I., Ruelle J. L., de Wilde M., Cabezon T. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect Immun. 1990 Sep;58(9):2895–2905. doi: 10.1128/iai.58.9.2895-2905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farizo K. M., Cochi S. L., Zell E. R., Brink E. W., Wassilak S. G., Patriarca P. A. Epidemiological features of pertussis in the United States, 1980-1989. Clin Infect Dis. 1992 Mar;14(3):708–719. doi: 10.1093/clinids/14.3.708. [DOI] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The sequence of enhanced cellular activity and protective humoral factors in murine pertussis immunity. Immunology. 1969 Dec;17(6):889–896. [PMC free article] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT H. J. EPIDEMIOLOGY OF A SMALL PERTUSSIS OUTBREAK IN KENT COUNTY, MICHIGAN. Public Health Rep. 1965 Apr;80:365–369. [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Hannah J. H., Stibitz S., Nguyen N. Y., Manclark C. R., Brennan M. J. Cloning and sequencing of the structural gene for the porin protein of Bordetella pertussis. Mol Microbiol. 1991 Jul;5(7):1649–1656. doi: 10.1111/j.1365-2958.1991.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Long S. S., Welkon C. J., Clark J. L. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990 Mar;161(3):480–486. doi: 10.1093/infdis/161.3.480. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Gillett N. A., Bice D. E. Comparison of systemic and local immune responses after multiple pulmonary antigen exposures. Reg Immunol. 1989 May-Jun;2(3):149–157. [PubMed] [Google Scholar]
- Mertsola J., Ruuskanen O., Eerola E., Viljanen M. K. Intrafamilial spread of pertussis. J Pediatr. 1983 Sep;103(3):359–363. doi: 10.1016/s0022-3476(83)80403-x. [DOI] [PubMed] [Google Scholar]
- Quintáns J., Lefkovits I. Precursor cells specific to sheep red cells in nude mice. Estimation of frequency in the microculture system. Eur J Immunol. 1973 Jul;3(7):392–397. doi: 10.1002/eji.1830030704. [DOI] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Robinson A., Ashworth L. A., Baskerville A., Irons L. I. Protection against intranasal infection of mice with Bordetella pertussis. Dev Biol Stand. 1985;61:165–172. [PubMed] [Google Scholar]
- Rose F. V., Cebra J. J. Isotype commitment of B cells and dissemination of the primed state after mucosal stimulation with Mycoplasma pulmonis. Infect Immun. 1985 Aug;49(2):428–434. doi: 10.1128/iai.49.2.428-434.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader C. E., George A., Kerlin R. L., Cebra J. J. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Int Immunol. 1990;2(6):563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Amsbaugh D. F., Leef M. F. Mucosal immunization with filamentous hemagglutinin protects against Bordetella pertussis respiratory infection. Infect Immun. 1992 Apr;60(4):1482–1488. doi: 10.1128/iai.60.4.1482-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Steed L. L., Setareh M., Friedman R. L. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukoc Biol. 1991 Oct;50(4):321–330. doi: 10.1002/jlb.50.4.321. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Kosco M. H., Tew J. G. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J Immunol. 1988 Jan 15;140(2):341–353. [PubMed] [Google Scholar]
- Weissman D. N., Bice D. E., Siegel D. W., Schuyler M. R. Murine lung immunity to a soluble antigen. Am J Respir Cell Mol Biol. 1990 Apr;2(4):327–333. doi: 10.1165/ajrcmb/2.4.327. [DOI] [PubMed] [Google Scholar]


