Abstract
Here we address the role of RIG-I and TLR3 in differential cytokine responses against Simian Virus 5 (SV5) and two distinct cytokine inducing SV5 mutants. IFN-beta and IL-6 secretion was induced by infection with P/V-CPI-, an SV5 mutant with P/V substitutions, and were reduced by either siRNA-mediated knockdown of RIG-I expression or by expression of a dsRNA-binding protein. TLR3 overexpression did not alter cytokine secretion induced by P/V-CPI- or by Le-(U5C, A14G), an SV5 promoter mutant. TLR3 signaling by addition of exogenously-added dsRNA was not blocked by WT SV5 or either SV5 mutant. Unexpectedly, TLR3 activation in infected cells led to enhanced IL-8 secretion which correlated with increased RIG-I expression. Dominant negative RIG-I and TRIF supported a model whereby TLR3 activation upregulates RIG-I expression and in turn hypersensitizes cells to RIG-I mediated cytokine secretion. Implications for crosstalk between different innate immunity pathways in mounting antiviral responses to paramyxoviruses are discussed.
INTRODUCTION
The innate immune response to viral infection is a critical factor in viral pathogenesis, adaptive immunity and viral tropism (Biron and Sen, 2007). Initial host cell defenses in response to viral infection include production of type I interferon (IFN) and secretion of proinflammatory cytokines such as interleukin-6 (IL-6) and IL-8 (Goodbourn et al, 2000). Cytokine secretion in response to virus infection can be triggered by intracellular signaling through two general types of pattern recognition receptors (PRRs): cytoplasmic RNA helicases or surface/endosomal Toll-like receptors (TLRs). These PRRs are able to recognize and respond to pathogen-associated molecular patterns (PAMPs) that are generated by viral infection, including dsRNA or viral RNA containing a 5′ triphosphate end. The present study addresses the role of the double stranded RNA (dsRNA)-sensing PRRs TLR3 and RIG-I in activation of cytokine secretion following infection by the paramyxovirus Simian virus 5 (SV5).
Retinoic-acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are cytosolic RNA helicases that both possess two N-terminal caspase recruitment domains (CARDs) followed by a DExD/H-box RNA helicase domain (Yoneyama et al., 2005). Upon recognition of dsRNA or RNA with an uncapped 5′-triphosphate, these PRRs initiate signaling pathways that can lead to the activation of transcription factors NFκB and Interferon Regulatory Factor (IRF)-3, which drive the expression of IFN-beta and proinflammatory cytokines (Kawai and Akira, 2008; Meylan and Tschopp, 2006). MDA-5 appears to be important for sensing infections by positive strand RNA viruses such as encephalomyocarditis virus (Kawai and Akira, 2008). RIG-I has been shown to play a role in antiviral responses to a range of negative strand RNA viruses, including Sendai virus, influenza A virus, and vesicular stomatitis virus (Kato et al., 2006; Le Goffic et al., 2007), as well as some positive strand viruses such as the flaviviruses (Chang et al., 2005).
Anti-viral responses can also be activated through TLR signaling (reviewed in Boehme and Compton, 2004; Meylan and Tschopp, 2006). The TLR family contains common structural features including an ectodomain harboring leucine-rich repeats (LRRs) and a cytoplasmic signaling domain termed the Toll/IL-1 receptor (TIR) homology domain (O’Neill and Dinarello, 2000). In addition to the cytosolic RNA helicases, TLR3 is also a key dsRNA-sensing PRR during RNA virus infection. Upon binding to dsRNA, TLR3 activates TIR-domain-containing adaptor inducing IFN-beta (TRIF)-dependent signaling pathways, leading to the upregulation of antiviral cytokines through downstream signaling pathways that in some cases overlap with those of the cytosolic PRRs (Kawai and Akira, 2008; Meylan and Tschopp, 2006). TLR3 has been shown to be an important PRR for responses to a range of RNA virus infections including West Nile virus, Influenza A virus, and the paramyxovirus respiratory syncytial virus (RSV) (Wang et al., 2004, Guillot et al., 2005, Rudd et al., 2005; Wilson et al., 2008).
The parainfluenza virus Simian Virus 5 (SV5), also known as parainfluenza virus 5 (PIV5), is a poor inducer of host cell responses, and infections of human epithelial cells result in only very low level synthesis of proinflammatory cytokines and IFN-beta (Choppin, 1964, He et al, 2002; Wansley and Parks, 2002). Members of the paramyxovirus family of negative strand RNA viruses employ a diverse range of mechanisms to circumvent host cell cytokine responses (reviewed in Conzelmann, 2005; Horvath, 2004; Goodbourn et al., 2000). Many of these mechanisms for counteracting IFN have been attributed to products of the P/V (or sometimes P/V/C) gene which encodes both the phosphoprotein P subunit of the RNA-dependent RNA polymerase and the accessory V protein (Didcock et al., 1999; Garcin et al., 1999; Lamb and Parks, 2007). A major function of the SV5 V protein is the inhibition of IFN signaling, through the targeting of STAT1 (signal transducer and activator of transcription 1) for degradation (Didcock et al., 1999; Horvath, 2004, Ulane et al., 2005). The SV5 V protein can also block activation of the IFN-beta promoter through V protein targeting the IFN-inducible RNA helicase mda-5 (Childs et al., 2007; 2009; Poole et al., 2002). Thus, the multifunctional V protein counteracts IFN responses at two steps, resulting in both limited induction of IFN synthesis and a block in IFN signaling.
The naturally-occurring CPI- strain of SV5 is defective in inducing STAT1 degradation and blocking type I IFN signaling (Chatziandreou et al., 2002). Mutational analyses have identified amino acid differences between WT SV5 and CPI- in the shared P/V region that are responsible for this loss of V protein function (Chatziandreou et al., 2002). We have previously engineered a recombinant SV5 mutant (P/V-CPI-) to encode these same six CPI- P/V substitutions in the background of the WT SV5 genome (Wansley and Parks, 2002). These P/V gene substitutions converted WT SV5 into a mutant that failed to target STAT1 for degradation as expected, but the chimeric virus was also found to overexpress viral mRNA and viral genomes and to replicate to higher-than-WT levels in tissue culture (Dillon and Parks, 2007; Wansley and Parks, 2002). Most importantly, the P/V-CPI- virus differed from WT SV5 by being a potent inducer of both IFN-beta and proinflammatory cytokines (Wansley and Parks, 2002).
We have recently reported the properties of second SV5 mutant Le-(U5C, A14G), that is isogenic to WT SV5 except for two nucleotide substitutions in the Leader, a non-coding region of the genome which acts as a promoter directing synthesis of viral mRNA and antigenomc RNA (Manuse and Parks, 2009). Despite encoding a functional V protein, this Leader mutant behaves very similarly to the P/V-CPI- mutant. Cells infected with the Le-(U5C, A14G) mutant are induced to express high levels of IFN-beta and proinflammatory cytokines and the mutant overexpresses viral mRNA (Manuse and Parks, 2009). We have previously demonstrated that the Leader mutant triggers cytokine production in a RIG-I dependent manner (Manuse and Parks, 2009).
The P/V-CPI- and Le-(U5C, A14G) viruses contain distinct mutations in separate gene products, but share the property of being potent inducers of cytokines. This raised the hypothesis that these two distinct mutants activated host cell responses through a common PRR. Here, we have examined the roles of RIG-I in activation of cytokines by P/V-CPI-, and in addition tested the involvement of TLR3 in activation of cytokines by both P/V-CPI- and Le-(U5C, A14G). Similar to the Le-(U5C, A14G) mutant (Manuse and Parks, 2009), the P/V-CPI- mutant activated cytokine synthesis through pathways that were dependent on RIG-I and dsRNA but not on expression of TLR3. Unexpectedly, stimulation of TLR3 by exogenous dsRNA resulted in elevated levels of RIG-I, and this led to increased expression of cytokines in cells infected with the SV5 mutants. We present a working model whereby activation of TLR3 signaling hypersensitizes cells to infection with the SV5 mutants by amplifying RIG-I mediated induction of cytokine synthesis.
RESULTS
RIG-I and dsRNA contribute to cytokine induction by P/V-CPI-
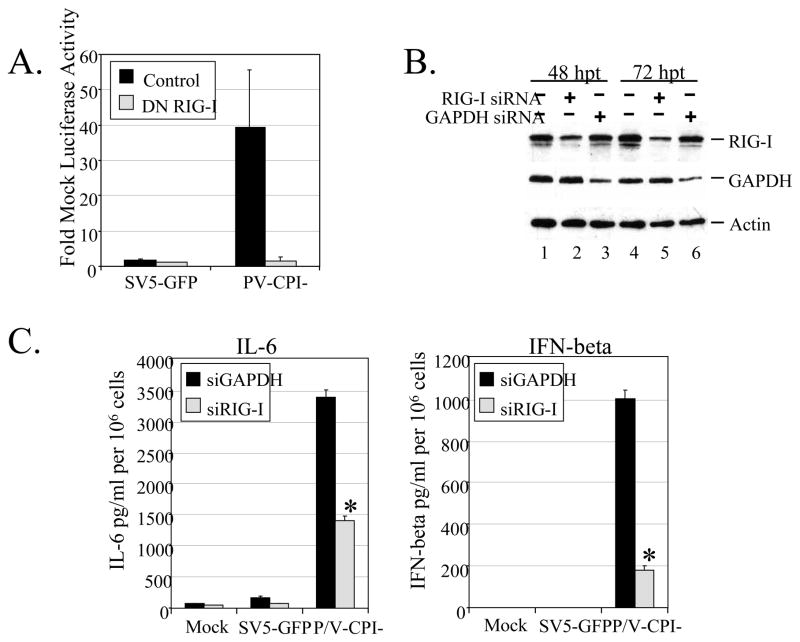
We hypothesized that cytokine secretion induced by P/V-CPI- would be dependent on RIG-I. To test this, we used a reporter gene assay to determine the effect of a dominant negative (DN) form of RIG-I on P/V-CPI-mediated activation of the IFN-beta promoter. A549 cells were co-transfected with a plasmid encoding luciferase under control of the IFN-beta promoter along with control plasmid or a plasmid expressing DN RIG-I. Sixteen h after transfection, cells were infected at a high moi with WT SV5-GFP or P/V-CPI-. At twenty four h pi, lysates were analyzed for luciferase activity. As shown in Fig. 1A, the IFN-beta promoter was potently activated by infection with P/V-CPI- but not WT SV5-GFP. In the presence of DN RIG-I, promoter activity was significantly reduced.
Figure 1. RIG-I contributes to cytokine induction by P/V-CPI-.
A) Reporter Gene Assay. A549 cells were co-transfected for 16 h with a plasmid expressing luciferase under control of the IFN-beta promoter and a either a RIG-I DN plasmid or its empty vector (control), and pSV-beta-galactosidase for normalization. Sixteen hrs pt, cells were infected at an moi of 10 with the indicated viruses. At 24 h pi lysates were harvested and analyzed for levels of luciferase activity. Results are expressed as fold over mock and error bars indicate standard deviation of triplicate samples. B) siRNA knockdown of RIG-I. A549 cells were left untreated (lanes 1 and 4) or transfected with 100 uM of siRNA targeting RIG-I (lanes 2 and 5) or GAPDH (lanes 3 and 6) as a control. At 48 and 72 h pt, levels of RIG-I, GAPDH, and actin were assayed by Western blotting. C) Cytokine secretion. A549 cells were transfected with siRNA targeting GAPDH or RIG-I and at 48 h pt cells were infected at an moi of 10 with SV5-GFP or P/V-CPI-. At 24 h pi (72 h pt), levels of IL-6 (left panel) or IFN-beta (right panel) were measured by ELISA. Results are expressed as mean values from triplicate samples with error bars representing standard deviation and values are normalized to 106 cells. *, p value less than 0.008 compared to corresponding samples from GAPDH siRNA treated control cells.
As an alternative approach, the role of RIG-I in activation of cytokines by P/V-CPI- was tested by reducing endogenous levels of protein by siRNA knockdown. As shown in Fig. 1B, levels of RIG-I and GAPDH control, were reduced by 48 h post transfection (h pt) with gene-specific siRNAs. Quatitation from multiple experiments showed an average reduction in RIG-I levels of 71.3% +/− 4.9, consistent with previous reports in A549 cells (Manuse and Parks, 2009). At 48 h pt, A549 cells were infected at high moi with WT SV5-GFP or with P/V-CPI-. At 24 h pi (72 h pt) media were analyzed by ELISA for presence of cytokines. As shown in Fig. 1C, WT SV5-GFP was a poor inducer of cytokines, and RIG-I knockdown had little effect. By contrast, control siRNA-treated cells that were infected with the P/V-CPI- mutant showed high levels of secreted IL-6 and IFN-beta. Infected cells in which RIG-I levels were decreased showed significant reduction in cytokine secretion. Together, these results support the hypothesis that RIG-I is involved in activation of cytokine secretion by P/V-CPI-.
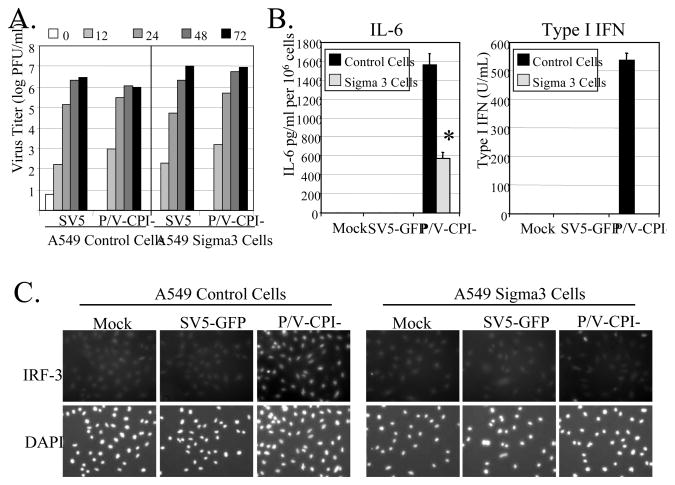
RIG-I is reported to respond to dsRNA produced during virus infection (Kato et al., 2008; Schlee et al. 2009). To determine the role of dsRNA in cytokine induction by P/V-CPI-, we used a previously described A549 cell line that stably expresses the reovirus sigma3 protein (Manuse and Parks, 2009), which has been shown to specifically bind dsRNA (Yue and Shatkin, 1997). We have previously shown that the efficiency of infection of sigma3-expressing cells does not differ significantly from that seen with control cells or between WT SV5 and the P/V mutant (Gainey et al, 2008; Manuse and Parks, 2009). As shown in Fig 2A, both WT SV5-GFP and the P/V mutant grew to ~1 log higher in sigma3-expressing cells compared to control cells, consistent with our previous results (Manuse and Parks, 2009). Control A549 cells or sigma3-expressing cells were infected at high moi with SV5-GFP or P/V-CPI- and levels of secreted type I IFN and IL-6 were determined by a VSV-based bioassay or by ELISA, respectively. As shown in Fig. 2B, A549-sigma3 cells infected with P/V-CPI- released significantly lower levels of both IL-6 and IFN compared to infection of control A549 cells.
Figure 2. Role of dsRNA in cytokine induction by P/V-CPI-.
A) Virus growth. Control or Sigma3-expressing A549 cells were infected at an moi of 0.05 with WT SV5-GFP or P/V-CPI- and virus titers were determined at the indicated times post infection. Results are representative of two independent experiments. B) Cytokine induction. Control or sigma3-expressing A549 cells were infected at an moi of 10 with SV5-GFP or P/V-CPI-. At 24 h pi, media was analyzed for levels of IL-6 by ELISA (left panel) or Type I IFN by bioassay (right panel). Results are expressed as mean values from triplicate samples with error bars representing standard deviation. For IL-6, values are normalized to 106 cells. *, p value less than 0.004 compared to corresponding control cells. C) IRF3 nuclear translocation. Control or sigma3 A549 cells were infected at an moi of 10 with the indicated viruses. At 24 h pi, cells were stained with DAPI and with antibodies specific for IRF-3.
Translocation of IRF-3 to the nucleus is required to initiate synthesis of the IFN-beta gene (Hiscott et al., 1999). To determine the extent to which IRF-3 activation was changed by expression of a dsRNA-binding protein, A549 control cells or A549-sigma3 cells were mock-infected or infected at high moi with SV5-GFP or P/V-CPI-. At 24 h pi, cells were permeabilized and stained with an IRF-3 antibody. In all cases, mock infected cells and cells infected with WT SV5-GFP showed a diffuse cytoplasmic staining of IRF-3, as reported previously (Dillon and Parks, 2007; Manuse and Parks, 2009). Infection of A549 control cells with the P/V mutant resulted in a bright nuclear staining for IRF-3, consistent with activation and translocation to the nucleus of infected cells (Fig 2C). By contrast, A549-sigma3 cells showed levels of IRF-3 staining that were similar to that of mock infected or WT SV5-GFP infected cells. Loss of nuclear IRF-3 staining in P/V mutant infected cells was not due to lower virus gene expression, since slightly higher levels of viral M mRNA were found in infected cells expressing sigma3 compared to control cells (data not shown). Although the reovirus sigma3 protein may exert other unrecognized mechanisms to alter innate immune responses, the well established dsRNA-binding property (Yue and Shatkin, 1997) supports the hypothesis that dsRNA produced during P/V-CPI- replication activates RIG-I and IRF-3 pathways that contribute to cytokine induction.
TLR3 does not mediate cytokine induction by SV5 mutants
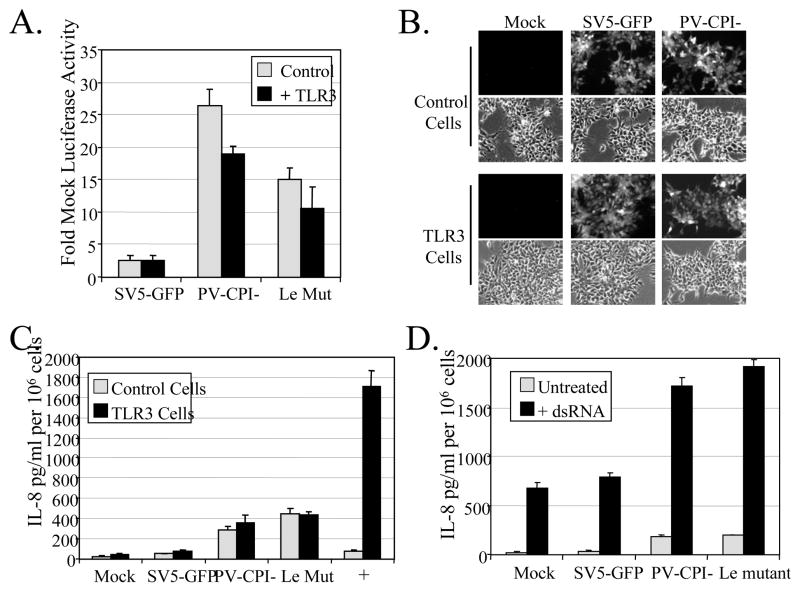
To test the role of TLR3 in SV5-mediated cytokine secretion, A549 cells were transfected with a reporter plasmid encoding luciferase under control of the IFN-beta promoter along with a plasmid expressing TLR3. Cells were then infected at an moi of 10 with WT SV5-GFP or the P/V mutant. As shown in Fig. 3A, the P/V mutant induced significant activation of the IFN-beta promoter, but importantly there was a very similar promoter activation in control cells and cells overexpressing TLR3.
Figure 3. WT and mutant SV5 do not activate TLR3 pathways, but they are also not able to block dsRNA-mediated TLR3 signaling.
A) Overexpression of TLR3. A549 cells were co-transfected with a plasmid expressing TLR3 or its empty vector (as a control) along with a plasmid encoding luciferase under the control of the IFN-beta promoter. At 16 h pt, cells were infected with indicated viruses at an moi of 10. At 24 h pi, lysates were harvested and luciferase activity was determined. B) Infection of 293 cells. 293 cells stably expressing TLR3 or LacZ (as a control) were mock infected or infected at an moi of 10 with WT SV5-GFP, or the mutants P/V-CPI-. At 24 h pi, cells were examined for GFP expression. C) 293 cells stably expressing TLR3 or LacZ (as a control) were mock infected or infected at an moi of 10 with WT SV5-GFP, or the mutants P/V-CPI- or Le-(U5C, A14G). As a positive control, cells were treated with exogenous dsRNA (PolyI:C; 5 ug/ml). At 24 h pi, media were analyzed by ELISA for levels of IL-8. D) 293-TLR3 were mock infected or infected at an moi of 10 with the indicated viruses and 6 h later were left untreated or challenged with exogenous dsRNA (PolyI:C; 5 ug/ml). At 24 h pi media was harvested and analyzed by ELISA for levels of IL-8. For all panels, results are expressed as mean values from triplicate samples with error bars representing standard deviation. IL-8 values are normalized to 106 cells. Le Mut; Le-(U5C, A14G).
As an alternative approach, we utilized 293 cells that inherently do not express TLRs or a 293 cell line that stably expresses TLR3. Cells expressing TLR3 or LacZ as a control were mock-infected or infected at a high moi with WT SV5-GFP or P/V-CPI-. The two cell lines showed similar levels of infection by these two viruses (Fig. 3B). Likewise, high moi titers were 10e7 PFU/ml for all infections, and did not differ by more than 0.5 log between each virus/cell combination (data not shown). At 24 h pi, media was analyzed by ELISA for presence of IL-8. As expected, treatment of the 293-TLR3 cells with exogenous dsRNA resulted in high levels of IL-8 secretion, but this was not seen with 293-LacZ control cells (Fig. 3C). WT SV5-GFP was a poor inducer of IL-8 in both cell lines. In the case of cells infected with the P/V-CPI- mutant, levels of IL-8 secretion were higher than that seen with WT SV5. Importantly, this elevated synthesis in P/V-CPI- infected cells did not differ between cells expressing TLR3 and the control LacZ. Together these data indicate that the P/V-CPI- mutant does not induce cytokine synthesis through TLR3-dependent pathways.
We have previously described a recombinant SV5 mutant Le-(U5C, A14G) that contains two nucleotide substitutions in the non-coding Leader promoter. Despite containing a functional V protein, this mutant is similar to P/V-CPI-, because it induces IL-6 and IFN-beta secretion through RIG-I signaling (Manuse and Parks, 2009). As shown in Fig. 3A, A549 cells infected with Le-(U5C, A14G) had IFN-beta promoter activation above that seen with WT SV5-GFP. However, in transfected A549 cells (Fig 3A) or in 293-TLR3 cells (Fig. 3C), overexpression of TLR3 did not significantly change the secretion of IL-8 or activation of the IFN-beta promoter. Therefore, the P/V-CPI- and Le-(U5C, A14G) mutants induce cytokine synthesis, but in both cases this is not affected by levels of TLR3.
The above findings raised the question of whether SV5 is capable of blocking activation of TLR3. To address this, 293-TLR3 cells were mock infected or infected at a high moi with WT SV5-GFP, or with the mutants P/V-CPI- or Le-(U5C, A14G). Cells were then challenged at 6 h pi with exogenous dsRNA and levels of secreted IL-8 were determined by ELISA at 24 h pi. As expected, mock infected cells showed a sharp increase in IL-8 secretion following treatment with exogenous dsRNA (black bars, Fig. 3D), and a very similar response was seen with WT SV5-GFP. Surprisingly however, dsRNA-treatment of cells infected with the P/V or leader mutant resulted in a much higher level of IL-8 secretion than that seen with challenged mock or WT SV5 infected cells. Together, these data are consistent with the proposal that WT SV5 cannot block TLR3-dependent responses in human epithelial cells.
dsRNA-mediated TLR3 signaling upregulates RIG-I synthesis, independent of IFN signaling
The above findings indicate that cytokine secretion from cells infected with the SV5 mutants: 1) is dependent on RIG-I, 2) is not altered by TLR3 overexpression and 3) is greatly enhanced when exogenous dsRNA is added to TLR3-expressing cells. These findings raise the hypothesis that TLR3 stimulation by dsRNA enhanced cytokine secretion from infected cells by increasing RIG-I expression.
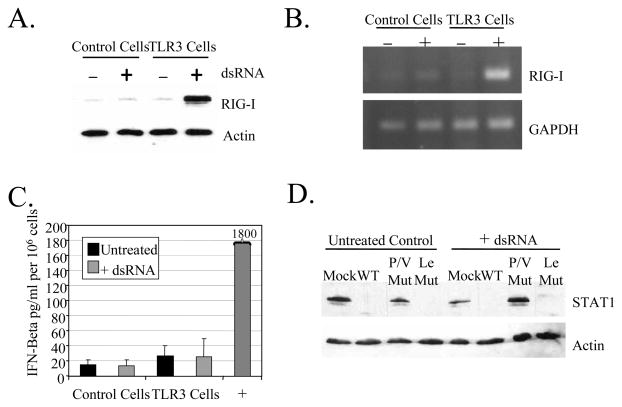
Consistent with this hypothesis, activation of TLR3 by exogenous dsRNA resulted in increased levels of RIG-I mRNA and protein. This is evident in Fig. 4, where levels of RIG-I protein (panel A) were enhanced ~40 fold, and mRNA (panel B) was similarly elevated after exogenous dsRNA treatment of 293-TLR3 cells. This was not seen in the case of control 293-LacZ cells that do not express TLR3. While the increase in RIG-I protein appeared to be greater than the increase in RIG-I RNA, our experiments do not distinguish between transcriptional and translational control of RIG-I expression.
Figure 4. TLR3 signaling increases RIG-I expression, but is not dependent on IFN signaling.
A and B) dsRNA increases RIG-I expression. 293 cells expressing TLR3 or LacZ (control) were treated without (−) or with (+) exogenous dsRNA (PolyI:C; 5 ug/ml). After 18 h, lysates were harvested and analyzed by Western blotting for levels of RIG-I and actin (panel A) or by RT-PCR for levels of RIG-I and GAPDH RNA. C) IFN-beta secretion. 293 cells expressing TLR3 or LacZ were treated without (−) or with (+) 5 ug/ml PolyI:C dsRNA and after 18 h media was collected and analyzed by ELISA for IFN-beta. Media from A549 cells infected with P/V-CPI- was used as a positive control. Data are from triplicate samples and error bars represent standard deviation. D) STAT1 levels. 293-TLR3 cells were mock infected or infected at an moi of 10 with the indicated viruses. At 6 h pi, cells were left untreated or treated with (+) dsRNA. Six hr later, cell lysates were analyzed by western blotting for STAT1 and actin.
Two experimental results support the contention that TLR3-mediated upregulation of RIG-I in 293 cells was independent of IFN signaling. First, 293-TLR3 and control 293-LacZ cells express very low levels of IFN-beta, and this was not further increased by treatment with exogenous dsRNA (Fig. 4C). Secondly, STAT1 levels were reduced in cells infected with WT SV5 and Leader mutant (Fig. 4D), consistent with these viruses encoding a functional V protein. STAT1 was not degraded by infection with the P/V mutant, which encodes a defective V protein (Wansley and Parks, 2002). Nevertheless, despite differences in STAT1 degradation between the P/V and Leader mutants (Fig. 4D), RIG-I synthesis was induced in response to dsRNA during virus infection (Fig. 5, below). These data indicate that TLR3 signaling to upregulate RIG-I expression is not dependent on IFN-beta signaling.
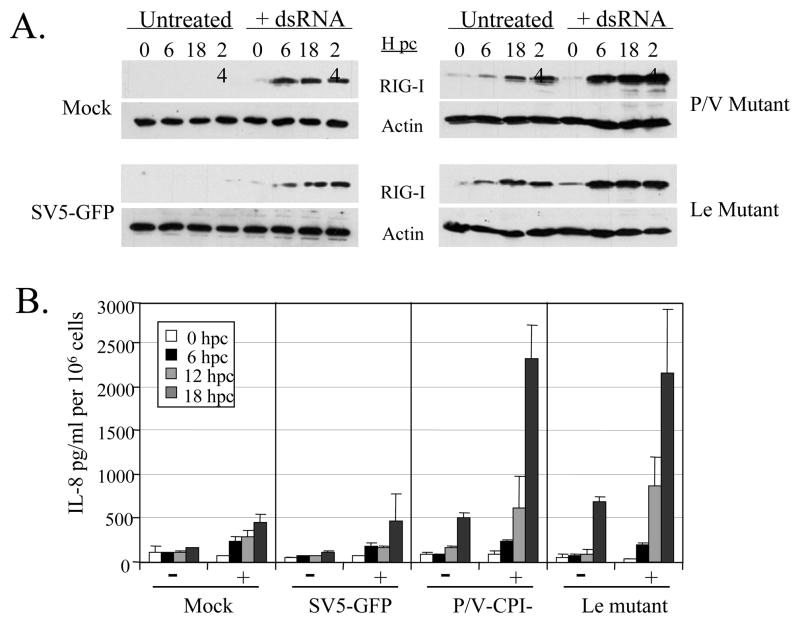
Figure 5. Kinetics of RIG-I induction and cytokine secretion following infection and treatment with dsRNA.
293-TLR3 cells were mock infected or infected at an moi of 10 with the indicated viruses. At 6 h pi, cells were left untreated or treated with (+) 5 ug/ml PolyI:C dsRNA. At the indicated times post challenge with dsRNA, cell lysates were analyzed for levels of RIG-I and actin (panel A) or media was analyzed for levels of IL-8 by ELISA (panel B). For panel B, data are from triplicate samples and error bars represent standard deviation from the mean.
To determine the relationship between RIG-I induction and cytokine synthesis, levels of RIG-I protein and IL-8 secretion were assayed at various times after challenge of infected cells with exogenous dsRNA (hours post-challenge, hpc). As shown in Fig. 5, 293-TLR3 cells that were mock infected or infected at high moi with WT SV5-GFP showed very low or undetectable levels of RIG-I, and these levels were greatly enhanced by exposure to dsRNA (panel A, left). This RIG-I expression profile correlated with a low level of IL-8 secretion in untreated cells, and a slightly enhanced IL-8 secretion by dsRNA treatment (panel B, mock and SV5-GFP panels). By contrast, unchallenged cells that were infected with the P/V or Leader mutants showed time-dependent increases in RIG-I, and these levels were further enhanced by dsRNA treatment (Fig. 5A, right panels). A small increase in secreted IL-8 in infected but untreated cells correlated with an induction in RIG-I levels by mutant virus infection alone. Likewise, much higher levels of IL-8 correlated with a further increase RIG-I after dsRNA treatment (Fig. 5B, right). Together, these data show that the level of RIG-I expression correlates with the amount of IL-8 synthesis induced by mutant virus infection.
dsRNA-mediated enhancement of IL-8 synthesis from SV5 mutant infected cells is dependent on both TRIF and RIG-I
The above data are consistent with a model whereby dsRNA signaling through TLR3 induces RIG-I expression, and this in turn enhances IL-8 synthesis in response to virus infection. A prediction of this model is that IL-8 promoter activity in dsRNA-treated cells infected with SV5 mutants will be reduced by dominant negative (DN) versions of both RIG-I and TRIF, the required adaptor molecule of the TLR3 signaling pathway.
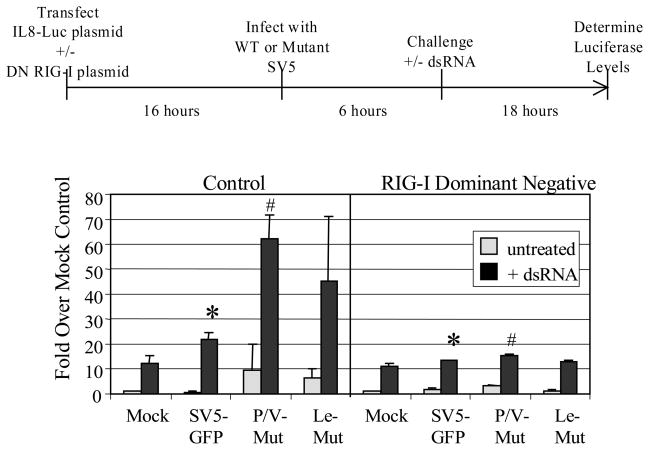
Fig. 6 shows a schematic diagram of the experimental procedure to test this model. 293-TLR3 cells were co-transfected with a plasmid encoding luciferase under control of the IL-8 promoter (Casola et al., 2000) and either a DN-RIG-I or a control plasmid. Sixteen h later, cells were mock infected or infected at high moi with WT SV5-GFP or the P/V or Leader mutants. Six h pi, cells were treated with exogenous dsRNA and luciferase activity was measured 18 h pt. As shown in the graph in Fig. 6, cells that were not treated with dsRNA showed relatively low levels of luciferase from the IL-8 promoter (gray bars, Fig. 6), with cells infected with the P/V or Leader mutants having ~5–10 fold increases in luciferase levels compared to cells infected with WT SV5-GFP. In control samples (left panel), treatment with dsRNA induced IL-8 promoter activity that was higher for cells infected with the P/V and Leader mutants (~45–60 fold increased) than with mock or WT SV5 infected cells (~10–20 fold increases). This was consistent with the IL-8 secretion data shown in Fig. 3D above. Most importantly, in the presence of the DN RIG-I plasmid (right panel), IL-8 promoter activation after dsRNA treatment of infected cells was significantly reduced to levels (~10 fold increases over untreated) similar to that seen with mock infected cells. These data support a role for RIG-I in increased activation of the IL-8 promoter upon dsRNA challenge of mutant virus infected cells.
Figure 6. dsRNA-induced enhancement of IL-8 synthesis in SV5 infected cells is dependent on RIG-I.
293-TLR3 cells were co-transfected with plasmids encoding luciferase under control of the IL-8 promoter, a DN form of RIG-I or the empty vector (control), and pSV-beta-galactosidase for normalization. Sixteen hrs pt, cells were infected with the indicated viruses at an moi of 10. Six hours later, cells were left untreated or treated with dsRNA (PolyI:C; 5 ug/ml). Lysates were harvested at 24 h pi and analyzed for luciferase activity. Results are expressed as fold over that seen with mock infected cells not treated with dsRNA. Data are from triplicate samples and error bars represent standard deviation. *; p<0.05 comparing SV5-GFP infected control cells with infected cells expressing DN RIG-I; #, p<0.02 comparing P/V mutant infected control cells with infected cells expressing DN RIG-I. P/V Mut, P/V-CPI-; Le Mut, Le-(U5C, A14G).
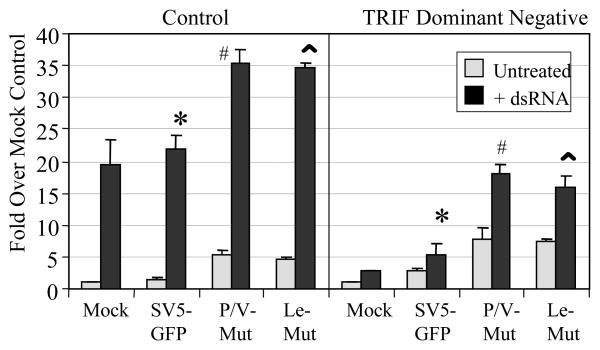
To determine if TLR3 signaling was also required for the dsRNA-mediated enhancement of IL-8 induction, the same experimental procedure was carried out, but in this case a plasmid encoding a DN version of TRIF was cotransfected with IL-8 reporter plasmid. As shown in Fig. 7, cotransfection of the DN TRIF plasmid significantly reduced the level of IL-8 promoter activity that resulted from addition of dsRNA to mock infected or virus infected cells. Taken together, these data are consistent with a model whereby dsRNA signaling through TLR3 results in upregulation of RIG-I, which functions to amplify cytokine responses in cells infected with SV5 mutants.
Figure 7. dsRNA-induced enhancement of IL-8 synthesis in SV5 infected cells is dependent on TRIF.
293-TLR3 cells were co-transfected with plasmids encoding luciferase under the control of the IL-8 promoter, a dominant negative form of TRIF or the empty vector (control), and pSV-beta-galactosidase for normalization. Sixteen hrs pt, cells were infected at an moi of 10 with the indicated viruses. Six hours later, cells were left untreated or treated with dsRNA (PolyI:C; 5 ug/ml). Lysates were harvested at 24 h pi and analyzed for luciferase activity. Results are expressed as fold over that seen with mock infected cells not treated with dsRNA. Data are from triplicate samples and error bars represent standard deviation. *; p<0.02 comparing SV5-GFP infected control cells with infected cells expressing DN TRIF; #, p<0.001 comparing P/V mutant infected control cells with infected cells expressing DN TRIF; ^, p<0.005 comparing Leader mutant infected control cells with infected cells expressing DN TRIF. P/V Mut, P/V-CPI-; Le Mut, Le-(U5C, A14G).
DISCUSSION
Viral dsRNA can activate antiviral responses through a number of cellular PRRs, including PKR (Gale and Katze, 1998), the RIG-I like helicases (Kato et al. 2006), and TLRs (Kawai and Akira, 2008; Meylan and Tschopp, 2006). In the cased of PKR, our previous work has shown that this PRR is activated at late times pi with the P/V-CPI- mutant but not WT SV5, resulting in eIF2-alpha phosphorylation and a global shut down of host and viral protein synthesis in P/V-CPI- infected cells (Gainey et al., 2008). For the RIG-I like helicases, MDA5 is targeted for inhibition by the paramyxovirus V protein through actions associated with the conserved C-terminal domain (Andrejeva et al, 2004). Consistent with this, the CPI- V protein, harboring changes only in the N-terminal domain, has been shown to be an effective inhibitor of MDA-5 (Andrejeva et al, 2004; Childs et al., 2007). Therefore, it is unlikely that this PRR is involved in responses to either WT or mutant SV5. Here, we have addressed the role of the dsRNA-sensing PRRs RIG-I and TLR3 in antiviral cytokine responses elicited by either WT SV5 or two distinct cytokine-inducing SV5 mutants. Our results show that despite harboring mutations in two separate viral components, these two SV5 mutants both signal through RIG-I, but not through TLR3. The most striking results were our data that support a model whereby activation of TLR3 by exogenously added dsRNA leads to hypersensitization of infected cells to RIG-I mediated induction of cytokines.
Infection with the P/V-CPI- mutant results in cytokine induction through pathways that involve the production of dsRNA and RIG-I signaling. This is consistent with previous results using RSV (Liu et al. 2007) and with natural and engineered mutants of Sendai virus (Melchjorsen et al., 2005; Strahe et al, 2006; 2007). Both the P/V-CPI- and the Le-(U5C, A14G) mutants activated cytokines through RIG-I (Fig. 1; Manuse and Parks, 2009), but infection with WT SV5 did not induce cytokines to any significant levels. Why is RIG-I activated by two different SV5 mutants that harbor alterations to two distinct gene products (P/V and Leader), but not by WT SV5? One possibility is that WT SV5 actively blocks some phase of RIG-I signaling, such as masking the inducing signal, inhibiting RIG-I directly, or by inhibiting downstream signaling of the RIG-I pathways. It has been reported that V protein can act as a decoy substrate for IKKalpha-mediated phosphorylation of IRF-3 and -7 in dendritic cells (Pfaller and Conzelmann, 2008) and IKKepsilon in epithelial cells (Lu et al., 2008). Thus, WT SV5 may inhibit signaling downstream of RIG-I, whereas the V protein encoded by P/V-CPI- might be defective in this function. This scenario, however, does not offer an explanation for the induction of cytokines by the Leader mutant, which encodes a WT V protein (Manuse and Parks, 2009).
Alternatively, differential RIG-I activation by WT SV5 and the mutants P/V-CPI- and Le-(U5C, A14G) might be due to the production of different levels or forms of viral RNA. This more likely explanation is consistent with our published data which shows that correcting overexpression of viral genes during P/V-CPI- mutant infection also leads to a reduction in antiviral responses (Dillon and Parks, 2008; Gainey et al., 2009). The finding that RIG-I responds to 5′-triphosphate RNA containing double-stranded structure (Schlee et al. 2009) suggests that origin of the activating RNA from the P/V and Leader mutants may be either unencapsidated genomic RNA or uncapped viral mRNA that has escaped the capping events carried out by the viral polymerase. It is well accepted in the field that the V protein blocks activation of MDA-5 but not RIG-I, and these findings raise the question of why one PRR is active in infected cells while others are inhibited. It is noteworthy that both the P/V-CPI- and the Le-(U5C, A14G) mutants overexpress viral mRNA, but genomic RNA is only overexpressed by P/V mutant (Dillon and Parks, 2008; Manuse and Parks, 2009). These findings are more consistent with these two distinct mutants activating RIG-I through a common defect that results in uncapped viral RNA.
Our results with overexpression of TLR3 indicate that this PRR is not a main contributing factor in cytokine responses to infection of epithelial cells with either P/V-CPI- or the Le-(U5C, A14G) mutant. This is consistent with previous work using transfected cells (Andrejeva et al., 2004) where it is reported that V protein cannot block TLR3 signaling (reviewed in Goodbourn and Randall, 2009), and with Sendai virus infection of myeloid dendritic cells where antiviral responses occurred in the absence of TLR-3, -7, -8, or -9 (Lopez et al., 2004). In RSV-infected A549 cells, RIG-I is a main sensing PRR at early times pi, and TLR3 plays a role only at late times pi (Liu et al., 2007). Importantly, TLR3 signaling in response to exogenous dsRNA was not inhibited by SV5. This finding raises the possibility that the lack of TLR3 signaling during the replication of the SV5 mutants was due to the generation of low levels of dsRNA that were below a threshold for TLR3 sensing, or that the location of TLR3 in intracellular vesicles or at the plasma membrane did not allow access to the cytoplasmic-localized dsRNA (Schroder and Bowie, 2005).
Our most striking finding was the strong enhancement of cytokine synthesis following dsRNA-treatment of TLR3-expressing cells that are infected with either of the SV5 mutants. Because cytokine synthesis induced by the SV5 mutants was dependent on RIG-I but not TLR3, we hypothesized that TLR3 stimulation led to upregulated expression of RIG-I. Indeed, the exogenous addition of dsRNA caused a robust increase in the level of RIG-I. An important distinction is that upregulation of RIG-I did not occur through cytoplasmic RNA helicase signaling because no increase in RIG-I expression was observed in 293 cells that did not express TLR3. While RIG-I has been shown to be an IFN-inducible gene (Yoneyama et al., 2004, Imaizumi et al., 2004), our data support the proposal that dsRNA-mediated upregulation of RIG-I in 293 cells is not fully dependent on IFN-beta signaling. It has been reported that IRF-1 can be an important transcription factor for activation of the RIG-I promoter (Su et al., 2007), and that TLR3 signaling can lead to the upregulation of IRF-1 (Goto et al., 2008). Our analysis using DN versions of RIG-I and TRIF link these two pathways. Taken together, our model suggests that dsRNA-mediated TLR3 signaling in infected cells leads to activation of IRF-1, which acts on the promoter of RIG-I to increase its expression, and this in turn sensitizes cells to RIG-I mediated activation of the IL-8 promoter.
While our data indicate that SV5 and the two cytokine-inducing mutants tested here do not directly activate TLR3, there may be consequences for TLR3 signaling from extracellular sources during in vivo infections. Various cell types can differ in their expression of both RIG-I-like helicases and TLRs (Kawai and Akira, 2008). Consistent with our model, dsRNA that is released from infected cells could stimulate TLR3-mediated antiviral responses in neighboring cells such that RIG-I levels are greatly enhanced. WT SV5 is largely noncytopathic (Choppin, 1964) and the release of dsRNA from infected cells would be predicted to be minimal. By contrast, the cytopathic SV5 mutants examined here would be predicted to release large amounts of dsRNA from lysed cells, and this could lead to inhibition of both virus replication and spread to neighboring cells through a TLR3-mediated enhancement of RIG-I signaling. TLR3-dependent upregulation of RIG-I could also have consequences in other paramyxovirus infections, such as RSV and Sendai virus, that signal through multiple PRRs. Secondly, TLR3 signaling can promote cross-priming of antigen from infected cells, and it has been proposed that this could promote immune responses in the case of viruses that do not directly infect dendritic cells or that are potent blockers of IFN production (Schulz et al., 2005). Work is in progress to test these hypotheses in animal models (Capraro et al., 2009).
In summary, our data show that two SV5 mutants with changes to two distinct gene products activate host cell responses through a common PRR, while these pathways are not activated by WT SV5 infection. Our new results showing TLR3-mediated sensitizing of RIG-I pathways indicate that cross talk between PRR pathways may be an important factor to consider during coinfections that result in activation of multiple TLR and non-TLR signaling.
MATERIALS AND METHODS
Cells and viruses
Monolayer cultures of A549 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS). A549 cells that constitutively express reovirus type 3 Dearing sigma3 protein were generated by transfection with pCXN-S4T3D (Kobayashi et al, 2007) followed by selection in DMEM containing 0.5 mg/ml G418. Colonies were picked, expanded in media containing G418, and screened by Western blotting with polyclonal rabbit serum against sigma3. The sigma3 plasmid and antiserum were kindly provided by T. Kobayashi and T. Dermody (Vanderbilt University School of Medicine). HEK-293 cells that stably express TLR3 or LacZ (Invivogen, Inc; San Diego, CA) were maintained in DMEM containing 10μg/ml Blastacidin.
WT SV5 expressing green fluorescent protein (SV5-GFP) was recovered as described previously (Wansley and Parks, 2002) from a cDNA plasmid (He et al., 1997) kindly provided by Robert Lamb (Northwestern University) and Biao He (Penn State University). SV5-GFP stocks were grown in MDBK cells. P/V-CPI- and Le-(U5C, A14G) were generated as described previously (Manuse and Parks, 2009; Wansley and Parks, 2002). Virus stock was generated in Vero cells by low moi infection as described previously (Gainey et al, 2008) in order to prevent generation of defective particles.
Western blotting
For Western blotting, 6-well dishes of cells were infected as described in the figure legends. At each time point, cells were washed with PBS and lysed in 1% SDS. Protein concentration was determined by BCA assay (Pierce Chemicals) and equivalent amounts of protein were analyzed by Western blotting with rabbit antiserum against the SV5 P protein (Dillon and Parks, 2007), RIG-I or GAPDH. As loading control, lysates were probed with anti-β-actin antibodies (Sigma A5316). Blots were visualized by horseradish peroxidase-conjugated antibodies and enhanced chemiluminescence (Pierce Chemicals). Band density was quantitated using ImageJ software.
RNA experiments
For knockdown of RIG-I, A549 cells in 24-well plates were transfected using TransIT siQuest (Mirus, inc.) with siRNA specific for RIG-I (Dharmacon M-0125-01; 100 nM final concentration) or non-target control RNA (Dharmacon D-001206-13; 100 nM final concentration) according to manufacturer’s instructions and as previously described (Gainey et al, 2008). Forty eight hrs post transfection, cells were infected as described above. For induction of TLR3, cells were treated with 5 ug/nl of PolyI:C (Invivogen, Inc; San Diego, CA). In the text, this is referred to as exogenously added dsRNA to emphasize that the dsRNA was added in the absence of a transfection reagent.
For measuring RNA levels, total RNA was purified by Trizol extraction and used in RT-PCR reactions with the Titan one-tube RT-PCR kit along with primers specific for RIG-I or GAPDH (sequences from Invivogen, Inc). PCR reactions were only semi-quantative. PCR parameters are available upon request.
Reporter gene assays and IFN bioassays
Cells in 6-well dishes were transfected with plasmids encoding luciferase under control of the IFN-β promoter and pSV-betagal (normalization control) at a concentration of 1μg/mL using Lipofectamine 2000 (Invitrogen). For IL-8 experiments, control LacZ- or TLR3-expressing 293 cells in 24-well dishes were transfected using Fugene (Roche) with plasmids (0.3 ug/well) encoding luciferase under control of the IL-8 promoter along with plasmids expressing dominant negative forms of RIG-I or TRIF (Invivogen, Inc) and with pSV-beta-gal to normalize transfections. After overnight incubation, cells were infected and cell lysates were harvested in reporter lysis buffer (Promega) at 24 h pi. Luciferase activity was determined using a TD-20/20 luminometer (Turner designs) and normalized to beta-galactosidase levels. Statistical significance was determined using Student’s t test.
For IFN bioassays, media from infected control cells or sigma3 A549 cells or a titration of Universal Type I IFN (PBL Biomedical Laboratories) was applied to freshly plated A549 cells in a 96-well dish for 24 hours. Cells were then infected with vesicular stomatitis virus (VSV, kindly provided by Doug Lyles) and 24 hours later the sensitivity of the cells to killing by VSV was determined by cell viability assay (see below).
Enzyme-linked immunosorbent assays and cell viability assays
Immunoreactive IL-6 (BD Opt EIA; BD Biosciences) or IFN-beta (PBL Biomedical Laboratories) extracellular media was quantified by a dual antibody sandwich ELISA according to manufacturer’s protocols. To allow comparison between experiments, cytokine levels were determined for the number of cells at the time of infection and values were normalized to 106 cells. Cell viability was measured by using a CellTiter 96 AQueaous One Solution cell proliferation assay (Promega) according to the manufacturer’s instructions.
Microscopy
Phase and fluorescent microscopy were carried out as described previously (Young et al., 2006). Analysis of IRF-3 nuclear translocation was performed as detailed elsewhere (Dillon and Parks, 2007). Images were captured using Qimaging digital camera and processed using Q-capture software. Exposure times were manually set to be constant between samples.
Acknowledgments
We thank members of the Parks lab for helpful comments on the manuscript. We thank Drs. A. Casola and A. Brasier (University of Texas Medical Branch, Galveston) for the kind gift of the IL-8 luciferase plasmid. This work was supported by NIH grants AI-42023. M.J.M. was supported by NIH Training Award Grant T32-AI007401.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrejeva J, Childs KS, Young DF, Carlos RS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Sen GC. Innate responses to viral infections. In: Fields B, Knipe D, Howley P, editors. Fields Virology. 5. Lippincott Williams and Wilkins Publishers; Philadelphia, Pa: 2007. pp. 249–278. [Google Scholar]
- Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capraro GA, Johnson JJ, Kock ND, Parks GD. Growth and antibody responses to respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5. Virology. 2008;376:416–428. doi: 10.1016/j.virol.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. Requirement of a novel upstream response element in respiratory syncytial virus-induced IL-8 gene expression. J. 2000;164:5944. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- Chang TH, Liao CL, Lin YL. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect. 2005;8(1):157–71. doi: 10.1016/j.micinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chatziandreou N, Young D, Andrejeva J, Goodbourn S, Randall RE. Differences in interferon sensitivity and biological properties of two related isolates of simian virus 5: a model for virus persistence. Virology. 2002;293:234–242. doi: 10.1006/viro.2001.1302. [DOI] [PubMed] [Google Scholar]
- Childs KS, Andrejeva J, Randall RE, Goodbourn S. Mechanism of mda-5 inhibition by paramyxovirus V proteins. J Virol. 2009;83:1465–1473. doi: 10.1128/JVI.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KS, Stock N, Ross C, Andrejeva J, Hilton L, Skinner M, Randall R, Goodbourn S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Choppin PW. Multiplication of myxovirus (SV5) with minimal cytopathic effects and without interference. Virology. 1964;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J Virol. 2005;79:5241–5248. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didcock L, Young DF, Goodbourn S, Randall RE. The V protein of simian virus 5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon PJ, Parks GD. A role for the P subunit of the paramyxovirus polymerase in limiting host cell antiviral responses. J Virol. 2007;81:11116–11127. doi: 10.1128/JVI.01360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- Gainey MD, Dillon PJ, Clark KM, Manuse MJ, Parks GD. Paramyxovirus induced shut off of translation: role of P and V proteins in limiting activation of PKR. J Virol. 2008;82:828–839. doi: 10.1128/JVI.02023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S, Didcock L, Randall RE. Interferons: cell signaling, immune modulation, antiviral responses and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Goodbourn S, Randall RE. The regulation of type I interferon production by paramyxoviruses. J Interferon Cytokine Res. 2009;29(9):539–47. doi: 10.1089/jir.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Arigami T, Kitago M, Nguyen SL, Narita N, Ferrone S, Morton DL, Irie RF, Hoon DS. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol Cancer Ther. 2008;7(11):3642–53. doi: 10.1158/1535-7163.MCT-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- He B, Paterson RG, Ward CD, Lamb RA. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- He B, Paterson RG, Stock N, Durbin JE, Durbin RK, Goodbourn S, Randall RE, Lamb RA. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology. 2002;303:15–32. doi: 10.1006/viro.2002.1738. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Pitha P, Genin P, Nguyen H, Heylbroeck C, Mamane Y, Algarte M, Lin R. Triggering the interferon response: the role of IRF-3 transcription factor. J Interferon Cytokine Res. 1999;19:1–13. doi: 10.1089/107999099314360. [DOI] [PubMed] [Google Scholar]
- Horvath CM. Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. Eur J Biochem. 2004;271:4621–4628. doi: 10.1111/j.1432-1033.2004.04425.x. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Hatakeyama M, Yamashita K, Yoshida H, Ishikawa A, Taima K, Satoh K, Mori F, Wakabayashi K. Interferon-gamma induces retinoic acid-inducible gene-I in endothelial cells. Endothelium. 2004;11(3–4):169–73. doi: 10.1080/10623320490512156. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;4;441(7089):101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor and RIG-1-like receptor signaling. Ann NY Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Antar AAR, Boehme KW, Danthi P, Eby EA, Gugliemi KM, Holm GH, Johnson EM, Maginnis MS, Naik S, Skelton WB, Wetzel JD, Wilson GJ, Chapell JD, Dermody TS. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe. 2007;1:147–157. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Sun L, Zhang H, Liu Q, Liu Y, Qin L, Shi G, Hu JH, Xu A, Sun YP, Li D, Shi YF, Zang JW, Chen Z, Wang ZG, Ge BX. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6(2):150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Fields B, Knipe D, Howley P, editors. Fields Virology. 5. Lippincott Williams and Wilkins Publishers; Philadelphia, Pa: 2007. pp. 1449–1496. [Google Scholar]
- Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178(6):3368–72. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and TLR 3 expression in respiratory syncytial virus infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol. 2004;173(11):6882–9. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- Lu LL, Puri M, Horvath CM, Sen GC. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for inhibitor of kB kinase epsilon (IKKe)/TBK1. J Biol Chem. 2008;283:14269–14276. doi: 10.1074/jbc.M710089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuse MJ, Parks GD. A Role for the Paramyxovirus Genomic Promoter in Limiting Host Cell Antiviral Responses and Cell Killing. J Virol. 2009;83:9057–9067. doi: 10.1128/JVI.01055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J, Jensen SB, Malmgaard L, Rasmussen SB, Weber F, Bowie AG, Matikainen S, Paludan SR. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79(20):12944–51. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22(5):561–9. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;(5):206–9. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- Pfaller CK, Conzelmann KK. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents TLR7/9-mediated interferon induction. J Virol. 2008;82:365–12373. doi: 10.1128/JVI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E, He B, Lamb RA, Randall RE, Goodbourn S. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology. 2002;303:33–46. doi: 10.1006/viro.2002.1737. [DOI] [PubMed] [Google Scholar]
- Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–7. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends in Immunology. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell TA, Liljestrom P, Reis E Sousa C. TLR3 promotes cross-priming to virus infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ML, Cardenas WB, Zamarin D, Palese P, Basler CF. Nuclear localization of the Nipah Virus W protein allows for inhibition of both virus- and TLR3-triggered signaling pathways. J Virol. 2005;79:6078–6088. doi: 10.1128/JVI.79.10.6078-6088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle L, Garcin D, Kolakofsky D. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology. 2006;351:101–111. doi: 10.1016/j.virol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Strahle L, Marq JB, Brini A, Hausmann S, Kolakofsky D, Garcin D. Activation of the beta interferon promoter by unnatural Sendai virus infection requires RIG-I and is inhibited by viral C proteins. J Virol. 2007;81:12227–12237. doi: 10.1128/JVI.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Barral PM, Fisher PB. Central role of interferon regulatory factor-1 (IRF-1) in controlling retinoic acid inducible gene-I (RIG-I) expression. J Cell Physiol. 2007;213(2):502–10. doi: 10.1002/jcp.21128. [DOI] [PubMed] [Google Scholar]
- Ulane CM, Kentsis A, Cruz CD, Parisien JP, Schneider KL, Horvath CM. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J Virol. 2005;79:10180–10189. doi: 10.1128/JVI.79.16.10180-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;(12):1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wansley EK, Parks GD. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J Virol. 2002;76:10109–10121. doi: 10.1128/JVI.76.20.10109-10121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82:8262–8271. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DexD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;1;175(5):2851–8. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Young VA, Dillon PJ, Parks GD. Variants of the paramyxovirus simian virus 5 with accelerated or delayed viral gene expression activate proinflammatory cytokine synthesis. Virology. 2006;350:90–102. doi: 10.1016/j.virol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Yue Z, Shatkin AJ. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology. 1997;234(2):364–71. doi: 10.1006/viro.1997.8664. [DOI] [PubMed] [Google Scholar]