Abstract
Advent of an effective schistosome vaccine would contribute significantly toward reducing the disease spectrum and transmission of schistosomiasis. We have targeted a functionally important antigen, Sm-p80, as a vaccine candidate because of its consistent immunogenicity, protective and antifecundity potentials, and important role in the immune evasion process. In this study, we report that using two vaccination approaches (prime boost and recombinant protein), Sm-p80-based vaccine formulation(s) confer up to 70% reduction in worm burden in mice. Animals immunized with the vaccine exhibited a decrease in egg production by up to 75%. The vaccine elicited strong immune responses that included IgM, IgA, and IgG (IgG1, IgG2a, IgG2b, and IgG3) in vaccinated animals. Splenocytes proliferated in response to Sm-p80 produced Th1 and Th17 response enhancing cytokines. These results again emphasize the potential of Sm-p80 as a viable vaccine candidate for schistosomiasis.
Introduction
Schistosomiasis has been a health concern since at least the ancient Middle Kingdom period (1500 BC) (El-Khoby et al. 2000); still, in the 21st century, it afflicts mankind in 76 different countries and carries an estimated yearly mortality rate of 280,000 (van der Werf et al. 2003). Estimates also indicate that 207 million people are infected and an additional 779 million people are at risk of acquiring this neglected tropical disease (Hotez et al. 2007; Steinmann et al. 2006). Traditionally, control of schistosomiasis has relied mostly on treatment with a single drug, praziquantel, to purge adult worms, however, this strategy has no prophylactic properties and the drug is ineffective against larval forms of the parasite (Utzinger et al. 2003; Wilson et al. 2008). In the last decade, consensus has been developing that to attain a long-lasting decrease in the disease spectrum and transmission of schistosomiasis, an approach based on vaccination coupled with chemotherapy needs to be designed and implemented (Bergquist et al. 2005, 2008). An efficacious anti-schistosome vaccine would contribute significantly to the lessening of morbidity associated with schistosomiasis via protective immune responses that should lead to reduced worm burdens and decreased egg production (Bergquist et al. 2005, 2008; Hagan and Sharaf 2003; Lebens et al. 2004; McManus and Loukas 2008; Siddiqui et al. 2008). As it relates to the vaccine development, protective and antifecundity potential of Sm-p80 in both murine (Ahmad et al. 2009a; Siddiqui et al. 2003a, b) and nonhuman primate (Ahmad et al. 2009b; Siddiqui et al. 2005b) models, has repeatedly demonstrated its potential as a viable vaccine candidate for the reduction of morbidity associated with schistosome infection. In addition, Sm-p80 was initially recognized to play a pivotal role in the schistosome immune evasion process of surface membrane turnover (Siddiqui et al. 1993; Silva et al. 1993; Young and Podesta 1986; Zhou and Podesta 1989), therefore, Sm-p80 can be characterized as a unique target to elicit protective immunity against schistosome infection.
This study was designed to further enhance and refine the vaccine efficacy of Sm-p80. In the present communication, we report that using a prime-boost approach and a recombinant protein immunization strategy in the presence of synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides, high levels of reduction in worm burden and egg production can be achieved that are comparable to levels previously recorded only with the irradiated cercarial vaccine in the murine model [for review, see (Hewitson et al. 2005)].
Materials and methods
Cloning, expression, and purification of Sm-p80
Full-length coding sequence of Sm-p80 (Siddiqui et al. 2003a) was cloned into pCold II vector (GenScript Corp., Piscataway, NJ, USA). Escherichia coli BL21 (DE3; Invitrogen Corp., Carlsbad, CA, USA) were used as the expression host strain. The expressed proteins were initially purified via Ni-nitrilotriacetic acid-agarose resin (Qiagen Inc., Valencia, CA, USA), followed by gel filtration chromatography using a Sephadex G-150 column. The fractions were analyzed by SDS-PAGE, the fractions containing a clear single protein band were pooled, and the protein concentrations were determined. Endotoxin levels in the protein samples were analyzed with a Limulus amebocyte lysate assay (Charles River Laboratories International, Inc., Wilmington, MA, USA).
Immunization protocol
C57BL/6 mice were purchased from Charles River Laboratories International Inc. (Wilmington, MA, USA). A total of 15 mice (three groups of five mice each) were immunized intramuscularly for naked DNA plasmid vaccine and subcutaneously for the protein vaccine formulation as follows: group 1 (control prime-boost), animals were immunized with 100 μg pcDNA3 at week 0 and boosted with 50 μg ODN #2137 (TGC TGC TTT TGT GCT TTT GTG CTT; Coley Pharmaceutical Group, Wellesley, MA, USA) at week 4 and 8. Group 2 (experimental prime-boost) mice were immunized with 100 μg Sm-p80-pcDNA3 (Siddiqui et al. 2003a, b) at week 0 and boosted on week 4 and 8 with 25 μg recombinant Sm-p80 protein mixed with 50 μg ODN #10104 (TCG TCG TTT CGT CGT TTT GTC GTT; Coley Pharmaceutical Group, Wellesley, MA, USA). Group 3 (control recombinant protein), animals were immunized with 50 μg ODN # 2137 (TGC TGC TTT TGT GCT TTT GTG CTT) at week 0 and boosted with the same amount of ODN #2137 on week 4 and week 8. Group 4 (experimental recombinant protein), mice were immunized with 25 μg recombinant Sm-p80 protein mixed with 50 μg ODN #10104 (TCG TCG TTT CGT CGT TTT GTC GTT) at week 0, and boosted with the same amount of protein plus ODN on week 4 and 8.
Challenge infection
Biomphalaria glabrata snails, infected with Schistosoma mansoni (Puerto Rican strain) were obtained from the Schistosomiasis Resource Center (Biomedical Research Institute, Rockville, MD, USA). Four weeks after the second boost, mice from the four groups were challenged with 150 cercariae of S. mansoni. The animals were sacrificed 6 weeks after challenge; adult worms were perfused from the hepatic portal system and also manually removed from the mesenteric veins. The number of worms recovered from each mouse (worm burden) was recorded and percentage reduction in worm burdens in vaccinated versus control animals was calculated (Ahmad et al. 2009a). After sacrifice, liver and intestine samples were collected from each animal and digested in 4% KOH. The number of eggs present in the tissue was determined and percent reduction in egg production was calculated (Ahmad et al. 2009a).
Enzyme-linked immunosorbent assays
Sera obtained following bleeding of all of the animals were pooled in their respective groups and ultimately used to determine the antibody responses. Briefly, 96-well microtiter plates were coated with 1.2 μg recombinant Sm-p80. The antigen-coated wells were sequentially incubated with serial dilutions of test sera and optimally diluted horseradish peroxidase-labeled secondary antibody. To estimate IgG and its subtypes, IgA and IgM, secondary antibodies specific for mouse (anti-IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM; Bio-Rad, Hercules, CA, USA) were used at a dilutions of 1:1,000. The enzyme-linked immunosorbent (ELISA) assays were performed as described previously (Ahmad et al. 2009a; Siddiqui et al. 2003a, b). All of the samples were assayed in triplicate. Results are expressed as end-point titers calculated from a curve of optical density verses serum dilution to a cut-off of two standard deviations above background control values. Results are expressed as the mean±S.E.
Cell proliferation and cytokine production assay
Single-cell suspensions were prepared from the spleens of control and experimental animals as described elsewhere (Siddiqui et al. 2005a). For determination of cell proliferation and cytokine production, 5×106 splenocytes/ml were cultured in a final volume of 200 μl in 96-well flat-bottom plates in the presence of either 1.2 μg recombinant Sm-p80 protein or 0.5 μg Con A. One hundred μl culture supernatants were collected after 48 h incubation to estimate IL-2, IL-4, IL-10, and INF-γ production. All of these cytokines were measured by using a murine cytokine Th1/Th2 ELISA panel kit (eBiosciences Inc., San Diego, CA, USA), following the procedure provided by the manufacturer. The remainders of the cell cultures were processed for cell proliferation assays as described previously (Siddiqui et al. 2005a).
RNA extraction, cDNA synthesis, and reverse transcription polymerase chain reaction
After sacrificing the animals, each spleen was removed aseptically then meshed through a fine screen in RPMI 1640 medium (HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS). The splenocytes were collected via centrifugation and the pellet was stored in Opti-Freeze DMSO Cryopreservation Medium (Fisher Scientific, Hampton, NH, USA), at −85°C until used. The day of assay, the cells were thawed, washed with Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% FBS and the cells were collected by centrifugation (Siddiqui et al. 2005a). The viability of cells was determined via Trypan Blue, >80% of cells were found to be viable. Cells were plated at 5×106 cells/ml in a final volume of 200 μl per well in 96-well flat-bottom microtiter plates containing RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% FBS and 100 μg/ml each of penicillin and streptomycin and 10 μg/ml gentamicin. Cells were cultured in the presence or absence of Sm-p80 antigen and as a positive control concanavalin A was used. The cultures were maintained at 37°C for 24 to 48 h. Total RNA were extracted from splenocytes before and after stimulation of cells with recombinant Sm-p80 via TRIzol method (Invitrogen Corp., Carlsbad, CA, USA). Reverse transcription reactions for first strand cDNA synthesis were carried out as described previously (Smith et al. 1999). Expression levels of different cytokines [IL-1α, IL-1β, IL-2, IL3, IL-4, IL-5, IL-6, IL-7, IL-8 (=MIP-2), IL-9, IL-10, IL-11, IL-12α, IL-12β, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IL-19, IL-20, IL-21, IL-22, IL-23, TNF-α, IFN-γ, TGF-β1 and TGF-β2], and GAPDH were determined via reverse transcription polymerase chain reaction (RT-PCR) using published protocols (Godinez et al. 2008; Montgomery and Dallman 1991; Lauwerys et al. 2000; Brandt et al. 2003; Shen et al. 2006; Ren et al. 2005; Yoshida et al. 2000). PCR products were analyzed on 2% agarose gels and relative differences in expression levels of cytokines were determined using Quantity One (version 4.6.2) computer program (Bio-Rad, Hercules, CA, USA).
Statistical analyses
To calculate the total sample size of experimental animals required per immunization, Fisher’s exact test (two-sided) was used, assuming the minimum detectable difference in response proportions PE and PC with 80% power and type I error rate of 0.05. If PC=0 (no response in control animals) and PE =0.5 (any measurable immune response, e.g., antibody production as determined by Western analysis or ELISA, T cell stimulation, cytokine production, protection from challenge infection, in 50% of the experimental animals) the number of animals needed per group is 15.
Data generated in this study was statistically analyzed as follows: significance between two groups was calculated via one-way analysis of variance and in group significance, by paired t test, using SPSS computer program. Bonferroni adjustments were included for multiple comparisons, to reduce the risk of reaching false conclusions based on chance. P values obtained from these methods were considered significant if they were <0.05.
Results
Expression of Sm-p80
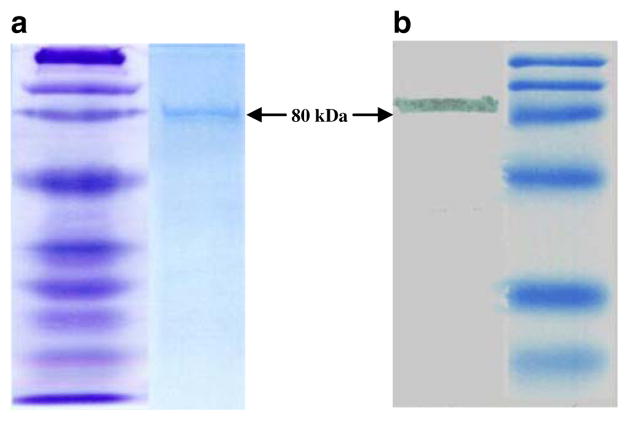
The expression of Sm-p80-pcDNA construct used in these studies has been described previously (Siddiqui et al. 2003a; b). Expression of Sm-p80 in E. coli BL21 (DE3) is shown in Fig. 1. Both Coomassie-stained polyacrylaime gel (Fig. 1a) and western blot analyses (Fig. 1b) of the bacterially expressed protein showed a discrete band at 80 kDa region, representing Sm-p80. The recombinant protein used in immunizations contained minimal endotoxin levels (approximately 0.06 EU/ml) which are acceptable levels, approved for human use by the Food and Drug Administration.
Fig. 1.
Protein expression of Sm-p80 in Escherichia coli BL21 (DE3). Coomassie-stained polyacrylamide gel showing a discrete band at 80 kDa representing Sm-p80 is shown in a Western blot of recombinant Sm-p80 is shown in b. This recombinant Sm-p80 was used in all of the immunization experiments
Protective and anti-fecundity effects of Sm-p80-based vaccines
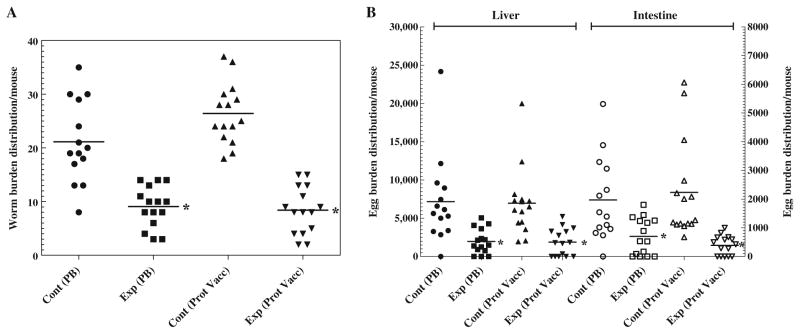
The protective potential of Sm-p80 was determined, first by using a vaccination regimen that involved the initial priming with plasmid DNA and boosting it twice at monthly intervals with the recombinant protein in the presence of ODN. In the group of mice immunized with this prime-boost approach, a 57% reduction in worm burden was recorded (Fig. 2a; Table 1). In the control group of mice, 42.56% of the worms recovered were males and 57.43% were females. Whereas, in the experimental prime-boost group, the worm count was as follows: males (44.11%) and females (55.88%). In the second approach, mice were immunized initially with the recombinant Sm-p80 protein in the presence of ODN as an adjuvant, and boosted twice with the same vaccine formulation, 4 weeks apart. Using this recombinant protein vaccination protocol, a 70% reduction in the worm burden was observed (Fig. 2a; Table 1). In the control group of mice (recombinant protein), 43.68% of the worms recovered were males and 56.31% were females. Similarly, in the experimental recombinant protein group, the worm count was as follows: males (42.85%) and females (57.14%). Both of these protection results were found to be statistically significant (p≤0.001). Similarly, antifecundity effect of the two vaccine formulations was even more pronounced. Specifically, animals immunized with the prime-boost approach exhibited a decrease in egg production by 71%, as determined by the total number of worms recovered (Fig. 2b; Table 1). Based on the number of female worms recovered, the antifecundity effect was 65%. Likewise, mice vaccinated using the recombinant Sm-p80 plus ODN approach, a reduction of 75% was observed in egg production by adult worms (Fig. 2b; Table 1). Based on the number of female worms obtained, the female fecundity reduction value for this group was 77%. Additionally, as previously reported, vaccination via DNA-prime/DNA-boost approach resulted in 59% reduction in worm burden and 84% decrease in egg production (Ahmad et al. 2009a).
Fig. 2.
Worm burden distribution (a) and egg load per gram of liver and intestine of individual mouse (b) for groups of immunized animals: control prime boost (Cont PB), pcDNA3-ODN-2137, experimental prime boost (Exp PB), Sm-p80-pcDNA3-rSm-p80-ODN-10104 control protein vaccine (Cont Prot Vacc) ODN-2137 and experimental protein vaccine (Exp Prot Vacc) rSm-p80-ODN-10104, respectively. Both reduction in worm burden (*p<0.002) and in egg counts (*p<0.001) was statistically lower in two experimental groups
Table 1.
Anti-worm and anti-fecundity effects in C57BL/6 mice following immunization either with prime boost regimen [(Sm-p80-pcDNA3)-(rSm-p80-ODN-10104)] or only with the recombinant protein (rSm-p80-ODN)
| Immunization group | Number | Worms burden (mean±SE) | Number of eggs/g liver (mean±SE) | Number of eggs/g intestine (mean±SE) | Number of eggs/g tissue(mean±SE) | Percent reduction in worm burden (p≤0.001) | Percent reduction in egg production (p≤0.001) |
|---|---|---|---|---|---|---|---|
| Control prime-boost (pcDNA-ODN-2137) | 14 | 21.14±2.04 | 7174.08±1544.82 | 1968.45±391.02 | 4571.26±2602.81 | – | – |
| Experimental prime boost (Sm-p80-pcDNA3-rSm-p80-ODN-10104) | 15 | 9.06±0.99 | 1967.05±422.37 | 699.85±165.44 | 1333.45±633.60 | 57.14 | 70.82 |
| Control protein vaccine (ODN-2137) | 15 | 26.40±1.45 | 6969.39±1147.60 | 2232.36±440.89 | 4600.87±2368.51 | – | – |
| Experimental protein vaccine (rSm-p80-ODN-10104) | 15 | 8.40±1.13 | 873.93±460.04 | 389.16±87.17 | 1131.54±742.38 | 69.69 | 75.40 |
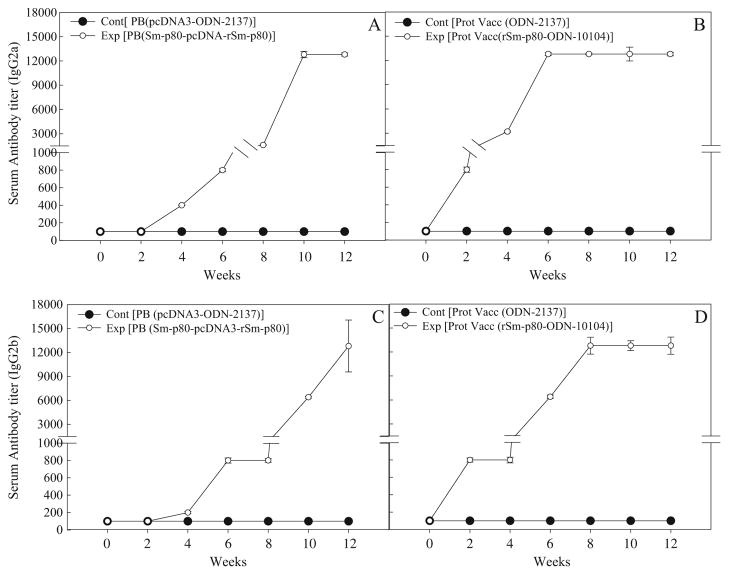
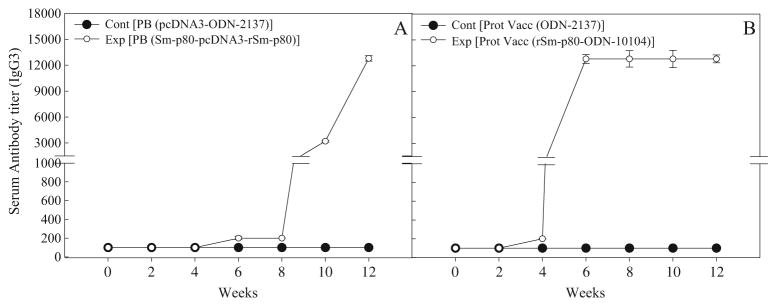
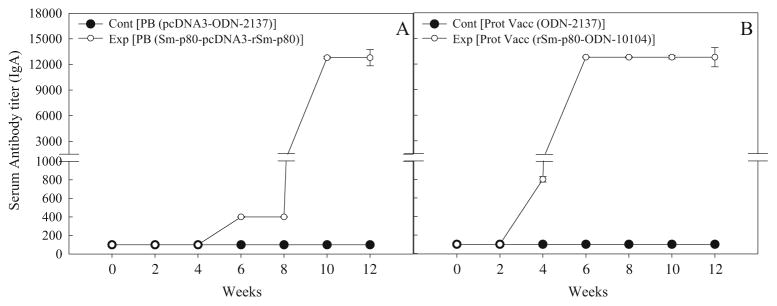
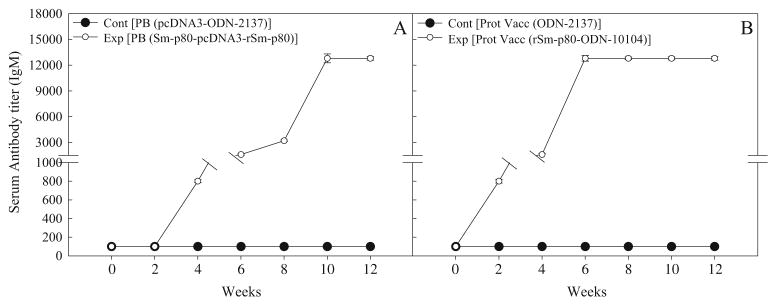
Antibody response to Sm-p80 in vaccinated mice
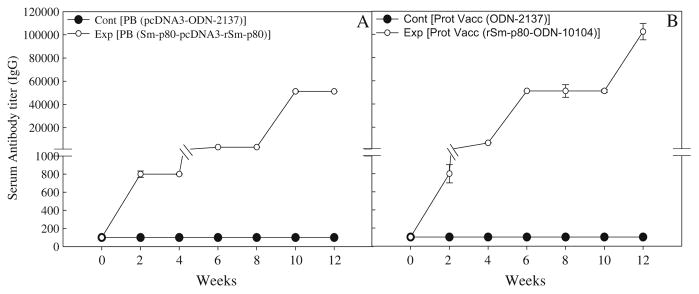
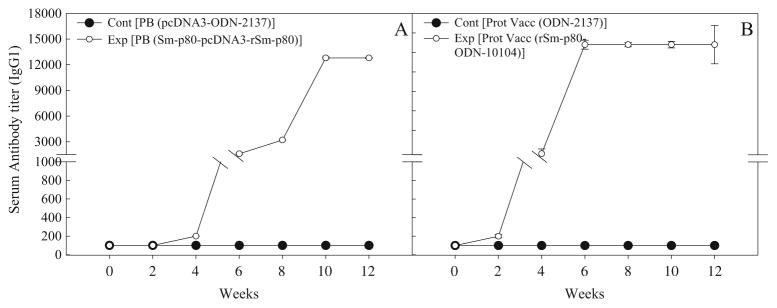
In the sera collected from mice, immunized using the prime-boost approach, distinct antibody titers were obtained for the total IgG (Fig. 3a) and its subtypes [IgG1 (Fig. 4a), IgG2A (Fig. 5a), IgG2B (Fig. 5c), and IgG3 (Fig. 6a]). Pronounced antibody titers for IgA (Fig. 7a) and IgM (Fig. 8a) were also recorded. No detectable levels of Sm-p80-specific antibodies (total IgG or IgG subtypes; IgA and IgM) were found in the group of mice immunized with control plasmid DNA and boosted ODN alone. Titer for the total IgG in the prime-boost group started to rise at week 6 and reached the highest level at week 10 (end-point titer= 51,200) and stayed at this level until challenge (Fig. 3a). For IgG subtypes, IgG1 titer in the prime-boost vaccinated group an increase was seen at week 6 and levels reached a peak at week 10 and stayed at this high level (end-point titer=12,800) until the cercarial challenge (Fig. 4a). Similarly, IgG2a titers reached a peak at week 10 (end-point titer=12,800; Fig. 5a) and IgG2b at week 12 (end-point titer=12,800; Fig. 5c). Similar patterns were observed for IgG3 (Fig. 6a) with highest levels reaching an end-point titer of 12,800 at week 12. IgA titers in the prime boost experimental group also rose sharply at week 8 and reached a peak level at week 10 (end-point titer=12,800; Fig. 7a)]. IgM titers reached a peak level (end-point titer=12,800; Fig. 8a) at week 10 in animals vaccinated using the prime-boost approach.
Fig. 3.
Antibody titers of anti-Sm-p80 total IgG in immunized mice. ELISA was performed with a pool of sera obtained by mixing equal volumes of serum collected from each mouse (biweekly) in their respective groups. Control prime boost (Cont PB; pcDNA3-ODN-2137), experimental prime boost (Exp PB; Sm-p80-pcDNA3-rSm-p80-ODN-10104), control protein vaccine (Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a represents the prime boost experiment while the protein vaccination experiment is shown in b. The values represent the mean of three replicates ± standard error
Fig. 4.
Antibody titers of anti-Sm-p80 total IgG1 in immunized mice. Control prime boost (Cont PB; pcDNA3-ODN-2137), experimental prime boost (Exp PB: Sm-p80-pcDNA3- rSm-p80-ODN-10104), control protein vaccine, Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a represents the prime boost experiment, while the protein vaccination experiment is shown in b. The values represent the mean of three replicates ± standard error
Fig. 5.
Antibody titers of anti-Sm-p80 total IgG2a and IgG2b in immunized mice. Control prime boost (Cont PB; pcDNA3-ODN-2137), experimental prime boost (Exp PB; Sm-p80-pcDNA3-rSm-p80-ODN-10104), control protein vaccine, (Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a and c represent the prime boost experiments (IgG2a and IgG2b). The protein vaccination experiment is shown in b and d for IgG2a and IgG2b, respectively. The values represent the mean of three replicates ± standard error
Fig. 6.
Antibody titers of anti-Sm-p80 total IgG3 in immunized mice. Control prime boost (Cont PB; pcDNA3- ODN-2137), experimental prime boost (Exp PB; Sm-p80-pcDNA3- rSm-p80-ODN-10104), control protein vaccine (Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a represents the prime boost experiment while the protein vaccination experiment is shown in b. The values represent the mean of three replicates ± standard error
Fig. 7.
Antibody titers of anti-Sm-p80 total IgA in immunized mice. Control prime boost (Cont PB; pcDNA3- ODN-2137), experimental prime boost (Exp PB: Sm-p80-pcDNA3- rSm-p80-ODN-10104), control protein vaccine (Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a represents the prime boost experiment while the protein vaccination experiment is shown in b. The values represent the mean of three replicates ± standard error
Fig. 8.
Antibody titers of anti-Sm-p80 total IgM in immunized mice. Control prime boost (Cont PB; pcDNA3-ODN-2137), experimental prime boost (Exp PB; Sm-p80-pcDNA3-rSm-p80-ODN-10104), control protein vaccine (Cont Prot Vacc; ODN-2137), and experimental protein vaccine (Exp Prot Vacc; rSm-p80-ODN-10104). a represents the prime boost experiment while the protein vaccination experiment is shown in b. The values represent the mean of three replicates ± standard error
Antibody titers were generally higher in the group of mice immunized with recombinant protein in the presence of ODN. Robust antibody titers were obtained for the total IgG (Fig. 3b) and its subtypes [IgG1 (Fig. 4b), IgG2A (Fig. 5b), IgG2B (Fig. 5d), and IgG3 (Fig. 6b]). Well-defined antibody titers for IgA (Fig. 7b) and IgM (Fig. 8b) was also seen. No detectable levels of Sm-p80-specific antibodies (total IgG or IgG subtypes; IgA and IgM) were found in the sera obtained from the control group of mice. Titer for the total IgG in the recombinant protein vaccinated group started to rise at week 4 and reached the highest level at week 12 (end-point titer=102,400; Fig. 3b). For IgG subtypes, titer of IgG1 in the vaccinated group showed an increase at week 4 and levels reached a peak at week 6 and stayed at this high level (end-point titer=12,800) until the cercarial challenge (Fig. 4b). Similarly, IgG2a titers reached a peak at week 6 (end-point titer=12,800; Fig. 5b) and IgG2b at week 8 (end-point titer=12,800; Fig. 5d), and stayed at these levels until the challenge. Similar patterns were observed for IgG3 (Fig. 6b) with highest levels reaching an end-point titer of 12,800 at week 6 and stayed at this level until they were infected with schistosome cercariae. IgA titers in the recombinant Sm-p80 plus ODN group, rose sharply at week 6 (end-point titer=12,800; Fig. 7b) and reached a peak level until week 12. IgM titers reached a peak level (end-point titer=12,800; Fig. 8b)] at week 6 in animals vaccinated with the Sm-p80 plus ODN and remained high until the challenge infection.
Cytokine profiles following in vitro stimulation of splenocytes with Sm-p80
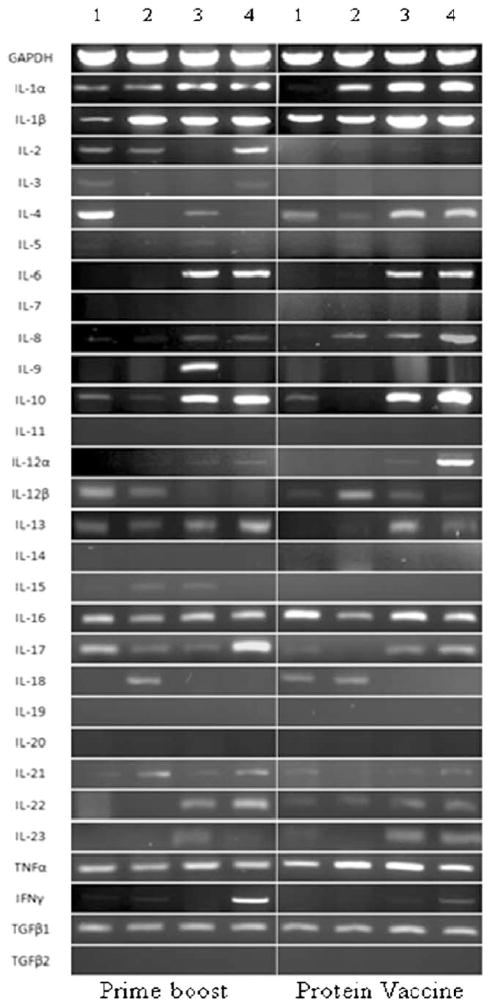
A large repertoire of cytokines were studied and relative differences in their expression were recorded in splenocytes following stimulation in vitro with recombinant Sm-p80 for both of the vaccination approaches (prime-boost and recombinant Sm-p80 plus ODN). As shown in Table 2, INF-γ and IL-2 production was significantly higher in vaccinated animals as compared with the control group. It was observed that there is no statistically significant difference in the IL-4 and IL-10 production when compared with the control group. Using RT-PCR studies, IL-1α, IL-1β, IL-2, IL3, IL-4, IL-5, IL-6, IL-7, IL-8 (=MIP-2), IL-9, IL-10, IL-11, IL-12α, IL-12β, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IL-19, IL-20, IL-21, IL-22, IL-23, TNF-α, IFN-γ, TGF-β1, and TGF-β2 expression was recorded and the agarose gels depicting the PCR products is shown in Fig. 9. Based on relative difference between the samples from control and experimental animals; in the group vaccinated using the prime-boost approach, IFN-γ appeared to highly upregulated followed by IL-17. In the Sm-p80 plus ODN group, proliferating splenocytes in response to recombinant Sm-p80, expressed higher levels of IFN-γ, IL-12α and to a lesser extent, IL-22 and IL-8 (=MIP 2).
Table 2.
Levels of cytokine production by splenocytes after 48hr stimulation with recombinant Sm-p80 in vitroa
| Immunization group | IL-2 (pg/mL) | IL-4 (pg/mL) | IL-10 (pg/mL) | IFN-γ (pg/mL) |
|---|---|---|---|---|
| Control prime-boost (pcDNA-ODN-2137) | 84.86±2.30 | 39.88±2.95 | 68.51±3.51 | 86.95±5.97 |
| Experimental prime boost (Sm-p80-pcDNA-rSm-p80-ODN-10104) | 471.47±18.61* | 55.64±4.69 | 62.64±1.18 | 297.52±26.60* |
| Control protein vaccine (ODN-2137) | 86.37±1.73 | 39.78±1.50 | 54.89±3.63 | 83.30±3.47 |
| Experimental protein vaccine (rSm-p80-ODN-10104) | 977.23±93.89* | 40.21±2.82 | 90.86±11.59* | 340.34±26.11* |
The values in the table represent mean±S.D.
p≤0.05 vs. corresponding control group stimulated by recombinant Sm-p80 respectively using independent sample test
Fig. 9.
Agarose gel electrophoresis of RT-PCR generated products of different cytokines for control prime boost (pcDNA3-ODN-2137) and experimental prime boost (Sm-p80-pcDNA3-rSm-p80-ODN-10104) groups and control protein vaccine (ODN-2137) and experimental protein vaccine (rSm-p80-ODN-10104) groups. In both panels, lanes 1 represent splenocytes from control group of animals without stimulation in vitro with recombinant Sm-p80; lanes 2 represent splenocytes from experimental group of animals without stimulation in vitro with recombinant Sm-p80. Lanes 3 show splenocytes from control group of animals with stimulation in vitro with recombinant Sm-p80; lanes 4 represent splenocytes from experimental group of animals with stimulation in vitro with recombinant Sm-p80
Discussion
For a molecularly defined schistosome vaccine to have a meaningful impact, it is likely to be judged against the cercarial radiation attenuated (RA) vaccine which is considered to be a “gold standard” (Hewitson et al. 2005). Immunization of mice with a single dose of RA-vaccine confers 56–80% protection against a challenge infection with S. mansoni (Sher et al. 1982; Minard et al. 1978; Ganley-Leal et al. 2005). To this effect, 57–70% protection observed in this study using Sm-p80-based vaccine formulations is comparable to RA-vaccine with respect to its prophylactic efficacy. Protection in mice receiving a single vaccination with the RA vaccine is apparently mediated by the IFN-γ-dependent mechanisms; however, there is evidence that IgG antibodies may also play a role in this protection (Hewitson et al. 2005; Jankovic et al. 1999). Humoral immunity appears to contribute to a greater extent in conferring protection achieved through the multiple exposures of RA-vaccine to mice (Hewitson et al. 2005; Jankovic et al. 1999). Similar to this, in our study, in mice vaccinated with prime-boost and recombinant protein approaches, IFN-γ was the apex cytokine produced by the proliferating splenocytes in response to Sm-p80 stimulation in vitro and this was coupled with high titers of IgG antibodies in the sera of immunized mice. Therefore, it appears that both of the vaccination strategies resulted in the induction of B cell- and IFN-γ-dependent mechanisms which is perhaps a requirement for an optimal vaccine against S. mansoni (Jankovic et al. 1999).
Strong total IgG antibody production was observed in the sera of vaccinated mice for both the prime-boost (endpoint titer=51,200) and recombinant protein (end-point titer=102, 400) approaches. Even though consistent reactivity to IgM, IgA, and all of the IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3), was detected in the sera obtained from mice immunized with both of the vaccination strategies; their highest titers plateaued at an end-point titer of 12,800. In toto, these antibody profiles suggest that a mixed Th1/Th2 type of response was generated. Generation of the mixed Th1/Th2 type of response has resulted in some improvement with regards to the reduction in worm burden and in antifecundity effects of the Sm-p80 compared to our previous studies in which Sm-p80 was used in a DNA vaccine formulation and a predominantly Th1-biased antibody response was observed in the mouse model (Ahmad et al. 2009a; Siddiqui et al. 2003a, b).
Splenocytes from mice immunized via either prime-boost or recombinant protein approaches, proliferated significantly higher following stimulation in vitro with the recombinant Sm-p80 protein, compared to their respective controls. These proliferating splenocytes expressed cytokines that have been linked with protection. Specifically, in the prime-boost approach, upregulation of IFN-γ and IL-17 was observed. There is now consensus on the important role IFN-γ plays in conferring vaccine-mediated protection against schistosomes challenge infection in animal models (Ahmad et al. 2009a; Ahmad et al. 2009b; Da’dara and Harn 2005; Da’dara et al. 2006; Hewitson et al. 2005; Jankovic et al. 1999; Siddiqui et al. 2003b, 2005a). Conversely, very little is known about the role of IL-17 in mediating protection even though T helper cell polarization studies have indicated that significant regulation of Th1, Th2, and interleukin (IL)-17-secreting lymphocytes is required to prevent severe liver pathology in murine schistosomiasis (Wilson et al. 2007). Recently, it has been reported that a significant increase in plasma levels of TGF-β and IL-17 cytokines in C57BL/6 mice experimentally infected with S. mansoni are directly related to worm burden reduction (Tallima et al. 2009). Additionally, in mice which were vaccinated using the recombinant protein approach, in addition to Th1 response enhancing cytokines, IFN-γ, and IL-12α, expression of IL-22 and IL-8 was also upregulated. IL-22 is one of the key effector cytokines produced by Th17 cells (Bettelli et al. 2008). It has been postulated that Th17 responses are likely to constitute an early response to a number of pathogens that are not managed well by Th1 or Th2 type of immunity and for which a strong tissue inflammation is needed to clear the infection (Bettelli et al. 2008). In addition, IL-8 or its murine counterpart macrophage inflammatory protein 2 (MIP-2) is a major macrophage and lymphocyte chemoattractant (Kishimoto et al. 2001). Taken together, the cytokine profile data indicate that a Th1 and Th17 type of response has been generated following vaccination with both of the approaches (prime-boost and recombinant protein).
In summary, the data presented here clearly indicate that using Sm-p80 as an antigen, reduction in worm burden (up to 70%, this study) as well as in egg production [up to 84% (Ahmad et al. 2009a)] can be achieved to high levels which previously could only be recorded with a RA-vaccine. Additionally, thus far, four “research groups” have independently shown reproducible and consistent protection levels with Sm-p80 in animal models {Nagoya City University Medical School, Nagoya, Japan (Ohta et al. 2004; Zhang et al. 2001); The John P. Robarts Research Institute and University of Western Ontario, London, Canada (Hota-Mitchell et al. 1997, 1999); Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD (Jankovic et al. 1996); Texas Tech University Health Sciences Center (Siddiqui et al. 2003a, b, 2005a, b; Ahmad et al. 2009a, b)}. Therefore, we believe that Sm-p80 is now ready to be moved into the next phase, i.e., scale up and cGMP production and prepare for Phase I/II human clinical trials.
Acknowledgments
This work was supported by a grant from the Thrasher Research Fund (Award No. 02824-5) to Afzal A. Siddiqui. Two summer stipends for High School Educators (Chad Haskins and Sue Diggs) were funded through Recovery Act Administrative Supplement (3R01AI071223-02S1) to Afzal A. Siddiqui.
References
- Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009a;31:156–161. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad G, Zhang W, Torben W, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Kennedy RC, Siddiqui AA. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine. 2009b;27:2830–2837. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–117. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- Da’dara AA, Harn DA. DNA vaccines against tropical parasitic diseases. Expert Rev Vaccines. 2005;4:575–589. doi: 10.1586/14760584.4.4.575. [DOI] [PubMed] [Google Scholar]
- Da’dara AA, Lautsch N, Dudek T, Novitsky V, Lee TH, Essex M, Harn DA. Helminth infection suppresses T-cell immune response to HIV-DNA-based vaccine in mice. Vaccine. 2006;24:5211–5219. doi: 10.1016/j.vaccine.2006.03.078. [DOI] [PubMed] [Google Scholar]
- El-Khoby T, Galal N, Fenwick A, Barakat R, El-Hawey A, Nooman Z, Habib M, bdel-Wahab F, Gabr NS, Hammam HM, Hussein MH, Mikhail NN, Cline BL, Strickland GT. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg. 2000;62:88–99. doi: 10.4269/ajtmh.2000.62.88. [DOI] [PubMed] [Google Scholar]
- Ganley-Leal LM, Guarner J, Todd CW, ‘Dara AA, Freeman GL, Jr, Boyer AE, Harn DA, Secor WE. Comparison of Schistosoma mansoni irradiated cercariae and Sm23 DNA vaccines. Parasite Immunol. 2005;27:341–349. doi: 10.1111/j.1365-3024.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Baumler AJ. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun. 2008;76:2008–2017. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P, Sharaf O. Schistosomiasis vaccines. Expert Opin Biol Ther. 2003;3:1271–1278. doi: 10.1517/14712598.3.8.1271. [DOI] [PubMed] [Google Scholar]
- Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 2005;27:271–280. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Hota-Mitchell S, Clarke MW, Podesta RB, Dekaban GA. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine. 1999;17:1338–1354. doi: 10.1016/s0264-410x(98)00391-0. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Aslund L, Oswald IP, Caspar P, Champion C, Pearce E, Coligan JE, Strand M, Sher A, James SL. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996;157:806–814. [PubMed] [Google Scholar]
- Jankovic D, Wynn TA, Kullberg MC, Hieny S, Caspar P, James S, Cheever AW, Sher A. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol. 1999;162:345–351. [PubMed] [Google Scholar]
- Kishimoto C, Kawamata H, Sakai S, Shinohara H, Ochiai H. Enhanced production of macrophage inflammatory protein 2 (MIP-2) by in vitro and in vivo infections with encephalomyocarditis virus and modulation of myocarditis with an antibody against MIP-2. J Virol. 2001;75:1294–1300. doi: 10.1128/JVI.75.3.1294-1300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–1853. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- Lebens M, Sun JB, Czerkinsky C, Holmgren J. Current status and future prospects for a vaccine against schistosomiasis. Expert Rev Vaccines. 2004;3:315–328. doi: 10.1586/14760584.3.3.315. [DOI] [PubMed] [Google Scholar]
- McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard P, Dean DA, Jacobson RH, Vannier WE, Murrell KD. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978;27:76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- Montgomery RA, Dallman MJ. Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J Immunol. 1991;147:554–560. [PubMed] [Google Scholar]
- Ohta N, Kumagai T, Maruyama H, Yoshida A, He Y, Zhang R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol Int. 2004;53:175–181. doi: 10.1016/j.parint.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ren F, Zhan X, Martens G, Lee J, Center D, Hanson SK, Kornfeld H. Pro-IL-16 regulation in activated murine CD4+ lymphocytes. J Immunol. 2005;174:2738–2745. doi: 10.4049/jimmunol.174.5.2738. [DOI] [PubMed] [Google Scholar]
- Shen L, Zhang C, Wang T, Brooks S, Ford RJ, Lin-Lee YC, Kasianowicz A, Kumar V, Martin L, Liang P, Cowell J, Ambrus JL., Jr Development of autoimmunity in IL-14alpha-transgenic mice. J Immunol. 2006;177:5676–5686. doi: 10.4049/jimmunol.177.8.5676. [DOI] [PubMed] [Google Scholar]
- Sher A, Hieny S, James SL, Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982;128:1880–1884. [PubMed] [Google Scholar]
- Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, Dekaban GA, Clarke MW. Characterization of Ca(2 +)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, Lloyd JD, Paz M, Villalovos RM, Pompa J. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003a;71:3844–3851. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, Lloyd JD, Pompa J, Villalovos RM, Paz M. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003b;20(21):2882–2889. doi: 10.1016/s0264-410x(03)00159-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Pinkston JR, Quinlin ML, Kavikondala V, Rewers-Felkins KA, Phillips T, Pompa J. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005a;12:3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Pinkston JR, Quinlin ML, Saeed Q, White GL, Shearer MH, Kennedy RC. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005b;23:1451–1456. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008;102:825–833. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- Silva EE, Clarke MW, Podesta RB. Characterization of a C3 receptor on the envelope of Schistosoma mansoni. J Immunol. 1993;151:7057–7066. [PubMed] [Google Scholar]
- Smith JK, Siddiqui AA, Modica LA, Dykes R, Simmons C, Schmidt J, Krishnaswamy GA, Berk SL. Interferon-alpha upregulates gene expression of aquaporin-5 in human parotid glands. J Interferon Cytokine Res. 1999;19:929–935. doi: 10.1089/107999099313479. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Tallima H, Salah M, Guirguis FR, El RR. Transforming growth factor-beta and Th17 responses in resistance to primary murine schistosomiasis mansoni. Cytokine. 2009 doi: 10.1016/j.cyto.2009.07.581. (in press) [DOI] [PubMed] [Google Scholar]
- Utzinger J, Keiser J, Shuhua X, Tanner M, Singer BH. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob Agents Chemother. 2003;47:1487–1495. doi: 10.1128/AAC.47.5.1487-1495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RA, Langermans JA, Van Dam GJ, Vervenne RA, Hall SL, Borges WC, Dillon GP, Thomas AW, Coulson PS. Elimination of Schistosoma mansoni adult worms by rhesus macaques: basis for a therapeutic vaccine? PLoS Negl Trop Dis. 2008;2:e290. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Kezuka T, Streilein JW. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 2. Generation of TGF-beta-producing regulatory T cells. Invest Ophthalmol Vis Sci. 2000;41:3862–3870. [PubMed] [Google Scholar]
- Young BW, Podesta RB. Complement and 5-HT increase phosphatidylcholine incorporation into the outer bilayers of Schistosoma mansoni. J Parasitol. 1986;72:802–803. [PubMed] [Google Scholar]
- Zhang R, Yoshida A, Kumagai T, Kawaguchi H, Maruyama H, Suzuki T, Itoh M, El-Malky M, Ohta N. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun. 2001;69:386–391. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Podesta RB. Effects of serotonin (5HT) and complement C3 on the synthesis of the surface membrane precursors of adult Schistosoma mansoni. J Parasitol. 1989;75:333–343. [PubMed] [Google Scholar]