Abstract
Epidemiological, clinical and laboratory studies have implicated solar ultraviolet (UV) radiation in various skin diseases including premature aging of the skin and melanoma and nonmelanoma skin cancers. Chronic UV radiation exposure-induced skin diseases or skin disorders are caused by the excessive induction of inflammation, oxidative stress and DNA damage, etc.. The use of chemopreventive agents, such as plant polyphenols, to inhibit these events in UV-exposed skin is gaining attention. Chemoprevention refers to the use of agents that can inhibit, reverse, or retard the process of these harmful events in the UV-exposed skin. A wide variety of polyphenols or phytochemicals, most of which are dietary supplements, have been reported to possess substantial skin photoprotective effects. This review article summarizes the photoprotective effects of some selected polyphenols, such as green tea polyphenols, grape seed proanthocyanidins, resveratrol, silymarin and genistein, on UV-induced skin inflammation, oxidative stress, and DNA damage, etc., with a focus on mechanisms underlying the photoprotective effects of these polyphenols. The laboratory studies conducted in animal models, suggest that these polyphenols have the ability to protect the skin from the adverse effects of UV radiation, including the risk of skin cancers. It is suggested that polyphenols may favorably supplement sunscreens protection, and may be useful for skin diseases associated with solar UV radiation-induced inflammation, oxidative stress and DNA damage.
Keywords: Interleukin, DNA repair, antioxidant, anti-inflammation, polyphenols, ultraviolet radiation, cyclooxygenase-2
Introduction
Polyphenols are a large family of naturally occurring plant products that are widely distributed in plant foods, including fruits, vegetables, nuts, seeds, flowers and bark. Important dietary sources of polyphenols are onions (flavonols); cacao, grape seeds (proanthocyanidins); tea, apples, and red wine (flavonols and catechins); citrus fruits (flavanones); berries and cherries (anthocyanidins); and soy (isoflavones) [56]. These polyphenols contribute to the beneficial health effects of vegetables and fruits. A brief description of the important classes of polyphenols and their sources is provided in Table 1. As we are concentrating on the role of polyphenols in skin photoprotection, we will briefly discuss the effects of solar ultraviolet (UV) radiation on the skin, and the role of plant polyphenols in skin photoprotection.
Table 1.
A brief description of various plant polyphenols and their sources
| Classes of polyphenols | Source and description |
|---|---|
| Phenolic acids | Phenolic acids are simple molecules such as caffeic acid, and coumaric acid. Phenolic acids form a diverse group that includes the widely distributed hydroxybenzoic and hydroxycinnamic acids. Hydroxycinnamic acid compounds (p-coumaric, caffeic acid, ferulic acid) occur most frequently as simple esters with hydroxy carboxylic acids or glucose, while the hydroxybenzoic acid compounds (p-hydroxybenzoic, gallic acid, ellagic acid) are present mainly in the form of glucosides. Ellagic acid is found in pomegranates. Coffee is particularly rich in bound phenolic acids, such as caffeic acid, ferulic acid, and p-coumaric acid. Phenolic acids found in blueberries include gallic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid and vanillic acid. |
| Flavonoids | Flavonoids are a subclass of polyphenols and are widely distributed in nature. The polyphenolic structure of flavonoids and tannins renders them quite sensitive to oxidative enzymes. |
| Anthocyanins | Anthocyanins and anthocyanidins are a large group of water-soluble pigments found in a large number of fruits, vegetables and flowers, particularly grapes, grape seed extract, and berries. Bilberry and other berries have a high concentration of anthocyanins. |
| Catechins or flavanols | These are primarily found in tea leaves. Tea leaves and grape seeds have the monomeric flavan-3-ols catechin, epicatechin, gallocatechin, epigallocatechin, epicatechingallate and epigallocatechin-3-gallate. |
| Flavones | Apigenin, luteolin. The herb chamomile has a good amount of apigenin. |
| Flavonols | Flavonols are found at high concentrations in onions, apples, red wine, broccoli, tea, and Ginkgo-Biloba. The most common flavonols are quercetin, kaempferol, and myricetin. Flavonols also include fisetin, isoquercitrin and hyperoside. |
| Flavanones | Flavanones are hesperidin and naringin. |
| Isoflavones | Genistein and daidzein are found in soy. |
| Lignans | Lignans are found in nuts and whole grain cereals. Flaxseed has a high content of lignan. |
| Proanthocyanidins | These are found in grapes, red wine, and pine bark. Pycnogenol is a pine bark extract. Grape seed extract provides a concentrated source of polyphenols, many of which are proanthocyanidins. Red wine is rich in the complex polyphenols, the proanthocyanidins. Proanthocyanidins share common properties with other polyphenols, in particular their reducing capacity and ability to chelate metal ions. |
| Procyanidins | Oligomeric catechins are found at high concentrations in red wine, grapes and grape seeds, cocoa, cranberry, apples, and some supplements such as pycnogenol. Apples contain many kinds of polyphenols, and the main components are oligomeric procyanidins. |
| Stilbenes | Resveratrol is found in the skin of dark colored grapes. |
| Tannins | Tannins are found in red wine, tea, and nuts. They are large molecules. Many flavonoids in foods also occur as large molecules (tannins). These include condensed tannins (proanthocyanidins), derived tannins and hydrolysable tannins. |
Solar ultraviolet radiation and the skin
The skin is the largest organ of the body and comprises a surface area of approximately 1.5–2.0 m2 which protects the internal organs of the body by acting as an effective barrier against the detrimental effects of environmental and xenobiotic agents. Exposure to solar UV radiation is the key factor in the initiation of several skin disorders, such as wrinkling, scaling, dryness, mottled pigment abnormalities including hypopigmentation and hyperpigmentation, and skin cancer [13, 28, 71].
Although many environmental and genetic factors contribute to the development of various skin diseases, the most important factor is chronic exposure of the skin to solar UV radiation. The solar UV spectrum can be divided into three segments based on the wave lengths of the radiation: short-wave (UVC; 200–290 nm), mid-wave (UVB; 290–320 nm), and long-wave (UVA; 320–400 nm). Each spectrum has a characteristic limit of efficiency in penetrating the epidermal and dermal layers of human and murine skin. A brief detail is as follows:
UVC (200–280 nm) spectrum. UVC radiation is largely absorbed by the atmospheric ozone layer and normally does not reach the surface of the earth. These wavelengths have enormous energy and are mutagenic in nature. UVC radiation can penetrate the skin to a depth of approximately 60–80 micrometer, and can damage DNA molecules.
UVB (280–320 nm) spectrum. UVB radiation constitutes approximately 5% of the total solar UV radiation and is mainly responsible for a variety of skin diseases including nonmelanoma and melanoma skin cancers. UVB radiation can penetrate the skin to a depth of approximately 160–180 micrometer. It can cross the whole epidermis layer and penetrate the dermis compartment of human skin. UVB radiation can induce both direct and indirect adverse biologic effects including induction of oxidative stress, DNA damage, premature aging of the skin [13, 28, 71], and multiple effects on the immune system [51, and reviewed in 64, 74], which together play important roles in the generation and maintenance of UV-induced neoplasms [25, 43, 85]. UVB can act as a tumor initiator [50], tumor promoter [40] and co-carcinogen [17, 104]. Although skin possesses an elaborate defense system consisting of enzymatic and non-enzymatic components to protect the skin from these adverse biological effects, excessive exposure to UV radiation overwhelms and depletes the cutaneous defense system leading to the development of various skin disorders including skin cancer [34, 40, 43, 66]
UVA (320–400 nm) spectrum. UVA comprises the largest spectrum of solar UV radiation (90–95%) and is considered as the “aging ray”. UVA penetrates deeper into the epidermis and dermis of the skin. UVA can penetrate the skin to a depth of approximately 1000 micrometer. It has been shown that extensive UVA exposure can lead to benign tumor formation as well as malignant cancers [5, and reviewed in 92]. The exposure to UVA induces the generation of singlet oxygen and hydroxyl free radicals, which can cause damage to cellular macromolecules, such as proteins, lipids and DNA [16]. In contrast to UVC or UVB, UVA is barely able to excite the DNA molecule directly and produces only a small number of pyrimidine dimers in the skin; therefore, it is assumed that much of the mutagenic and carcinogenic action of UVA radiation is mediated through reactive oxygen species [14, 76]. This, however, is still a matter of debate. It has been suggested that bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation [18]. UVA is a significant source of oxidative stress in human skin, which causes photoaging in the form of skin sagging rather than wrinkling [53] and can suppress some immune functions [87].
There is ample clinical and experimental evidence to suggest that immune factors contribute to the pathogenesis of solar UV-induced skin cancer in mice and probably in humans as well [88, 101]. Chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high rate of skin cancer [48]. This observation is consistent with the hypothesis that immune surveillance is an important mechanism designed to prevent the generation and maintenance of neoplastic cells. Further, the incidence of skin cancers, especially squamous cell carcinoma, is also increased among organ transplant recipients [12, 20, 72]. The increased frequencies of squamous cell carcinoma, especially in transplant patients, are presumably attributable to a long-term immunosuppressive therapy [15], however nonimmune mechanisms may also play a role [24]. These studies provide evidence in support of the concept that UV-induced immune suppression promotes skin cancer risk.
Polyphenols and skin photoprotection
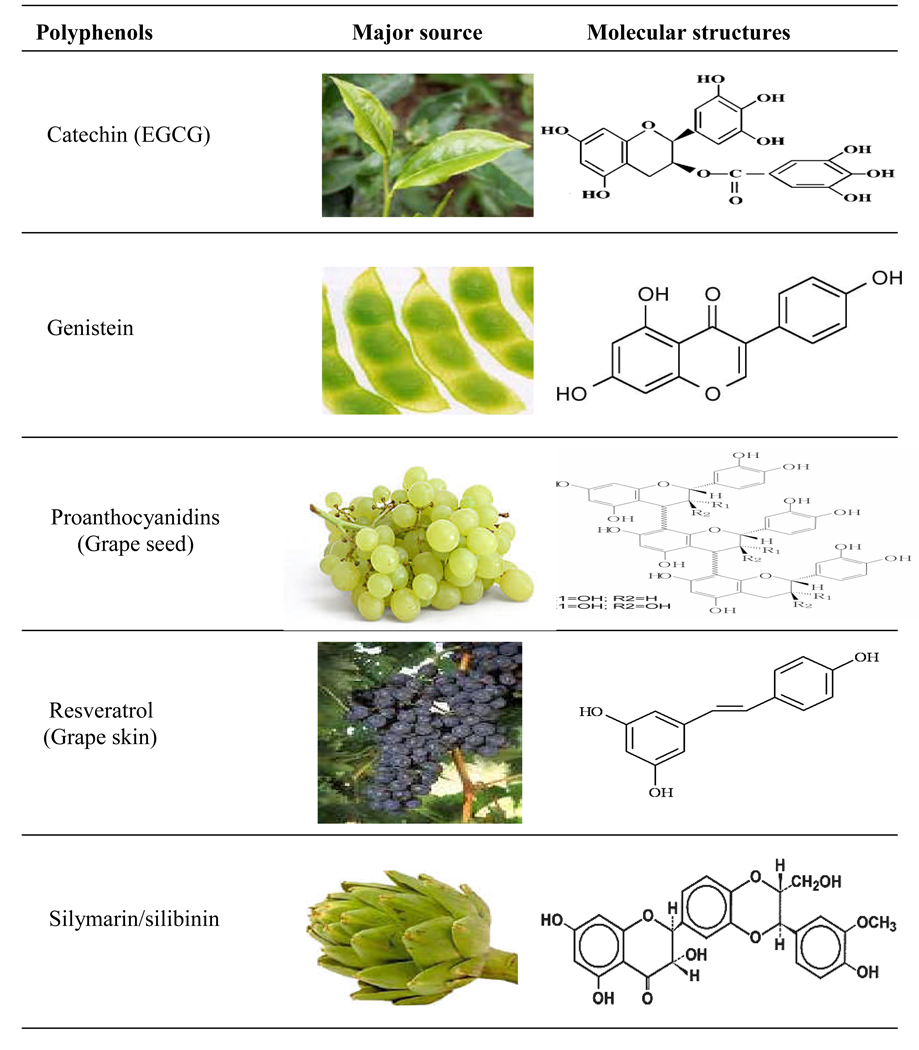
There has been considerable interest in the use of naturally occurring plant products, including polyphenols, for the prevention of UV-induced skin photodamage primarily including the risk of skin cancer. Polyphenols, specifically dietary, possessing anti-inflammatory, immunomodulatory and anti-oxidant properties are among the most promising group of compounds that can be exploited as ideal chemopreventive agents for a variety of skin disorders in general and skin cancer in particular. Recent advances in our understanding at the cellular and molecular levels of carcinogenesis have led to the development of promising strategies for the prevention of cancer or so called ‘chemoprevention’ strategy. Chemoprevention is a means of cancer control that is based on the use of specific natural or synthetic chemical substances that can suppress, retard or reverse the process of carcinogenesis. In this respect, chemoprevention offers a realistic strategy for controlling the risk of cancers. Furthermore, a chemopreventive approach appears to have practical implications in reducing skin cancer risk because, unlike the carcinogenic environmental factors that are difficult to control, individuals can modify their dietary habits and lifestyle in combination with a careful use of skin care products to prevent the photodamaging effects in the skin. Studies from our laboratory have shown the efficacy of naturally occurring polyphenols, such as green tea polyphenols (GTPs), silymarin from milk thistle and proanthocyanidins from grape seeds (GSPs), against UV radiation-induced inflammation, oxidative stress, DNA damage and suppression of immune responses. Here, we will briefly summarize and discuss the photoprotective potential of some polyphenols, such as polyphenols from green tea and grape seeds as these polyphenols have been the object of extensive in vitro and in vivo studies. The photoprotective role of other plant polyphenols such as silymarin, genistein, and resveratrol also will be discussed. A summary of molecular targets or mechanism of action of these selected polyphenols is given and their sources and molecular structures are described in Table 2 and Figure 1.
Table 2.
A summary of molecular targets or mechanism of action of some selected polyphenols in skin photoprotection
| Polyphenols | Source | Molecular targets/ Mechanisms |
References |
|---|---|---|---|
| Catechins | Tea leaves and buds | Inhibits H2O2, NO, iNOS, LPO, MPO | 6,19,33–35,38 |
| 41,43,45,47 | |||
| 67,90,91 | |||
| Inflammation, COX-2, PGs, IL | 19,37,39,41 | ||
| 43,59 | |||
| NF-κB, IKKα, AP-1, MAPK proteins, Enhance antioxidant defense enzymes |
6,33 | ||
| 36,38,44,45,99 | |||
| Inhibition of DNA damage | 11,46,59,61,70 | ||
| 96,102 | |||
| DNA repair mechanism | 61,63 | ||
| Proanthocyanidins | Grape seeds, nuts, bark |
Inflammation | 6 |
| Inhibition of H2O2, iNOS, LPO, MPO | 6,66,82 | ||
| NF-κB, IKKα, AP-1, MAPK proteins, Anti-oxidant defense enzymes |
57, 60,82 | ||
| 57,82 | |||
| Resveratrol | Grape skin, peanuts, red wine, & mulberries |
Inhibition of inflammation, H2O2, LPO, COX-2, PGs |
1–3 |
| 2 | |||
| NF-κB, IKKα, MAPK proteins | 1,2,6 | ||
| Silymarin | Milk thistle | Inhibits H2O2, LPO, NO, iNOS, MPO | 31,32 |
| Inflammation, COX-2, PGs, PCNA, NF-κB, IKKα, AP-1, MAPK proteins |
40 | ||
| 21 | |||
| Cell cycle proteins | 21 |
Figure 1. Nichols and Katiyar.
Polyphenols: their sources and molecular structures
Inhibition of photocarcinogenesis
Nonmelanoma skin cancers, including basal cell and squamous cell carcinomas, represent the most common malignant neoplasms in humans [65, 84, 88]. Epidemiological, clinical and biological studies have indicated that solar UV radiation is the major etiological agent in the development of skin cancers [7, 65, 81, 84]. Various animal models have been employed to examine the anti-photocarcinogenic effects of phytochemicals, like polyphenols. Following standard photocarcinogenesis protocols, it has been found that oral administration of GTPs (a mixture of green tea polyphenols or catechins) in drinking water of mice resulted in significant protection against skin tumorigenesis in terms of tumor incidence, tumor multiplicity and tumor size per group compared to non-GTPs-treated animals [reviewed in 36, 38, 44, 45, 99]. A water extract of green tea leaves, which primarily contained a mixture of polyphenolic ingredients, when provided as the sole source of drinking water to mice afforded protection against UVB radiation-induced tumorigenesis [94], and also promoted partial regression of established skin papillomas in mice [95]. Topical treatment of SKH-1 hairless mouse skin with GTPs or (−)-epigallocatechin-3-gallate (EGCG) in a hydrophilic ointment significantly inhibited UVB-induced skin tumor development [67]. Dietary grape seed proanthocyanidins (GSPs, 0.2 and 0.5%, w/w) supplementation of a control AIN76A diet inhibited photocarcinogenesis in SKH-1 hairless mice in terms of tumor incidence (% mice with tumors), tumor multiplicity and tumor size [66]. Dietary GSPs also resulted in prevention of malignant progression of UVB-induced papillomas to carcinomas as compared to the malignant progression observed in non-GSPs-treated UVB-exposed control mice [66]. Resveratrol is found in the skin of colored grapes, peanuts, red wine and mulberries. Topical application of resveratrol inhibits UVB-induced skin tumor initiation, promotion and progression [4, 29]. Silymarin, a flavonoid obtained from milk thistle, also has been shown to have anti-photocarcinogenic activity in laboratory animals. We [40] have shown that topical application of silymarin to SKH-1 hairless mice inhibited UVB-induced skin tumor development in terms of tumor incidence, tumor multiplicity and growth of the tumors. Silibinin, which is a major component of silymarin, has been shown to inhibit photocarcinogenesis in mice when applied topically or in the diet [21, 22]. As multiple in vivo animal studies suggest that plant polyphenols possess anti-photocarcinogenic activity, we will briefly summarize and discuss the molecular targets or mechanisms of action of these selected polyphenols against photocarcinogenesis.
Mechanism of Action and Molecular Targets of Polyphenols
Sunscreen effects
Most of the natural polyphenols are pigments, typically yellow, red or purple, and can absorb UV radiation. Therefore, when applied topically, they can prevent penetration of the radiation into the skin. The radiation that polyphenols can absorb includes the entire UVB spectrum of wavelengths and part of the UVC and UVA spectra. Thus polyphenols may act as a sunscreen. This ability of natural polyphenols to act as sunscreens can reduce inflammation, oxidative stress and DNA damaging effects of UV radiation in the skin and, thus, on topical application the photoprotective effects of polyphenols are due in part to this sunscreen effect.
Anti-inflammatory effects
UV radiation-induced erythema, edema and hyperplastic epithelial responses are considered as inflammatory markers, and play crucial roles in skin tumor promotion [reviewed in 71]. UVB-induced cyclooxygenase-2 (COX-2) expression and a subsequent increase in the production of prostaglandin (PG) metabolites in the skin is a characteristic response of keratinocytes to acute or chronic exposure to UVB radiation. COX-2 is a rate-limiting enzyme for the generation of PG metabolites from arachidonic acid [54], and COX-2 expression has been linked to the pathophysiology of inflammation and cancer [10]. A number of studies have demonstrated overexpression of COX-2 in chronically UVB-irradiated skin, as well as in UVB-induced premalignant lesions and squamous- and basal-cell carcinomas of the skin [8, 89]. Mechanistic studies of photocarcinogenesis have revealed that oral administration of GTPs (through addition to the drinking water) to SKH-1 hairless mice resulted in significant inhibition of UV radiation-induced cutaneous edema, erythema, and bi-fold skin thickness (a biomarker of inflammation). Treatment with GTPs also inhibits UVB-induced expression of COX-2 and its prostaglandin metabolites, which have been implicated in skin carcinogenesis and play a role in promoting tumors in the skin [59]. Topical treatment with GTPs prior to UV exposure reduced the UV-induced hyperplastic response, myeloperoxidase activity and the numbers of infiltrating inflammatory leukocytes in the skin [37, 39, 43]. The relevance of the extensive in vitro and in vivo data that have been generated using animal models to the photoprotective effects of GTPs in human skin is not yet clearly understood. We have found, however, that topical application of GTPs prior to UV irradiation of the un-tanned backs of humans resulted in significantly less development of erythema as compared to the UV-irradiated skin that was not treated with GTPs [19, 41]. We also found that topical treatment of human skin with GTPs or EGCG (<1mg/cm2 skin area) prior to UVB exposure significantly reduced UVB-induced infiltration of inflammatory leukocytes and myeloperoxidase activity [41], which is used as a marker of tissue infiltration. Topical application of EGCG also resulted in inhibition of UVB-induced production of prostaglandin metabolites, including PGE2, PGF2α and PGD2, which play a critical role in inflammatory disorders and in proliferative skin diseases [41]. Exposure of the skin to UV radiation is known to enhance the levels of proinflammatory cytokines. As the elevated levels of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, contribute to the tumor promotion process, this effect would be expected to result in an earlier occurrence of tumors and more rapid progression [reviewed in 71]. The administration of GTPs in the drinking water of mice significantly reduced the levels of these proinflammatory cytokines in UVB-irradiated skin [59]. The GTPs also reduced the levels of biomarkers of cellular proliferation in the UV-irradiated skin, including proliferating cell nuclear antigen (PCNA) and cyclin D1. The inhibitory effects of GTPs on these biomarkers of inflammation in UV-exposed skin provide further mechanistic evidence of the anti-carcinogenic effects of GTPs. Jeon et al. have examined the effects of dietary EGCG (1500 ppm in control diet) on UVB-induced inflammation in hairless mice [30]. They observed that regular intake of EGCG strengthens the skin’s tolerance and appears to do so by increasing the minimal dose of radiation required to induce erythema thereby inhibiting the UV-induced perturbation of epidermal barrier function and skin damage. Zhao et al. [102] demonstrated that oral administration of green tea extract prior to and during multiple treatments with psoralen plus UVA reduced hyperplasia, hyperkeratosis, erythema and edema formation in murine skin. Treatment of EpiDerm, a reconstituted human skin equivalent, with a green tea extract also has been shown to inhibit the 8-methoxypsoralen-DNA adduct formation and p53 protein accumulation associated with exposure to psoralen plus UVA irradiation [102]. Mnich et al. [68] found that topical treatment of human skin with green tea extract reduced UV-induced p53 expression and the number of apoptotic keratinocytes, suggesting that green tea extract ma y b e a suitable everyday photochemopreventive agent. These in vivo observations that have been generated using both animal and human systems provide insights into the possible protective mechanisms involved in the anti-inflammatory effects of green tea polyphenols.
The in vivo effects of other polyphenols like resveratrol, grape seed proanthocyanidins and silymarin also have been examined using animal models [reviewed in 6]. As was found for the GTPs and EGCG, topical treatment or dietary intake of the GSPs and/or silymarin inhibited UVB radiation-induced edema, erythema, infiltration of inflammatory leukocytes and myeloperoxidase activity in the mouse skin [21, 22, 40, 66]. Silymarin has been shown to inhibit UVB-induced COX-2 expression and subsequently the production of PG metabolites, which are considered to be tumor promoters in the skin. Silymarin also has been shown to inhibit the expression of ornithine decarboxylase, an enzyme required for polyamine biosynthesis, which has a role in tumor promotion in UVB-exposed skin [40]. Topical application of resveratrol prior to UVB irradiation resulted in significant inhibition of UVB-induced increases in bi-fold skin thickness (a marker of edema development), hyperplastic response, leukocyte infiltration, and COX-2 and ornithine decarboxylase activity in SKH-1 hairless mouse skin [2, 3]. Collectively, the results concerning the inhibitory effects of these polyphenols on UVB-induced inflammatory responses revealed that anti-photocarcinogenic effects of polyphenols are mediated in part through their anti-inflammatory effects.
Anti-oxidant effects
The skin possesses an elaborate antioxidant defense system to deal with UV-induced oxidative stress; however, excessive and chronic exposure to UV radiation can overwhelm the cutaneous antioxidant capacity, leading to oxidative stress and oxidative damage which may result in skin disorders, immunosuppression, premature aging of the skin and development of melanoma and non-melanoma skin cancers. GTPs have been shown to inhibit photo-enhanced lipid peroxidation [35]. Topical treatment of the mouse and human skin with EGCG prior to UV exposure significantly reduced UVB-induced nitric oxide and hydrogen peroxide production, as well as leukocyte infiltration [19, 41, 43]. It is well established that the infiltrating leukocytes are the major source of nitric oxide and hydrogen peroxide production, which create the state of oxidative stress. EGCG has been shown to have the ability to block UVB-induced leukocyte infiltration in mouse as well as in human skin, and thus may be able to inhibit UVB-induced production of reactive oxygen species by these infiltrating leukocytes [34, 37, 41, 43]. Although reactive oxygen species help the host to destroy invading microorganisms [49], excessive and uncontrolled production can also damage host tissues and predispose it to various disease states [23, 49]. Thus, the application of EGCG may prove beneficial in ameliorating the harmful effects caused by UVB radiation through its ability to reduce the generation of reactive oxygen species. Treatment with EGCG also has been shown to result in a reduction in the numbers of hydrogen peroxide producing and inducible nitric oxide synthase expressing cells, as well as a reduction in the production of hydrogen peroxide and nitric oxide both in the epidermis and dermis of UVB-irradiated skin sites [43]. Similar effects also have been observed in human skin when EGCG was applied topically before exposure to UVB (4x minimal erythema dose) [34]. This EGCG treatment also inhibited UV-induced epidermal lipid peroxidation and protected the antioxidant defense enzymes in the UVB-exposed human skin [34]. Based on the evidence of the photoprotective effects of GTPs/EGCG in animal and human systems, it appears that both GTPs and EGCG can induce preventive effects by acting at different active sites within the cascade of events that generates reactive oxygen species. Kim et al. [47] observed that EGCG treatment of the skin of guinea pigs inhibits UVB-induced lipid peroxidation and the erythema response. They also found that EGCG treatment of human fibroblasts in culture blocked the UV-induced increase in collagen secretion and collagenase mRNA levels, and also inhibited the binding activities of the UV-induced nuclear transcription factors nuclear factor-kappaB (NF-κB) and activated protein (AP)-1 [47]. Wei et al. [98] have demonstrated that aqueous extracts of green tea have potent scavenging effects on oxygen species and block UV-induced oxidative DNA damage in the calf thymus, which may, at least in part, explain the mechanisms by which green tea inhibits photocarcinogenesis. Collectively, these data suggest that green tea may have the potential to reduce the risk of UV-induced oxidative stress-mediated skin diseases or disorders in humans, including premature aging of the skin and development of cutaneous malignancies.
Oxidation of some amino acid residues, such as lysine, arginine and proline, leads to the formation of carbonyl derivatives that affect the nature and function of the proteins [83]. The presence of carbonyl groups in proteins has become a widely accepted measure of oxidative damage of proteins under conditions of oxidative stress. Multiple exposures of the skin to UV radiation results in a several-fold increase in the levels of protein carbonyls in comparison to non-UV exposed skin. In separate experiments, it has been shown that topical treatment with EGCG, GTPs or GSPs significantly inhibits acute or chronic UV irradiation-induced protein oxidation in the skin of mice [82, 91]. The inhibition of UVB-induced protein oxidation by green tea polyphenols or proanthocyanidins could result in a reduction in skin photodamage and, more specifically, may prevent premature aging of the skin.
Treatment of normal human epidermal keratinocytes with EGCG in vitro was found to inhibit UVB-induced intracellular release of hydrogen peroxide concomitantly with the inhibition of UVB-induced oxidative stress-mediated phosphorylation of epidermal growth factor receptor and mitogen-activated protein kinases signaling pathways [33]. Similar effects also were observed when HaCaT cells were treated with (−)-epicatechin-3-gallate (ECG) and exposed to UVB radiation. These in vitro studies suggest that ECG can act as a free radical scavenger when keratinocytes are photodamaged [26, 27]. The treatment of HaCaT cells with ECG also demonstrated its free radical scavenging effects when cells were irradiated with UVA radiation. These observations indicate that EGCG could play an important role in the attenuation of oxidative stress-mediated cellular signaling responses, which are essential factors in various skin diseases in humans. Topical cream-based formulations of EGCG or GTPs for human use have been developed, and their photoprotective effects evaluated in vivo using an animal model. An exceptionally high photoprotective effect of EGCG or GTPs was observed against UV radiation-induced oxidative stress in the mouse skin when evaluated in terms of lipid peroxidation, hydrogen peroxide production and analysis of anti-oxidant defense enzymes [67, 90]. Topical treatment of EGCG or oral administration of GTPs in the drinking water of mice also has been shown to inhibit UVB radiation-induced depletion of antioxidant defense enzymes, such as catalase, glutathione peroxidase, superoxide dismutase and the levels of glutathione [90]. A study has been conducted in an attempt to determine whether the sunscreen-containing green tea extracts protect human subjects from UV irradiation-induced photoaging and photoimmunosuppression [55]. The investigators reported that a sunscreen containing different concentrations of green tea extracts conferred significant protection against biological events associated with photoaging (MMP-2, MMP-9) and photoimmunology (CD1a+ Langerhans cells). Similar to green tea, chemopreventive effects also were noted when mice were given a GSPs-supplemented AIN76A diet. The provision of dietary GSPs (0.2 and 0.5%, w/w) to mice exposed to either acute or chronic UVB irradiation was found to inhibit depletion of glutathione peroxidase, catalase, and glutathione, and to inhibit UVB-induced hydrogen peroxide, lipid peroxidation, protein oxidation and nitric oxide, in mouse skin [82]. As UVB-induced oxidative stress mediates activation of mitogen-activated protein kinases (MAPK) and NF-κB signaling pathways, the effects of GSPs in vivo in the same animal model on these pathways also were examined. It was observed that the treatment with GSPs inhibited UVB-induced phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun-N-terminal-kinase and p38 proteins of the MAPK family, which seemed to be mediated through reactivation of MAPK phosphatases [82]. It has been shown that the GSPs can inhibit the UVB-induced activation of NF-κB through inhibition of degradation of IκBα and activation of IκB kinase α. Using an identical mouse model, it has been further demonstrated that dietary administration of GSPs resulted in inhibition of the expression of PCNA, cyclin D1, inducible nitric oxide synthase (iNOS) and COX-2 in the skin, which are NF-κB-targeted proteins. Similar studies were conducted in vitro using normal human epidermal keratinocytes with and without treatment with GSPs and UVB irradiation. The results were identical to those obtained in the mouse model, which suggests that results generated in this animal model of the photoprotective effects of GSPs can be extrapolated to the human system [57]. Study also has been conducted to determine the effects of oligomeric proanthocyanidins on UV-induced melanogenesis of human melanocytes in vitro. The results of this study suggested that oligomeric proanthocyanidins have potential photoprotective effects on human melanocytes including scavenging of intracellular reactive oxygen species and adjustment of cell cycle check points [103]. In an in vitro cell culture model, treatment of human epidermoid carcinoma A431 cells with GSPs resulted in inhibition of cell proliferation and induction of apoptotic cell death. This effect of GSPs was associated with the inhibition of constitutive expression of NF-κB/p65 and its targeted genes, such as COX-2, iNOS, PCNA, cyclin D1 and matrix metalloproteinase (MMP)-9 [60]. These observations provide a molecular basis for the photoprotective effects of GSPs and GTPs in an in vivo model. Studies conducted by the authors have shown that topical treatment of SKH-1 hairless mouse skin with silymarin resulted in inhibition of UVB-induced intracellular production of H2O2 in both the epidermis and dermis when analyzed by immunohistochemistry and biochemical analytical procedures and compared with the results obtained using non-silymarin-treated control mice [31]. In these experiments, it was found that the significant inhibition of the UVB-induced oxidative stress was associated with significant inhibition of UV-induced infiltration of activated macrophages and neutrophils. Treatment with silymarin also inhibits UVB-induced expression of inducible nitric oxide synthase and subsequently nitric oxide production [31, and reviewed in 32]. Resveratrol is also a potential polyphenolic antioxidant. Pretreatment of human epidermal keratinocytes with resveratrol inhibited UVB-mediated activation of the NF-κB pathway [1, 2]. In SKH-1 hairless mice, topical application of resveratrol inhibited UVB-induced inflammatory responses and hydrogen peroxide production, which is a stable source of oxidative stress, in the skin [3]. Inhibition of these critical events by resveratrol may have contributed to the prevention of UV radiation-induced skin cancer in these mice. Park and Lee [73] have demonstrated that treatment of HaCaT cells with resveratrol before UVB irradiation resulted in an increase in cell survival of UVB-irradiated cells which was associated with the reduction of reactive oxygen species production. Additionally, the activation of caspase-3 and -8 was partially reduced in the resveratrol-pretreated HaCaT cells, implying that the attenuation of caspase-3 and -8 activation is involved in cell survival after UVB irradiation. Soybeans are a rich source of the isoflavones, genistein and daidzein, and are photoprotective [reviewed in 97]. Studies using SENCAR mice have shown that topical genistein treatment reduced UV radiation-induced activation of c-fos and c-jun in a dose-dependent manner [93]. Genistein also has been shown to reduce UV radiation-induced oxidative and photodynamic DNA damage [69]. Treatment of the human keratinocyte cell line NCTC 2544 with genistein prevented UV-induced enhancement of the DNA-binding activity of the signal transducer and activator of transcription-1 by acting as a tyrosine kinase inhibitor, thus limiting lipid peroxidation and increases in reactive oxygen species generation [58].
To further illustrate the role of polyphenols in dermatologic conditions as well as in skin photoprotection, we are providing an in-depth review of the studies on the effects of polyphenols from green tea.
Green tea polyphenols rapidly remove or repair UVB-induced DNA damage
UV-induced DNA damage in skin cells is an important initiator of signaling pathways. The DNA photoproducts generated by UV-induced DNA damage are altered DNA structures that activate a cascade of responses, beginning with the initiation of cell cycle arrest and activation of DNA repair mechanisms. The biologically harmful effects associated with UV radiation exposure are largely the result of errors in DNA repair, which can lead to oncogenic mutations [reviewed in 86]. UV-induced DNA damage in the form of cyclobutane pyrimidine dimers (CPD) is considered as a molecular trigger for the induction of immunosuppression and initiation of photocarcinogenesis [52, 100]. Several studies have documented that exposure of the skin to UV radiation results in immediate formation of CPDs in skin cells [42]. Most of the UVB-induced CPDs were found in the epidermis, but some were detected in the dermis. The location of the damage depends on the ability of the UV radiation to penetrate the skin [42]. It has been found that UV exposure of less than one minimal erythema dose is sufficient to cause damage DNA in target cells of human skin [42].
UVB-induced CPDs are formed immediately after the interaction of photons with the DNA molecule. In an in vitro study using cultured human cells (lung fibroblasts, skin fibroblasts, and epidermal keratinocytes), EGCG resulted in a dose-dependent reduction in UV-induced DNA damage in all three cell types [70]. When applied topically to the mouse skin, GTPs (a mixture of green tea polyphenols) significantly inhibited UVB-induced DNA damage as assessed using a 32P-postlabelling technique [11]. Topical treatment of human skin with GTPs prior to UV exposure resulted in a dose-dependent inhibition of formation of CPDs [46]. Camouse et al. [9] found that topical application of green tea or white tea extracts provided human skin protection from solar-simulated ultraviolet light. These tea extracts were shown to provide protection against the detrimental effects of UV light on cutaneous immunity. The investigators concluded that these protective effects were not due to direct UV absorption or sunscreen effects as both products had a sun protection factor of 1.
Extensive studies of the effects of polyphenols, particularly green tea polyphenols, on the repair kinetics and repair mechanisms of UV-induced CPDs have been carried out in the laboratory of Dr. Katiyar. One of these studies showed that topical treatment of skin with EGCG does not prevent UVB-induced formation of CPDs immediately after UVB irradiation, which indicated that EGCG does not have a significant filtering effect on the UVB radiation. However; in skin samples obtained at 24 hours or 48 hours after UVB exposure, the numbers of CPD-positive cells were significantly reduced (or repaired) in the EGCG-treated C3H/HeN mouse skin as compared to the control group of mice which were not treated with EGCG [61]. Studies of the DNA repair mechanisms suggested that the rapid repair of UV-induced CPDs by EGCG was mediated through stimulation of a cytokine (IL-12) on application of the EGCG onto the mouse skin [61]. IL-12 has been shown to have the capacity to induce DNA repair [62, 79, 80] and this concept was confirmed by testing the effect of EGCG on UV-induced CPD formation in IL-12 knockout mice. EGCG does not remove or repair UV-induced CPDs in the skin of IL-12 knockout mice, further confirming the role of IL-12 in rapid repair of DNA damage by this polyphenol [61]. Studies of the effects of oral administration of GTPs in the drinking water of mice on UVB-induced DNA damage also were carried out and it was found that UV-induced DNA damage (CPDs) was resolved rapidly in the GTPs-treated mice when compared to GTPsuntreated mice [59]. This DNA repairing effect of GTPs was less pronounced in IL-12 knockout mice, as was observed in the case of EGCG treatment. Schwarz et al. [78] observed that treatment of normal human keratinocytes and “human skin equivalent” with GTPs reduced UVB-induced DNA damage and that this effect was mediated through the induction of IL-12. Collectively, these data suggest that the difference in the GTPs-associated DNA repair capacity between IL-12 knockout mice and their wild-type counterparts may be due to the absence of IL-12 in the IL-12 knockout mice. The mechanisms by which GTPs repaired CPDs were identical to the mechanisms by which EGCG repaired CPDs.
Wei et al. [96] have shown that an aqueous extract of green tea scavenges H2O2 and inhibits UV-induced oxidative DNA damage in an in vitro system. Zhao et al. [102] demonstrated that application of green tea extract to Epiderm, a reconstituted human skin equivalent, also inhibited psoralen-UVA-induced formation of 8-methoxypsoralen-DNA adducts [102]. Treatment of skin with a 5% green tea extract significantly inhibited DNA damage induced by solar simulator radiation when assessed using a 32P-postlabeling technique [11]. These observations demonstrate the potential chemopreventive effects of green tea polyphenols against UVB-induced DNA damage.
Repair of UV-induced DNA damage by green tea polyphenols is mediated through nucleotide excision repair (NER) mechanism
Further studies have been conducted to verify the green tea polyphenol-associated DNA repair mechanisms in UVB-irradiated skin. Meeran et al. postulated that an NER mechanism is involved in the repair of photodamaged DNA by green tea polyphenols, and that IL-12 has a role in this process [61, 63]. To determine whether the NER mechanism is required for the EGCG-induced IL-12-mediated repair of UVB-induced CPDs, NER-deficient fibroblasts from xeroderma pigmentosum complementation group A (XPA) patients and NER-proficient fibroblasts from a healthy person (XPA-proficient) were exposed to UVB with or without prior treatment with EGCG. The CPD-positive cells were detected by immunostaining at different time points after UVB exposure of the cells. It was observed that the numbers of CPD-positive cells were significantly lower at 24 hours after UVB exposure in the XPA-proficient cells, but that treatment with EGCG did not significantly remove or repair UVB-induced CPDs in NER-deficient cells. This observation indicated that EGCG-induced DNA repair is mediated through a functional NER mechanism.
Repair of UVB-induced DNA damage by green tea polyphenols leads to a reduction in UVB-induced inflammation in the skin
Exposure of the skin to UV radiation induces inflammation, and there is increasing evidence that chronic inflammation promotes the initiation of various skin diseases, including the development of skin cancers [16, and reviewed in 71]. Both UV-induced inflammatory responses and UV-induced skin tumorigenesis are causally related to UV-induced DNA damage. Therefore, it was of interest to explore the effects of green tea polyphenols on DNA repair and their relationship with inflammatory effects. CPDs are formed immediately after the exposure of the skin to UV radiation, and inflammation develops thereafter. Following UV exposure, it was observed that UV-induced DNA damage in the form of CPDs was repaired or removed more rapidly in the skin of mice that had been treated either with topical application of EGCG or orally administered GTPs. Subsequently, the levels of UVB-induced inflammation was lower in the treated mice than the non-treated mice with the levels of inflammation in the mouse skin from the different treatment groups being assessed through analysis of biomarkers of inflammation, such as COX-2 expression, PGE2 production and the levels of pro-inflammatory cytokines. Interestingly, this effect of EGCG or GTPs was not observed in IL-12-deficient or knockout mice. This may be due to the fact that the treatment with EGCG or GTPs was not able to repair UV-induced DNA damage significantly in the IL-12 knockout mice, as detailed [59]. This new information supports the concept that UV-induced DNA damage and inflammatory responses are causally related with the increased risk of photocarcinogenesis. This in vivo experimental evidence indicates that the prevention of UVB-induced skin cancer by GTPs or EGCG is mediated through inhibition of UVB-induced inflammation, which in turn is mediated, at least in part, through rapid repair of damaged DNA. The outcome of this study therefore suggests that regular consumption of green tea or green tea polyphenols may be considered as an effective strategy for the prevention of inflammation-associated skin diseases including skin cancers.
Bioavailability and metabolism of polyphenols
The bioavailability and metabolism of polyphenols may influence their effectiveness. The considerable structural diversity among the polyphenols can influence the bioavailability of the individual components. Small molecules, like catechin monomers, can be easily absorbed through the gut barrier, whereas the large molecular weight polyphenols, such as proanthocyanidins and even (−)-epigallocatechin-3-gallate, are poorly absorbed. Once absorbed, polyphenols are conjugated to glucuronide, sulphate and methyl groups in the gut mucosa and inner tissues. Non-conjugated polyphenols are virtually absent in plasma. Such reactions facilitate their excretion and limit their potential toxicity, if any [77]. During digestion in the intestine, the large polyphenolic molecules break into multiple small molecules or metabolites and these may systemically induce beneficial effects in the body. Polymeric proanthocyanidins are not absorbed as such in the gut. Detection of proanthocyanidin dimers B1 and B2 in human plasma indicated that the absorption of these dimers was ~100-fold lower than that of the monomeric flavanols. In the case of topical delivery of the polyphenols, the penetration of polyphenols into the skin is limited and successful delivery of plant polyphenols requires cream-based, organic solvent-based or lipid soluble topical formulations that can enhance the penetration of the polyphenols.
Conclusion
The polyphenols discussed in this review article show significant anti-inflammatory, anti-oxidant and anti-DNA damaging effects. These protective effects of polyphenols may contribute to their anti-photocarcinogenic effects and act to abrogate the various biochemical processes induced or mediated by solar UV radiation. Based on the epidemiological evidence and laboratory studies conducted using in vitro and in vivo systems, it is suggested that routine consumption or topical treatment of these polyphenols may provide efficient protection against the harmful effects of solar ultraviolet radiation in humans. For appropriate conversion of drug or chemopreventive agent doses from animal studies to human studies, the body surface area normalization method has been prescribed [reviewed in 75]. Based on this reference, the human equivalent dose (HED) of any chemopreventive agent can be calculated using the following formula:
(Km factor for mouse = 3; Km factor for adult human = 37).
Further, the use of polyphenols in combination with sunscreens or skin care lotions may provide an effective strategy for mitigating the effects of UV radiation that will lead to the protection of the skin from various skin diseases caused by excessive sun exposures.
Acknowledgments
The work reported from Dr. Katiyar’s laboratory was supported by the funds from National Institutes of Health (CA104428, AT002536) and Veteran Affairs Merit Review Award (SKK). The content of this article does not necessarily reflect the views or policies of the funding sources. Grateful thanks are also due to our former and current colleagues and postdoctoral fellows for their outstanding contributions.
Abbreviations used
- COX-2
cyclooxygenase-2
- EGCG
epigallocatechin-3-gallate
- GSPs
grape seed proanthocyanidins
- GTPs
green tea polyphenols
- IL
interleukin
- NER
nucleotide excision repair
- NFκB
nuclear factor-kappaB
- UV
ultraviolet
References
- 1.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 3.Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet B radiation - damage by resveratrol in mouse skin is mediated via modulation in Survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 4.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 5.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 7.Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckman SY, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 9.Camouse MM, Domingo DS, Swain FR, et al. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp Dermatol. 2009;18:522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapple KS, Cartwright EJ, Hawcroft G, et al. Localization of cyclooxygenase-2 in human sporadic colorectal adenomas. Am J Pathol. 2000;156:545–553. doi: 10.1016/S0002-9440(10)64759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee ML, Agarwal R, Mukhtar H. Ultraviolet B radiation-induced DNA lesions in mouse epidermis: an assessment using a novel 32P-postlabelling technique. Biochem Biophys Res Comm. 1996;229:590–595. doi: 10.1006/bbrc.1996.1848. [DOI] [PubMed] [Google Scholar]
- 12.Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J Am Acad Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- 13.de Gruijl FR, van der Leun JC. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of stratospheric ozone depletion. Health Phys. 1994;67:319–325. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 14.de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Singlet oxygen, UVA, and ozone. Methods Enzymol. 2000;319:359–366. doi: 10.1016/s0076-6879(00)19035-4. [DOI] [PubMed] [Google Scholar]
- 15.DiGiovanna JJ. Posttransplantation skin cancer:scope of the problem, management and role for systemic retinoid chemoprevention. Transplant Proc. 1998;30:2771–2775. doi: 10.1016/s0041-1345(98)00806-9. [DOI] [PubMed] [Google Scholar]
- 16.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 17.Donawho CK, Kripke ML. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res. 1991;51:4176–4181. [PubMed] [Google Scholar]
- 18.Douki T, Reynaud-Angelin A, Cadet J, et al. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42:9221–9226. doi: 10.1021/bi034593c. [DOI] [PubMed] [Google Scholar]
- 19.Elmets CA, Singh D, Tubesing K, et al. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 20.Fortina AB, Caforio AL, Piaserico S. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J Heart Lung Transplant. 2000;19:249–255. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 21.Gu M, Dhanalakshmi S, Singh RP, et al. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 22.Gu M, Dhanalakshmi S, Singh RP, et al. Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev. 2005;14:1344–1349. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 24.Hojo M, Morimoto T, Maluccio M. Cyclosporin induces cancer progression by a cell-autonomous mechanism. Nature (Lond.) 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 25.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 26.Huang CC, Fang JY, Wu WB, et al. Protective effects of (−)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296:473–481. doi: 10.1007/s00403-005-0540-5. [DOI] [PubMed] [Google Scholar]
- 27.Huang CC, Wu WB, Fang JY, Chiang HS, Chen SK, Chen BH, Chen YT, Hung CF, et al. (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 2007;12:1845–1858. doi: 10.3390/12081845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichihashi M, Ueda M, Budiyanto A. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 29.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 30.Jeon HY, Kim JK, Kim WG, et al. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and UV-induced skin damage. Skin Pharmacol Physiol. 2009;22:137–141. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 31.Katiyar SK. Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int J Oncol. 2002;21:1213–1222. [PubMed] [Google Scholar]
- 32.Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects. Int J Oncol. 2005;26(1):169–176. [PubMed] [Google Scholar]
- 33.Katiyar SK, Afaq F, Azizuddin K, et al. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176:110–107. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 34.Katiyar SK, Afaq F, Perez A, et al. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 35.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of spontaneous and photoenhanced lipid peroxidation in mouse epidermal microsomes by epicatechin derivatives from green tea. Cancer Lett. 1994;79:61–66. doi: 10.1016/0304-3835(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 36.Katiyar SK, Ahmad N, Mukhtar H. Green tea and skin. Arch Dermatol. 2000;136:989–994. doi: 10.1001/archderm.136.8.989. [DOI] [PubMed] [Google Scholar]
- 37.Katiyar SK, Challa A, McCormick TS, et al. Prevention of UVB-induced immunosuppression in mice by green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 38.Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection. Int J Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 39.Katiyar SK, Elmets CA, Agarwal R, et al. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 40.Katiyar SK, Korman NJ, Mukhtar H, et al. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 41.Katiyar SK, Matsui MS, Elmets CA, et al. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- 42.Katiyar SK, Matsui MS, Mukhtar H. Kinetics of UV light-induced cyclobutane pyrimidine dimers in human skin in vivo: An immunohistochemical analysis of both epidermis and dermis. Photochem Photobiol. 2000;72:788–793. doi: 10.1562/0031-8655(2000)072<0788:koulic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen presenting cells and oxidative stress. J Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 44.Katiyar SK, Bergamo BM, Vayalil PK, Elmets CA. Green tea polyphenols: DNA photodamage and photoimmunology. J Photochem Photobiol B: Biology. 2001;65:109–114. doi: 10.1016/s1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 45.Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell Biochem (S) 1997;27:59–67. [PubMed] [Google Scholar]
- 46.Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clinical Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- 47.Kim J, Hwang J-S, Cho Y-K, et al. Protective effects of (−)-epigallocatechin-3-gallate on UVA- and UVB-induced skin damage. Skin Pharmacol Appl Skin Physiol. 2001;14:11–19. doi: 10.1159/000056329. [DOI] [PubMed] [Google Scholar]
- 48.Kinlen L, Sheil A, Peta J. Collaborative United Kingdom-Australia study of cancer in patients treated with immunosuppressive drugs. Br J Med II. 1979:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klebanoff SJ. In: Inflammation: Basic Principles and Clinical Correlates. Gallin JI, Goldstein IM, Snyderman R, editors. New York, NY: Raven Press; 1988. pp. 391–444. [Google Scholar]
- 50.Kligman LH, Akin FJ, Kligman AM. Sunscreens prevent ultraviolet photocarcinogenesis. J Am Acad Dermatol. 1980;3:30–35. doi: 10.1016/s0190-9622(80)80221-0. [DOI] [PubMed] [Google Scholar]
- 51.Kripke ML. Photoimmunology. Photochem Photobiol. 1990;52:919–924. doi: 10.1111/j.1751-1097.1990.tb08703.x. [DOI] [PubMed] [Google Scholar]
- 52.Kripke ML, Cox PA, Alas LG, et al. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krutmann J. The role of UVA rays in skin aging. Eur J Dermatol. 2001;11:170–171. [PubMed] [Google Scholar]
- 54.Langenbach R, Loftin CD, Lee C, et al. Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis. Ann N Y Acad Sci. 1999;889:52–61. doi: 10.1111/j.1749-6632.1999.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 55.Li YH, Wu Y, Wei HC, et al. Protective effects of green tea extracts on photoaging and photommunosuppression. Skin Res Technol. 2009;15:338–345. doi: 10.1111/j.1600-0846.2009.00370.x. [DOI] [PubMed] [Google Scholar]
- 56.Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 57.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Rad Biol Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Maziere C, Dantin F, Dubois F, et al. Biphasic effect of UVA radiation on STAT1 activity and tyrosine phosphorylation in cultured human keratinocytes. Free Radic Biol Med. 2000;28:1430–1437. doi: 10.1016/s0891-5849(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 59.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeran SM, Katiyar SK. Proanthocyanidins inhibit mitogenic and survival-signaling in vitro and tumor growth in vivo. Front Biosci. 2008;13:887–897. doi: 10.2741/2729. [DOI] [PubMed] [Google Scholar]
- 61.Meeran SM, Mantena SK, Elmets CA, et al. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 62.Meeran SM, Mantena SK, Meleth S, et al. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. 2006;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 63.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 64.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–254. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 65.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 66.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 67.Mittal A, Piyathilake C, Hara Y, et al. Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: Relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5:555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mnich CD, Hoek KS, Virkki LV, et al. Green tea extract reduces induction of p53 and apoptosis in UVB-irradiated human skin independent of transcriptional controls. Exp Dermatol. 2009;18:69–77. doi: 10.1111/j.1600-0625.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 69.Moore JO, Wang Y, Stebbins WG, et al. Photoprotective effect of isoflavone genistein on ultraviolet B induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis. 2006;27:1627–1635. doi: 10.1093/carcin/bgi367. [DOI] [PubMed] [Google Scholar]
- 70.Morley N, Clifford T, Salter L, et al. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol Photomed. 2005;21:15–22. doi: 10.1111/j.1600-0781.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 71.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 72.Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 73.Park K, Lee JH. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol Rep. 2008;19:413–417. [PubMed] [Google Scholar]
- 74.Parrish JA. Photoimmunology. Adv Exp Med Biol. 1983;160:91–108. doi: 10.1007/978-1-4684-4406-3_10. [DOI] [PubMed] [Google Scholar]
- 75.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 76.Runger TM. Role of UVA in the pathogenesis of melanoma and non-melanoma skin cancer. A short review. Photodermatol Photoimmunol Photomed. 1999;15:212–216. doi: 10.1111/j.1600-0781.1999.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 77.Scalbert A, Morand C, Manach C, et al. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002;56:276–282. doi: 10.1016/s0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- 78.Schwarz A, Maeda A, Gan D, et al. Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12. Photochem Photobiol. 2008;84:350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 79.Schwarz A, Maeda A, Kernebeck K, et al. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwarz A, Stander S, Berneburg M, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 81.Scotto J, Fears TR. Skin cancer epidemiology: research needs. Natl Cancer Inst Monogr. 1978;50:169–177. [PubMed] [Google Scholar]
- 82.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 83.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann NY Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 84.Strom S. In: Basal and squamous cell skin cancers of the head and neck. Weber R, Miller M, Goepfert H, editors. Baltimore, MD: Williams and Wilkins; 1996. pp. 1–7. [Google Scholar]
- 85.Taylor CR, Stern RS, Leyden JJ, et al. Gilchrest, Photoaging/photodamage and photoprotection. J Am Acad Dermatol. 1990;22:1–15. doi: 10.1016/0190-9622(90)70001-x. [DOI] [PubMed] [Google Scholar]
- 86.Timares L, Katiyar SK, Elmets CA. DNA damage, apoptosis and Langerhans cells-activators of UV-induced immune tolerance. Photochem Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ullrich SE. Potential for immunotoxicity due to environmental exposure to ultraviolet radiation. Hum Exp Toxicol. 1995;14:89–91. doi: 10.1177/096032719501400118. [DOI] [PubMed] [Google Scholar]
- 88.Urbach F. Incidences of nonmelanoma skin cancer. Dermatol Clin. 1991;9:751–755. [PubMed] [Google Scholar]
- 89.Vanderveen EE, Grekin RC, Swanson NA, et al. Arachidonic acid metabolites in cutaneous carcinomas. Arch Dermatol. 1986;122:407–412. doi: 10.1001/archderm.122.4.407. [DOI] [PubMed] [Google Scholar]
- 90.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 91.Vayalil PK, Mittal A, Hara Y, et al. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122:1480–1487. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 92.Wang SQ, Setlow R, Berwick M. Ultraviolet A and melanoma: a review. J Am Acad Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Zhang X, Lebwohl M, et al. Inhibition of ultraviolet B (UVB)-induced cfos and c-jun expression in vivo by a tyrosine kinase inhibitor genistein. Carcinogenesis. 1998;19:649–654. doi: 10.1093/carcin/19.4.649. [DOI] [PubMed] [Google Scholar]
- 94.Wang ZY, Huang MT, Ferraro T, et al. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–1170. [PubMed] [Google Scholar]
- 95.Wang ZY, Huang MT, Ho CT, et al. Inhibitory effect of green tea on the growth of established skin papillomas in mice. Cancer Res. 1992;52:6657–6665. [PubMed] [Google Scholar]
- 96.Wei H, Ca Q, Rahn R, et al. DNA structural integrity and base composition affect ultraviolet light-induced oxidative DNA damage. Biochemistry. 1998;37(18):6485–6490. doi: 10.1021/bi972702f. [DOI] [PubMed] [Google Scholar]
- 97.Wei H, Saladi R, Lu Y, et al. Isoflavone genistein: photoprotection and clinical implications in dermatology. J Nutr. 2003;133 11 Suppl 1:3811S–3819S. doi: 10.1093/jn/133.11.3811S. [DOI] [PubMed] [Google Scholar]
- 98.Wei H, Zhang X, Zhao JF, et al. Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radic Biol Med. 1999;26:1427–1435. doi: 10.1016/s0891-5849(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 99.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 100.Yarosh D, Alas LG, Yee V, et al. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–4231. [PubMed] [Google Scholar]
- 101.Yoshikawa T, Rae V, Bruins-Slot W. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 102.Zhao JF, Zhang YJ, Jin XH, et al. Green tea protects against psoralen plus ultraviolet A-induced photochemical damage to skin. J Invest Dermatol. 1999;113:1070–1075. doi: 10.1046/j.1523-1747.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 103.Zi SX, Ma HJ, Li Y, et al. Oligomeric proanthocyanidins from grape seeds effectively inhibit ultraviolet-induced melanogenesis of human melanocytes in vitro. Int J Mol Med. 2009;23:197–204. [PubMed] [Google Scholar]
- 104.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]