Abstract
Cardiovascular disease is the number one cause of death in the United States. Deployment of stents and vascular grafts has been a major therapeutic method for treatment. However, restenosis, incomplete endothelialization, and thrombosis hamper the long term clinical success. As a solution to meet these current challenges, we have developed a native endothelial ECM mimicking self-assembled nanofibrous matrix to serve as a new treatment model. The nanofibrous matrix is formed by self-assembly of peptide amphiphiles (PAs), which contain nitric oxide (NO) donating residues, endothelial cell adhesive ligands composed of YIGSR peptide sequence, and enzyme-mediated degradable sites. NO was successfully released from the nanofibrous matrix rapidly within 48 hours, followed by sustained release over period of 30 days. The NO releasing nanofibrous matrix demonstrated a significantly enhanced proliferation of endothelial cells (51 ± 3 % to 67 ± 2 %) but reduced proliferation of smooth muscle cells (35 ± 2 % to 16 ± 3 %) after 48 hrs of incubation. There was also a 150-fold decrease in platelet attachment on the NO releasing nanofibrous matrix (470 ± 220 platelets/cm2) compared to the collagen-I (73 ± 22 × 103 platelets/cm2) coated surface. The nanofibrous matrix has the potential to be applied to various cardiovascular implants as a self-assembled coating, thereby providing a native endothelial extracellular matrix (ECM) mimicking environment.
Keywords: self-assembly, nitric oxide, endothelium, biomimetic material, peptide, stents, vascular grafts
Introduction
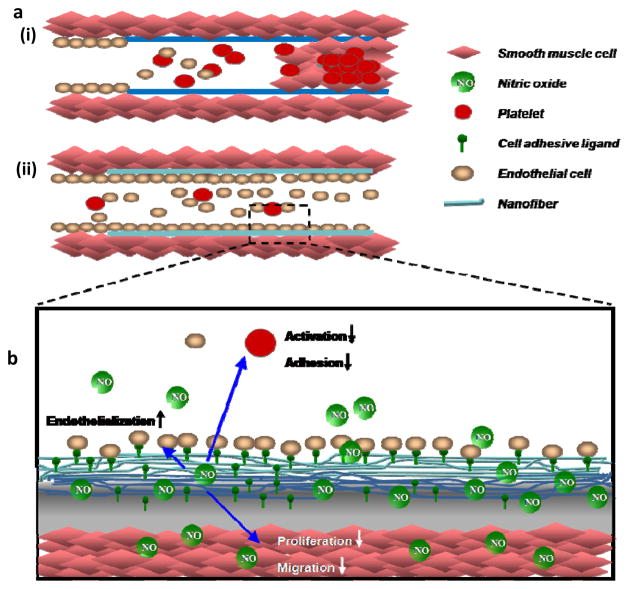
Cardiovascular disease is the leading cause of death in the United States [1]. Currently, stents and vascular grafts are the primary therapeutic methods for treatment of cardiovascular diseases. However, restenosis, incomplete endothelialization, and thrombosis hamper their long term clinical success [2–4]. Native endothelium consists of a monolayer of endothelial cells that adhere to the underlying nanofibrillar basement membrane and modulate vascular tone by release of soluble factors, such as nitric oxide (NO). The local release of NO plays a critical role in controlling the function of the human cardiovascular system by regulating vascular cell homeostasis [5–7]. Thus, the inevitable loss of this multi-functional endothelium associated with vascular stretch and injury at the implant sites of stents and vascular grafts triggers a cascade of restenosis by smooth muscle cells proliferation with accompanying extracellular matrix production. The risk of late thrombosis by platelet adhesion also compromises long term patency. Altogether, currently used stents and vascular grafts remain limited by incomplete re-endothelialization, restenosis, and late-thrombosis (Figure 1a) [2–4, 8].
Figure 1.
(a)Schematic comparison of current stent/vascular grafts ( ) and endothelial ECM-mimic nanofibrous matrix (
) and endothelial ECM-mimic nanofibrous matrix ( ). (i) Endothelial disruption exposes vessel wall and leading to restenosis, thrombosis and incomplete endothelialization. (ii) Native endothelial ECM-mimic nanofibrous matrix will prevent restenosis and thrombosis while promoting endothelialization. (b) Components of the native endothelial ECM-mimic nanofibrous matrix. NO released from the nanofibrous matrix prevents platelet activation, adhesion, smooth muscle cell proliferation, migration and promotes endothelialization.
). (i) Endothelial disruption exposes vessel wall and leading to restenosis, thrombosis and incomplete endothelialization. (ii) Native endothelial ECM-mimic nanofibrous matrix will prevent restenosis and thrombosis while promoting endothelialization. (b) Components of the native endothelial ECM-mimic nanofibrous matrix. NO released from the nanofibrous matrix prevents platelet activation, adhesion, smooth muscle cell proliferation, migration and promotes endothelialization.
Numerous therapeutic approaches have been investigated to overcome these problems with limited success. It is believed that the incorporation of endothelium specific factors will provide an enhanced clinical treatment, specifically tailoring biomaterials for cardiovascular implant coatings. To this effect, several NO releasing materials have been studied in the form of films or hydrogels and found to reduce platelet adhesion and intimal hyperplasia, both in vitro and in vivo [9–12]. However, none of the above materials are presently able to completely tackle all current clinical challenges, as they are limited by their inability to mimic the properties of native endothelium. Instead, a more multifunctional approach is required, which would provide a native endothelial extracellular matrix (ECM) mimicking environment on the surface of stents or vascular grafts to prevent restenosis and thrombosis by inhibiting smooth muscle proliferation and platelet adhesion, while enhancing re-endothelialization by promoting endothelial cell proliferation. Therefore, the goal of this study is to develop a native endothelial ECM mimicking nanofibrous matrix that consists of NO releasing peptide amphiphiles, and to study the behavior of endothelial cells, smooth muscle cells and platelets in vitro on this nanofibrous matrix.
Peptide amphiphiles (PAs) that consist of hydrophobic tails coupled to hydrophilic functional peptide sequences are attractive templates for biomimetic scaffolds because cell adhesion ligands and enzyme-mediated degradable sites can be incorporated into the hydrophilic domains of the PAs to mimic biochemical properties of the extracellular matrix (ECM) [13, 14]. In order to mimic properties of a native endothelium, the designed hydrophilic functional peptide sequences consist of a matrix metalloprotease-2 (MMP2) mediated cleavage site, Gly-Thr-Ala-Gly-Leu-Ile-Gly-Gln (GTAGLIGQ), [15] coupled to an endothelial cell-adhesive ligand, Tyr-Ile-Gly-Ser-Arg (YIGSR), [16] or a polylysine (KKKKK) group to form NO (or nitrogen oxide) donating residues [17–19]. This study utilizes a bottom-up approach to achieve a unique synergistic effect by combining multiple components, including cell-adhesive ligand (YIGSR), cytokine molecule (NO), enzyme-mediated degradation (MMP-2), and self-assembly into a nano-fibrillar structure. NO is a natural mediator of vascular homeostasis and is produced by endothelial cells. It has been known to reduce platelet adhesion and smooth muscle cell proliferation, while concurrently stimulating endothelial cell proliferation [18, 19]. Therefore, this nanofibrous matrix comprised two different PAs, PA-YIGSR (C16-GTAGLIGQYIGSR) and PA-KKKKK (C16-GTAGLIGQKKKKK). The nanofibrous matrix is designed to act as a surrogate reservoir of NO by replenishing the NO supply to the native artery during renewal of the injured endothelium. The incorporation of NO donating residues into the PA will allow controlled release of NO from the nanofibrous matrix coated on stents or vascular grafts into the local blood stream. NO will limit smooth muscle cell proliferation and platelet adhesion, while enhancing re-endothelialization onto stents or vascular grafts.
Materials and Methods
Synthesis of Peptide Amphiphiles
Two thirteen-amino acid peptides consisting of MMP-2 sensitive sequences (GTAGLIGQ) with cell-adhesive sequence YIGSR (PA-YIGSR) or NO donating residue KKKKK (PA-KKKKK) were synthesized using standard Fmoc-chemistry on an Advanced Chemtech Apex 396 peptide synthesizer, as similarly described before. These peptides were alkylated to be linked to a 16 carbon palmityl chain, thereby creating an amphiphile [13, 14].
Self-Assembly of Peptide Amphiphiles (PAs) into Nanofibrous Matrix Coating
Self-assembly of PAs into nanofibers was characterized using transmission electron microscope (TEM). 5 μl of each 0.1 wt % PA solutions were cast on a carbon coated formvar copper grid (400 mesh). This grid was dried overnight. Before imaging, the dried samples were negatively stained with 10 μl of 2% phosphotungstenic acid (PTA) for 30s. The samples were imaged (42000x, 52000x) on a FEI Tecnai T12 TEM microscope at 60 kV accelerating voltage.
Preparation of Nanofibrous matrix Coated Culture Chambers
For cell adhesion and spreading studies, 0.1 wt % PA solutions were prepared in DI water (pH 7.4). 50 μl of PA solution were placed in 12-well silicone flexiPERM cell-culture chambers attached to glass coverslips. The chambers were placed in a chemical fume hood for 24 hours to induce self-assembly by solvent evaporation. The chambers were further dried for another 48 hours in a 37°C incubator and sterilized under UV for 4 hours.
Cell Maintenance
Human umbilical vein endothelial cells (HUVECs) were grown in endothelium growth medium (EGM) complete medium (2% FBS, 0.1% hEGF, 0.1% hydrocortisone, 0.1% gentamycin A, 0.4% bovine brain extract). Human aortic smooth muscle cells (AoSMCs) were grown in smooth muscle cell basal medium (SmBM) SingleQuot® Kit complete culture medium (5% FBS, 0.1% Insulin, 0.2% hFGF-B, 0.1% gentamycin A, 0.1% hEGF). All cells and media were purchased from Lonza Inc. (Walkersville, MD).
Optimization of Ratio of PA-YIGSR and PA-KKKKK
HUVECs were seeded at density of 40,000 cells/cm2 on different molar ratios of PA-YIGSR and PA-KKKKK, designated as YK 90 (90% PA YIGSR, 10% PA KKKKK), YK 75 (75% PA YIGSR, 25% PA KKKKK), YK 50 (50% PA YIGSR, 50% PA KKKKK), YK 25 (25% PA YIGSR, 75% PA KKKKK), and YK 10 (10% PA YIGSR, 90% PA KKKKK). Cells were stained for morphology and spreading with a LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR) and counted after 2 hours using Nikon NIS Elements imaging software (Melville, NY).
Preparation and Characterization of NO-Releasing Nanofibrous Matrix (PA-YK-NO)
Scrubbed NO gas was reacted with 1 wt% PA-YK solution under argon gas in a 100 mL round bottom flask overnight. The resulting PA-YK-NO solution was cast into films by dropping 150 μl on 13mm glass coverslips. The PA-YK-NO films were dried in a chemical fume hood for first 24 hours and at 37°C for the following 48 hours. To account for nitrite bound to the PAs, PA-YIGSR-NO films were also prepared in the same way as a control. The films were incubated in 500μl of HEPES buffer saline (HBS) in a 24 well tissue culture plate. The incubated HBS was collected, frozen (−80°C), and replaced by fresh HBS at 0 hr, 2 hr, 4 hr, 6 hr, 24hr, 1 day, and every alternate day up to one month. NO release from the PA-YK-NO nanofibrous matrix was quantified using the Greiss assay to measure collected nitrite, which is the primary degradation product of NO [19]. At the end of one month, each collected sample was mixed with 100 μl of the Griess reagent. The Greiss reagent consists of sulfanilamide and N-1-napthylethylenediamine dihydrochloride (NED), which react sequentially with nitrite to produce an azo compound that is responsible for providing colorimetric analysis. After incubation for 15 minutes at room temperature, the samples were read at 540 nm using an absorbance microplate reader (EL x 800, BIO-TEK Instrument, VT). The results were obtained by normalizing the NO content released from PA-YK-NO with the NO released from PA-YIGSR-NO.
Evaluation of Cellular Behaviors on Nanofibrous Matrix Coatings
HUVECs and AoSMCs were seeded on PA-YK nanofibrous matrix coated culture chamber at densities of 30,000 cells/cm2 and 15,000 cells/cm2, respectively. After 2 hours of incubation cells were morphologically stained with the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR) and analyzed for cell adhesion and spreading using Nikon NIS Elements imaging software (Melville, NY). To evaluate the effect of NO on cell proliferation, 0.1 wt% PA-YK-NO and PA-YK nanofibrous matrix coatings were prepared as describe before. HUVECs and AoSMCs were seeded at densities of 30,000 cells/cm2 and 15,000 cells/cm2, respectively. Proliferation of HUVECs and AoSMCs was evaluated by proliferating cell nuclear antigen (PCNA) staining. After 48 hrs of incubation, cells were fixed in 10% formalin, permeabilized in methanol, and blocked using a 3% hydrogen peroxide solution. Cells were then incubated with tris-buffered saline, followed by incubation with mouse IgG anti-PCNA primary antibody (Dako Corp., Carpinteria, CA) diluted 1:100 in PBS with 3% FBS. Then, cells were incubated with anti-mouse IgG HRP (Dako Corp., Carpinteria, CA) diluted 1:100 in PBS with 3% FBS, followed by incubation with aminoethylcarbazole chromogen (Dako Corp., Carpinteria, CA). The cells were counterstained with Mayer’s hematoxylin and rinsed with 37mM ammonium hydroxide. The percentage of proliferating cells per field of view (20 X) was determined by counting the red proliferating cells and blue non-proliferating cells using phase contrast microscopy. For all cell quantifications, five random fields were imaged and averaged for each well. Furthermore, four samples were tested for each condition making up the experiments. The results shown in the graphs depict an average of over 120 images from 3 independent experiments (n=12).
Evaluation of Platelet Adhesion on Nanofibrous Matrix Coating
PA-YK and PA-YK-NO nanofibrous matrix coatings were prepared on 316L Stainless steel surfaces (1×1 cm2). A solution of 2.5 mg/ml collagen I was prepared in 3% glacial acetic acid to serve as a positive control and cast into films in the same manner described for the PAs. Whole blood from a healthy volunteer was collected in BD Vacutainer® Heparin Tubes (BD, NJ) and mixed with 10uM mepacrine to fluorescently label the platelets. PA-YK, PA-YK-NO, collagen films, and uncoated stainless steel surfaces were separately incubated with mepacrine-labeled blood at 37°C for 90 minutes and then rinsed with PBS. The number of adherent platelets per field of view (10x) was determined using a fluorescent microscope by averaging five random fields per sample. The results shown in the graphs depict data from 3 independent experiments (n=12) with four samples each.
Statistical Analysis
All experiments were performed at least three independent times. All data were compared with one-way ANOVA tests to evaluate statistical significance using SPSS software. Within the ANOVA analysis, Tukey multiple comparisons test was performed to find significant differences between pairs. A value of p<0.05 was considered to be statistically significant.
Results and Discussion
Developing an endothelial ECM mimic coating is deemed as a vitally important solution needed to meet the current challenges faced by vascular grafts and stents. Towards this goal, we have synthesized and characterized a native endothelial ECM mimic nanofibrous matrix designed to reconstitute the properties of native endothelium onto the cardiovascular implant surface. Two PAs, PA-YIGSR and PA-KKKKK were successfully synthesized for this purpose. The PAs contain enzyme-mediated degradable MMP-2 sensitive sequences, along with YIGSR or a polylysine (KKKKK) group to form NO donating residues. PA-YKs were also designed by mixing different molar ratios of PA-YIGSR and PA-KKKKK. Thus, densities of cell adhesive ligands and NO could be tuned by varying the ratios of PA-YIGSR and PA-KKKKK. Self-assembly of the PAs into nanofibrous matrices was achieved by a solvent evaporation method. TEM images showed that all different PAs were successfully self-assembled into nanofibers with uniform diameter between 7–8 nm and several microns in length (Figure 2). Consistent multilayered nanofibrous matrix coatings were found.
Figure 2.
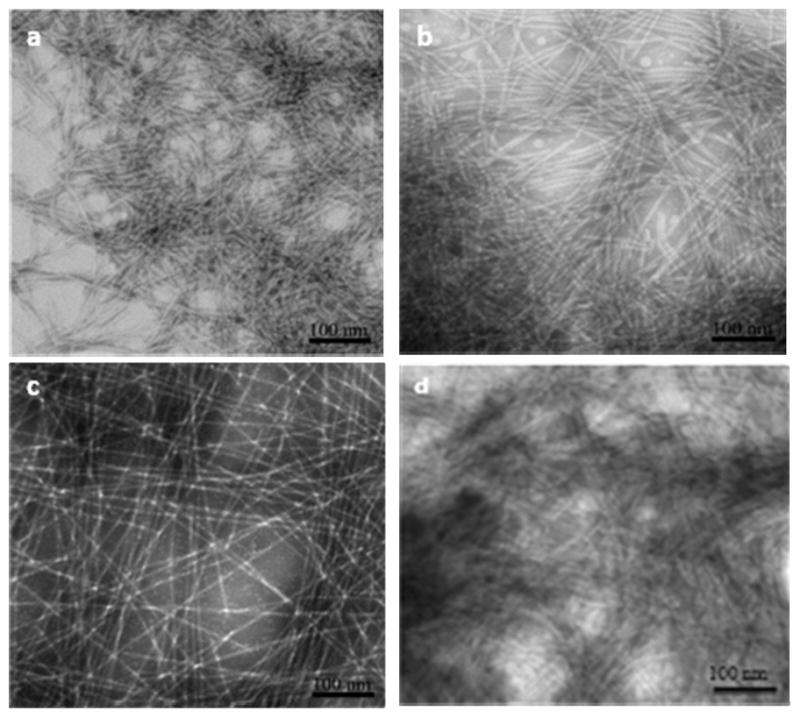
TEM images of self-assembled nanofibrous matrices by solvent evaporation method. (a) PA-YIGSR (b) PA-KKKKK (c) PA-YK (d) PA-YK-NO. PA-YK and PA-YK-NO are 90 to 10 molar ratio mixture of PA-YIGSR and PA-KKKKK.
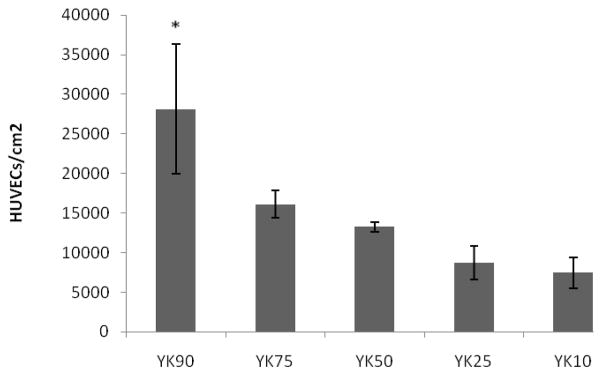
To determine the optimal matrix composition, endothelial cells were seeded on various ratios of PA-YKs and cell adhesion was found to be significantly greater with increasing PA-YIGSR concentration (Figure 3). Thus, PA-YK (9:1 mol/mol) was used for all further studies. PA-YK was reacted with NO gas to form PA-YK-NO, as previously described [18, 19], and allowed to form a self-assembled coating by solvent evaporation. Notably, PA-YK-NO also self-assembled into nanofibers with similar features (Figure 2d). Thus, the reaction with NO did not affect self-assembly of the PA-YK-NO into nanofibers, indicating that NO binding to side groups of PA-YK does not interfere with the self-assembly process. This could be explained by the fact that only amino acids closer to the core are critical in self-assembling process, as evidenced previously. [21] This also demonstrates that the side groups within PAs can be modified with biological functional groups without adversely affecting the self-assembly process.
Figure 3.
HUVEC attachment on different molar ratios of PAYIGSR and PAKKKKK. YK 90 (90% PA YIGSR, 10% PA KKKKK), YK 75 (75% PA YIGSR, 25% PA KKKKK), YK 50 (50% PA YIGSR, 50% PA KKKKK), YK 25 (25% PA YIGSR, 75% PA KKKKK), YK 10 (10% PA YIGSR, 90% PA KKKKK) (*p<0.05 compared to all other YKs).
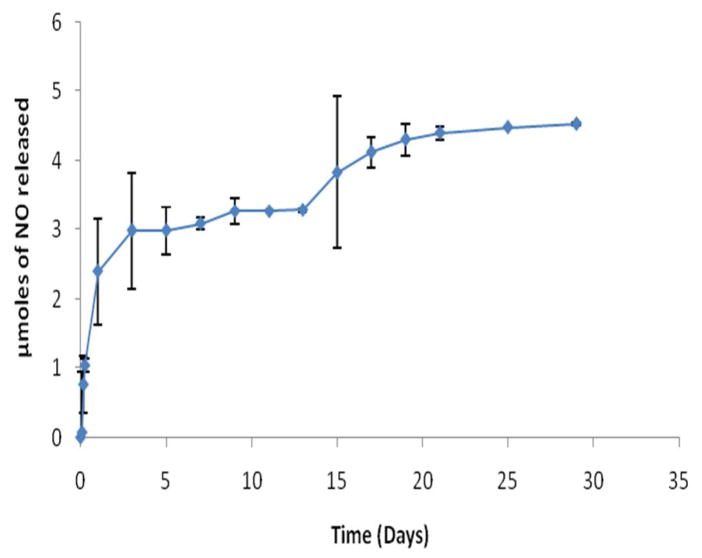
The NO release profile from the PA-YK-NO nanofibrous matrix was evaluated using the Greiss assay. Successful NO release was observed, occurring in a two-stage process. An initial burst release occurred in first 48 hours, followed by a slow sustained release over a period of one month that resulted in a 53% recovery of NO (Figure 4). The initial burst release could potentially be explained as NO release from the surface of the PA-YK-NO nanofibrous matrix that was easily accessible for local delivery. The subsequent sustained slow release may be attributed to NO release from the bulk of the nanofibrous matrix by a combination of diffusion and enzyme degradation. Over time, due to the presence of MMP-2 degradable sites, the nanofibrous matrix degrades slowly, and this is believed to aid in the sustained release of NO from the bulk of the matrix, locally dispensing NO as a concentration gradient. Furthermore, the release profile slightly increases after approximately 15 days, which may be due to the continued slow degradation of the nanofibrous matrix. Based on these results, if this PA-YK-NO nanofibrous matrix is self-assembled onto the surface of a metal stent with a surface area of 0.396 cm2 (circumference diameter 3mm, length 15 mm), the total amount of NO released into the blood stream over a month would be 0.32 μmoles. This amount is of the same order of magnitude as cumulative NO released by endothelial cells at a rate of 1×10−10mol cm−2 min−1 [22]. Moreover, the amount of NO has the potential to be easily tuned in future applications by changing the number of lysine moieties in PA-KKKKK. This is especially important when one considers that the proliferation of smooth muscle cells, which is one of the key events in restenosis, begins as early as one day after stent-induced injury [23]. Therefore, the 48 hour burst release of NO is critical to arrest neointimal hyperplasia. The following slow sustained release over a longer period is required to maintain the non-proliferative state of smooth muscle cells, anti-thrombogenicity of the vessel wall, and promote endothelialization during the recovery period that can take up to several weeks.
Figure 4.
NO release from PA-YK-NO films in HBS at pH 7.4, 37 °C. Data represent the mean of four samples. Error bar represents mean ± standard deviation.
Within an in vivo setting, it is believed that the NO will recruit surrounding endothelial cells and circulating satellite endothelial progenitor cells from the blood stream and surrounding tissues onto the nanofibrous matrix coated stents or grafts to promote re-endothelialization [20]. The presence of the endothelial cell adhesive ligand, YIGSR, is expected to promote their retention on the nanofibrous matrix against blood flow induced shear stress. NO release from the reservoir embedded in the nanofibrous matrix will stimulate endothelial cell proliferation and be maintained long-term due to slow the MMP-2 mediated degradation as triggered enzymatically by the local cells. The slow degradation will allow for a NO concentration gradient within the surrounding implant environment. Thus, all of these therapeutic factors provided by nanofibrous matrix will work in concert to produce an adhesive endothelial layer with similar characteristics to the native endothelium, further reducing the risk of restenosis and thrombosis as shown in Figure 1b.
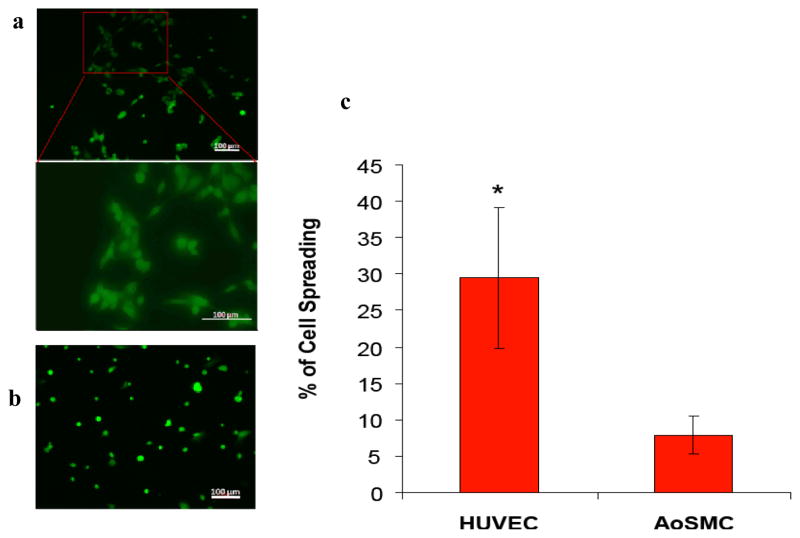
The effect of the incorporated YIGSR on cell adhesion and spreading was evaluated by seeding HUVECs and AoSMCs on PA-YK nanofibrous matrix coatings. A significant difference was found in the spreading behavior of HUVECs compared to AoSMCs on the PA-YK nanofibrous matrix after 2 hours (Figure 5). HUVECs were found to spread three fold more than AoSMCs. These different cellular behaviors clearly indicate that the endothelial cell adhesive ligand, YIGSR-containing nanofibrous matrix (PA-YK) supports endothelial cell, but not AoSMCs, adhesion and spreading.
Figure 5.
Fluorescent images of (a) HUVECs and (b) AoSMCs on PA-YK after 2 hours using Live/Dead assay. HUVECs attain their regular spread morphology within 2 hours. AoSMCs do not display any signs of spreading. (c) Initial spreading of HUVECs and AoSMCs on PA-YK nanofibrous matrix. HUVECs show significantly greater spreading than AoSMCs after 2 hrs (*p<0.05). Error bar represents means ± standard deviation for n=12.
To evaluate the effect of NO on proliferation, HUVECs and AoSMCs were seeded on PA-YK and PA-YK-NO nanomatrices, and their proliferation was evaluated after 48 hrs of incubation. As shown in Figure 6, the percentage of PCNA positive HUVECs on the PA-YK-NO nanofibrous matrix (67±2%) was found to be significantly greater compared to the PA-YK nanofibrous matrix (51 ± 3 %). However, the percentage of PCNA positive AoSMCs on the PA-YK-NO (16±3%) was significantly lower than on PA-YK (35±2%). These results indicate that the PA-YK-NO nanofibrous matrix enhances endothelial cell growth but limits smooth muscle cell growth, which is consistent with earlier studies [18, 19]. Enhancement of endothelialization, while preventing smooth muscle growth, is deemed to be a pivotal step towards preventing stent/graft failure due to restenosis and late thrombosis [24].
Figure 6.
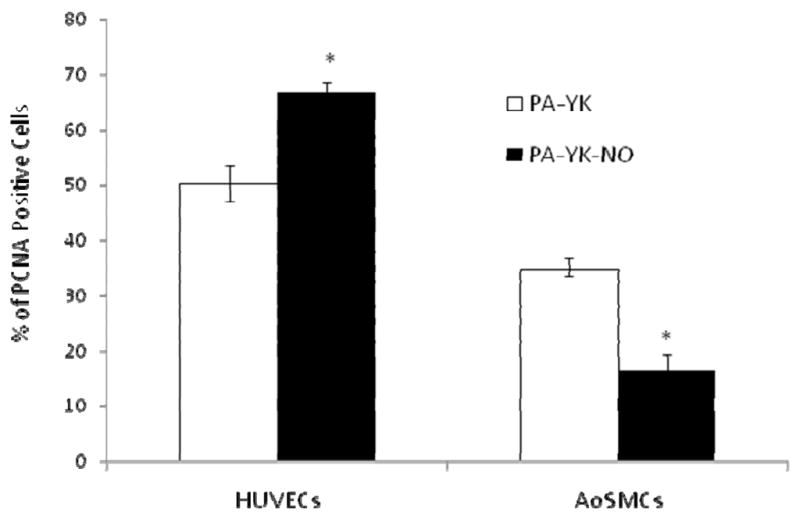
Proliferation of HUVECs and AoSMCs seeded on PA-YK and PA-YK-NO nanomatrices after 48 hours, quantitatively assessed by PCNA staining. Results are expressed as the percentage of PCNA positive cells. (*p < 0.05). Data represent the mean of four samples. Error bar represents mean ± standard deviation.
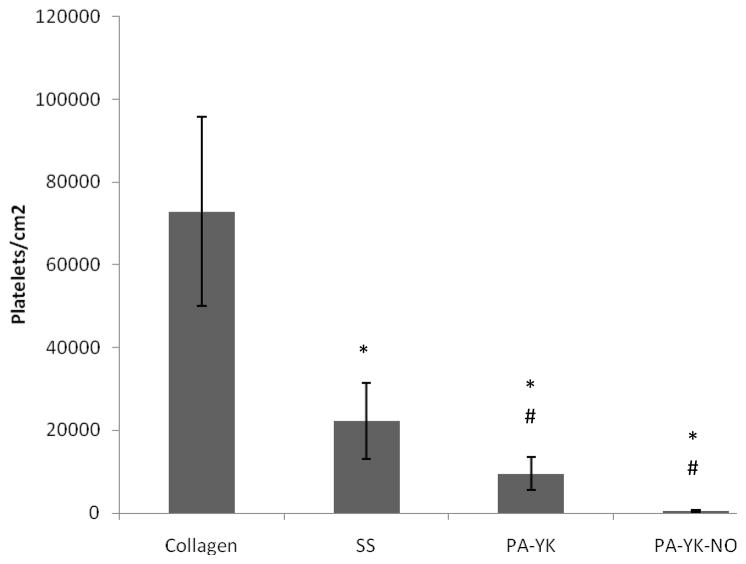
Activation and aggregation of platelets at the implant site has been implicated both in stent-restenosis and late thrombosis [2, 3, 25]. NO is known to be a natural potent anti-thrombogenic agent, serving in one of its many functions to prevent activation and aggregation of platelets in blood vessels [6, 7, 10]. Therefore, the thrombo-resistance of the nanofibrous matrix was evaluated by incubating with fluorescently labeled blood from a healthy human volunteer. As shown in figure 7, platelet adhesion was dramatically reduced on the PA-YK (9 ± 3 × 103 platelets/cm2) and the PA-YK-NO (470 ± 220 platelets/cm2) nanofibrous matrices compared to the controls, Collagen I (73 ± 22 × 103 platelets/cm2) and stainless steel (22 ± 9 × 103 platelets/cm2). Notably, there was a 150-fold decrease in platelet adhesion on PA-YK-NO as compared to that on Collagen-I. This result indicates that the PA-YK-NO nanofibrous matrix prevents platelet adhesion, which may contribute to limiting late thrombosis.
Figure 7.
Platelet adhesion on Collagen-I (Collagen), Stainless Steel (SS), PA-YK, and PA-YK-NO nanomatrices evaluated by incubating these surfaces with fluorescently labeled human blood for 90 minutes. Results are expressed as platelets adhered per unit area. Data represent the mean of four samples. Error bar represents mean ± standard deviation. (*: p < 0.05 compared to collagen, #: p<0.05 compared to SS).
Therefore, this nanofibrous matrix presents an attractive case for use as a biomimetic scaffold for coating cardiovascular implants. Current implants are limited by restenosis, thrombosis, and lack of endothelialization. NO is known to promote endothelialization, while simultaneously limiting smooth muscle cell proliferation and platelet activation and adhesion. Additionally, several other NO releasing materials have emerged recently and been shown to reduce platelet adhesion and intimal hyperplasia, both in vitro and in vivo. However, none of the above materials have so far been able to completely tackle all current clinical challenges, as they are limited by their inability to mimic the essential properties of native endothelium [10–12, 19, 26]. Native endothelium plays a critical role in controlling the function of the human cardiovascular system [5–7]. Thus, the approach of this study was to utilize bottom-up methodology to achieve a unique synergistic effect that combined multiple components, including endothelial cell-adhesive ligands (YIGSR), cytokine molecules (NO) retained by donor peptide moieties (KKKKK), enzyme-mediated degradation (MMP-2), and self-assembly into a nanofibrillar structure. All of these features of the nanofibrous matrix worked together to release NO in a controlled manner, while the cell adhesive ligands ensured the recruitment and adhesion of endothelial cells. NO also prevented smooth muscle cell proliferation, providing the scaffold with an anti-thrombotic character. It is therefore our belief that this nanofibrous matrix has great potential for application on implantable cardiovascular devices.
Conclusions
A native endothelial ECM mimicking nanofibrous scaffold was developed with tunable properties. This matrix consisted of two different self assembled peptide amphiphiles, containing either an endothelial cell adhesive ligand or polylysine NO donor. The peptide amphiphiles were mixed in a 9:1 molar ratio based on endothelial cell adhesion and reacted with pure NO under high pressure. This endothelial ECM mimicking nanofibrous matrix showed increased initial adhesion of endothelial cells due to the presence of endothelial cell specific ligands. The proliferation of endothelial cells was also increased by the nanofibrous matrix, while the proliferation of smooth muscle cells was limited. This property of the nanofibrous matrix is essential to promote re-endothelialization and the prevention of neointimal hyperplasia. Finally, the endothelial ECM mimicking nanofibrous matrix was found to significantly limit the adhesion of platelets. A vital characteristic needed to limit thrombosis, which plagues conventional cardiovascular implants. In summary, this nanofibrous matrix has great potential to be applied to various cardiovascular implants as a self-assembled coating to provide a native endothelium ECM mimicking environment that may limit restenosis and thrombosis, while enhancing re-endothelialization. Therefore, this study presents a multifunctional strategy to overcome many of the current challenges faced in the treatment of cardiovascular disease.
Acknowledgments
Authors gratefully acknowledge Melissa Chimento for TEM images. This project has been supported by Wallace H. Coulter Foundation (H.W.J), T32EB004312 from the NIBIB (J. A), Caroline P. Ireland Research Scholarship (M.K., A.A), and HL71189 and HL074391 from NIH (C.B., J.R.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Shuhaiber JH, Evans AN, Massad MG, Geha AS. Mechanisms and future directions for prevention of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg. 2002;22(3):387–396. doi: 10.1016/s1010-7940(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 3.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 5.Kuo PC, Schroeder RA. The emerging multifaceted roles of nitric oxide. Ann Surg. 1995;221(3):220–235. doi: 10.1097/00000658-199503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3′, 5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981;57(5):946–955. [PubMed] [Google Scholar]
- 8.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27(7):1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 9.Hanson SR, Hutsell TC, Keefer LK, Mooradian DL, Smith DJ. Nitric oxide donors: a continuing opportunity in drug design. Adv Pharmacol. 1995;34:383–398. doi: 10.1016/s1054-3589(08)61099-6. [DOI] [PubMed] [Google Scholar]
- 10.Kaul S, Makkar RR, Nakamura M, Litvack FI, Shah PK, Forrester JS, et al. Inhibition of acute stent thrombosis under high-shear flow conditions by a nitric oxide donor, DMHD/NO. An ex vivo porcine arteriovenous shunt study. Circulation. 1996;94(9):2228–2234. doi: 10.1161/01.cir.94.9.2228. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151(3):305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds MM, Frost MC, Meyerhoff ME. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radic Biol Med. 2004;37(7):926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Jun HW, Yuwono V, Paramonov SE, Hartgerink JD. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv Mater. 2005;17:2612–2617. [Google Scholar]
- 14.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G. In: Handbook of Proteolytic Enzymes. Barrett AJ, Rawlings ND, Woessner JF, editors. San Diego, CA: Academic; 1998. p. 1199. [Google Scholar]
- 16.Massia SP, Hubbell JA. Human endothelial cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and two polymeric biomaterials. J Biomed Mater Res. 1991;25(2):223–242. doi: 10.1002/jbm.820250209. [DOI] [PubMed] [Google Scholar]
- 17.Marin J, Rodriguez-Martinez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther. 1997;75(2):111–134. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- 18.Jun HW, Taite LJ, West JL. Nitric oxide-producing polyurethanes. Biomacromolecules. 2005;6(2):838–844. doi: 10.1021/bm049419y. [DOI] [PubMed] [Google Scholar]
- 19.Bohl KS, West JL. Nitric oxide-generating polymers reduce platelet adhesion and smooth muscle cell proliferation. Biomaterials. 2000;21(22):2273–2278. doi: 10.1016/s0142-9612(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 20.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 21.Paramonov SE, Jun HW, Hartgerink JD. Self-assembly of peptide-amphiphile nanofibers: the roles of hydrogen bonding and amphiphilic packing. J Am Chem Soc. 2006;128(22):7291–7298. doi: 10.1021/ja060573x. [DOI] [PubMed] [Google Scholar]
- 22.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274(6 Pt 2):H2163–2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100(5A):3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91(11):2793–2801. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekar B, Tanguay JF. Platelets and restenosis. J Am Coll Cardiol. 2000;35(3):555–562. doi: 10.1016/s0735-1097(99)00596-3. [DOI] [PubMed] [Google Scholar]
- 26.Lipke EA, West JL. Localized delivery of Nitric Oxide from hydrogels inhibits neointima formation in rat carotid balloon injury model. Acta Biomaterials. 2005;1(6):597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]