Abstract
Purpose
Infection with oncogenic human papillomaviruses has been linked to the development of cervical neoplasia and cancer. The exclusive expression of E7, a viral oncogene, in infected cells makes this protein an ideal target for immunotherapy. We recently reported on the results of a trial in women with cervical carcinoma-in-situ using HspE7, a protein vaccine consisting of full length HPV16 E7 linked to a heat shock protein from M. bovis. The stimulating effects of HspE7 on specific cytotoxic T lymphocytes have been demonstrated in vitro and in (pre-)clinical trials. The induction of a B-cell response by HspE7 and its association with clinical outcome is unknown, and is the purpose of this study.
Experimental design
We measured the serum IgG levels against HPV16 E7 and HPV16 and -18 VLPs using a multiplexed Luminex based assay in 57 women with CIS who received the HspE7 vaccine.
Results
Vaccination with HspE7 results in a modest, yet maintained increase in HPV16 E7 specific IgG levels. While not significant, increased HPV16 E7 IgG levels appear to be correlated with a positive therapeutic effect. Women who were previously treated for recurrent disease (by LEEP) had significantly higher HPV16 E7 IgG levels compared with subjects without recurrent disease (p=0.01). In women with recurrent disease, higher IgG levels correlated with complete pathological response.
Conclusions
This study suggests that IgG levels could potentially be used as a marker for response to a therapeutic vaccine. Further translational investigations of the ‘priming’ of local immune responses using extirpative procedures should be explored.
Keywords: HPV, cervical carcinoma, HspE7, therapeutic vaccine, IgG
Introduction
Accumulated data has demonstrated an etiologic role for high risk (HR) HPVs in a wide range of (genital tract) neoplasms and cancers [1, 2]. The identification of HR HPV as the causative agent in cervical carcinogenesis sparked interest in the development of vaccines to prevent HPV16 and -18 infections and reduce cervical cancer [3, 4]. Although the recently commercialized prophylactic vaccines are highly effective in preventing type specific infection, they do not appear to have therapeutic benefit [5].
The current treatment for high-grade dysplasia mainly consists of extirpative or ablative procedures aimed at removing visible lesions. However, these procedures do not target the causative HPV infection resulting in recurrent or persistent CIN [6]. The highest incidence rates of high grade CIN and CIS are noted during a woman’s prime reproductive years [7], and these therapeutic extirpative cervical procedures potentially affect reproductive success [8]. Therefore, HPV-targeted, non-surgical treatment methods for pre-cancerous lesions would be beneficial. Since HPV16 accounts for more than 50% of cervical cancer cases worldwide [1], therapeutic vaccines targeting HPV16 are of particular interest. The viral oncogenes (E6 and E7) are critical to the induction and maintenance of cellular transformation [9]. These two proteins are only expressed in infected host cells and represent ideal target antigens for therapeutic HPV vaccine development [10]. The goal of therapeutic vaccines is to enhance the body’s anti-tumor immunity, facilitating natural resolution of lesions and infections [11].
We previously reported on the effects of an HspE7 therapeutic vaccine (Nventa Biopharmaceuticals Corp. San Diego, CA) in women with CIN III [12] (NCI-5850, Clinicaltrials.gov #NCT00075569). HspE7 is a fusion peptide of the full length HPV16 E7 antigen fused to the Heat Shock Protein 65 from Mycobacterium bovis BCG (Hsp65). Hsp fusion proteins have been shown to induce antigen-specific cytotoxic T-lymphocytes, Type 1 cytokines and anti-tumor immunity [13]. In the present study we wished to determine whether vaccination with HspE7 induced a B-cell response and whether the mounting of an anti-HPV16 E7 IgG response upon vaccination is associated with a clinical response in women with carcinoma-in-situ of the cervix.
Materials and Methods
Study design
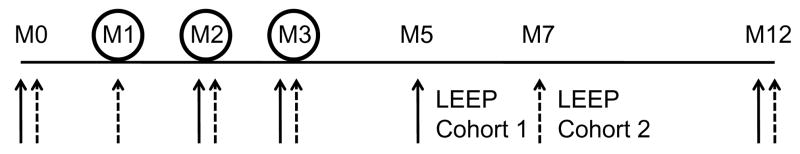
Details of the study were reported previously [12]. Briefly, this was an NCI-sponsored single-arm, open label, phase II study conducted to establish the response rate and toxicity of HspE7 in women with CIN III. The study was divided into two cohorts. For baseline determination blood was drawn at the enrolment visit (M0; see Figure 1 for schema). During the study, blood was drawn immediately prior to administration of vaccine dose 2 and 3 (M2 and M3). At M5 or M7 for cohort 1 or 2 respectively, blood was drawn prior to the LEEP procedure. A final blood draw was performed at M12. At M1, M2 and M3 500 μg subcutaneous doses of HspE7 were administered on alternating thighs.
Figure 1.
Schema of study design. At M1, M2 and M3 500 μg subcutaneous doses of HspE7 were administered on alternating thighs. Blood for serology was drawn (solid arrows for cohort 1, dashed arrows for cohort 2) at the enrolment visit (M0), M1 (cohort 2 only). During the study, blood was drawn immediately prior to administration of vaccine dose 2 and 3 (M2 and M3). At M5 or M7 for cohort 1 and cohort 2 respectively, blood was drawn prior to the LEEP procedure. A final blood draw was performed at M12.
In our previous report [12] we did not identify any differences between cohorts with regards to epidemiologic or HPV baseline types, thus both cohorts were combined for further analyses. Women in cohort 2 had two baseline pre-vaccine serum samples (M0 and M1); statistical analysis showed no differences in serum IgG levels between both samples, so they were averaged as the baseline value.
Production of Recombinant Antigen
The recombinant HPV16 E7 protein was affinity purified from E. Coli as a GST fusion protein using glutathione-agarose (GE Healthcare Bio-Sciences Corp.). The GST tag was removed by digestion with a precision protease (for details see supplementary files online).
The isolated E7 protein was estimated to be 95% pure based on the presence of a single band migrating at 17 kDa on a coomassie blue-stained SDS page gel. Western Blot with monoclonal anti-HPV16 E7 antibody (Zymed, South San Francisco, CA) confirmed that the 17 kDa fragment was the HPV16 E7 protein (data not shown).
To generate virus like particles (VLPs), the L1/L2 proteins for HPV16 and L1 protein for HPV18 were expressed in Trichoplusia ni cells (High Five™, Invitrogen, Carlsbad, CA) infected with recombinant baculovirus vectors and VLPs were purified as described previously [14–16].
Coupling of the recombinant proteins to Luminex Xmap carboxylated biospheres
For each set of Luminex Xmap carboxylated biospheres (Luminex corporation, Austin, TX), 6.25×106 beads were activated by resuspension in monobasic sodium phosphate containing 2.5 mgeach freshly prepared 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide Hydrochloride (EDC; Pierce, Rockford, IL) and N-hydroxysulfosuccinimide (Sulfo-NHS; Pierce). The activated beads were coupled to antigen (Table 1) during a 3 hour incubation at 25°C. The coupled beads were washed with PBS-TBN (0.02%Tween 20, 0.1% bovine serum albumin [BSA], 0.02% azide) and stored at 4°C until further use. For a more detailed description of the bead coupling procedure please consult the supplementary materials.
Table 1.
Antigen coupled to Luminex beads
| Bead region# | Antigen | Ag concentration |
|---|---|---|
| 47 | Goat Anti-human IgG (Jackson Immunoresearch) | 5 ug |
| 52 | Human IgG (Pierce) | 5 ug |
| 53 | HPV16 E7 | 30 ug |
| 54 | HPV16 VLP | 30 ug |
| 55 | HPV-18 VLP | 7.5 ug |
| 56 | GST (GenScript Corporation) | 25 ug |
Luminex Assay
The Luminex assay (xMAP; Luminex Corp.) used polystyrene beads containing a mixture of two fluorescent dyes allowing for their identification. The analyzer aspirates the beads; while excitation with the red laser allows identification of the bead set, the green laser is used to excite the reporter fluorescent label. Serum samples were diluted 1:125 in blocking buffer (PBS, 1% BSA and 2.5% CBS-K reagent (Millipore, Danvers, MA)). For each plate (96 wells) we added 2.5 ul of each bead set (five sets total) to 2500 ul blocking buffer. To block non-specific binding, both the diluted serum samples and the diluted bead sets were incubated overnight at 4°C. The next morning, 25 ul diluted serum and 25 ul beads were mixed in a multiscreen HTS 96 well plate (Millipore) and incubated for 3 hours at RT (final serum dilution 1:250). Using a vacuum manifold, the beads were washed with PBS-BSA and incubated with 50 ul biotinylated goat anti-human IgG (Jackson Immuno research, Westgrove, PA; diluted 1:300 in PBS-BSA) for 1 hour at RT. After another wash step, the beads were incubated in 50 ul Streptavidin-phycoetheryin (Invitrogen) for 15 minutes, followed by analysis on a Luminex 200.
All serum MFI values fell within the linear range of the assay as determined by a serial dilution of monoclonal anti-HPV16 E7 IgG (Zymed; data not shown).
ELISA assay for the detection of HPV VLPs
The ELISA protocol utilized 0.5% polyvinyl alcohol (PVA; Sigma, St. Louis, MO) as a blocking agent and 0.8% polyvinylpyrrolidone (PVP; sigma) as a secondary antibody adsorption enhancer, as previously described [15, 17].
Median Fluorescent Intensity normalization
To allow for intra- and interplate normalization we utilized human IgG and anti-human IgG coupled beads as controls. Since these beads measure assay conditions (i.e., IgG coupled beads) and total IgG in human blood (i.e., anti-human IgG coupled beads), the average MFI values for each of these bead sets should be identical across all plates given a normal distribution of IgG in the subject. For both control beads, we calculated the mean MFIs for each plate (value A) and an average for all the plates combined (value B). The mean MFI for each plate (value A) was divided by the mean MFI across all the plates (value B). These calculations provided two values for each plate (value CIgG and value Canti-IgG). The average of these two C-values was used to normalize the MFI for every bead in each well of a specific plate. We controlled for intra-plate variability by running each sample in duplicate on every plate. Inter-plate variability was controlled by running all serum samples for a subset of patients on the same plate, MFI values for samples on one plate were not different from samples ran on different plates (p=0.23).
In order to control for possible non-specific interactions with the beads proper and/or with contaminating GST in the HPV16 E7 preparation, MFI values for GST coupled beads were measured in each well. One patient (patient 13) repeatedly reacted with GST and subtracting the GST MFI resulted in negative values; this patient was excluded from statistical analyses. To validate the Luminex assay, we compared detection of antibodies to HPV16 and -18 VLP using Luminex and ELISA data; there was excellent agreement as indicated by a kappa value of 0.873 (95% CI 0.814–0.931) and 0.888 (0.823–0.952), respectively.
Clinical response evaluation
As described previously [12], the outcome to HspE7 vaccination was evaluated by post-vaccination colposcopic evaluation and histopathology of post-vaccination LEEP specimens. A pathologic complete response (CRpath) was defined as a post-vaccine LEEP specimen negative for CIN. A partial response (PR) was defined as a 50% or more reduction of lesion size post-vaccination based on quantitative colposcopic measurements made on grid colposcopy forms [18, 19]. Stable disease (SD) was defined as persistent CIN III with less than 50% reduction of lesion size. Progressive disease (PD) was defined as any colposcopically measured increase in lesion size or invasion identified on any biopsy performed during the study or in the final LEEP pathology. In our cohort only two women showed PD. For statistical analysis SD and PD were combined.
Statistical analysis
This longitudinal study examined mean E7 antibody levels at baseline (M0), each vaccination and exit (M12) comparing levels using paired t- tests. Within subject effects of HPV16 and HPV 18 VLP levels were tested using the multivariate ANOVA approach to repeated measures. One-way analysis of variance was used to compare individual overall mean E7 antibody levels in HPV16 positive and negative patients, as well as for clinical response. Generalized linear model (GLM) repeated measures were used to assess the influence of individual patient characteristics; specifically clinical outcome and whether a woman had a LEEP prior to entering the study, on changes in E7 antibody level over the study period. Logistic regression was used to assess the association of mean E7 MFI and previous LEEP status to clinical response. A p<0.05 was considered statistically significant in all analyses. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Serological response to prophylactic vaccination
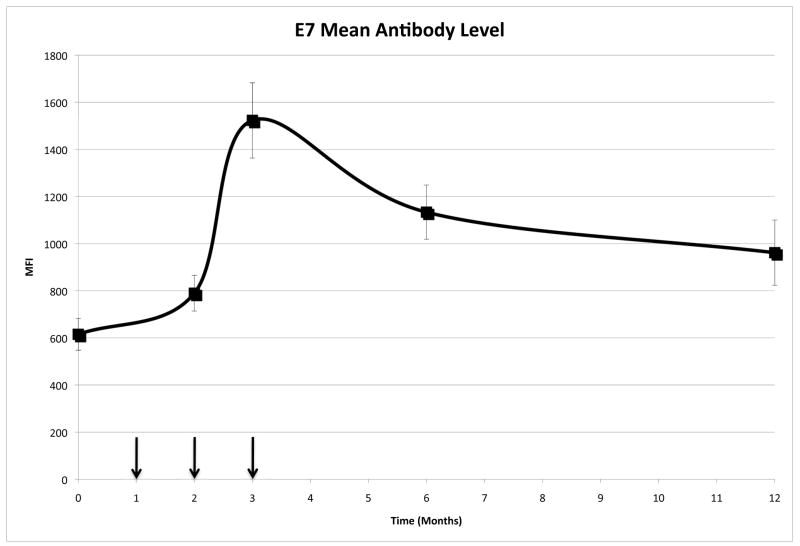
57 women completed all three vaccination doses, and were tested for HPV antibody levels. A multiplexed Luminex based assay was used to determine the serum IgG levels against HPV16 E7 (Figure 2). At enrolment (M0), women had HPV16 E7 specific MFIs of 615.17±67.62 (mean ± SEM). The average anti-HPV16 E7 IgG levels peaked at M3 (1522.80±159.59), two months after the first vaccine dose. 81% (n=46) of the subjects showed an increase in MFI from M0 to M3. Three months after the last dose there was a significant drop in Ab levels compared to M3 (average change in mean IgG levels=−424.2 from M3-M5/7; p=0.003), however the anti-E7 IgG levels were still higher than pre-vaccination (p<0.001). Nine months (M12) after the last vaccination, Ab levels remained elevated (961.46±138.42) in comparison with those at M0 (p=0.012).
Figure 2.
HPV16 E7 specific IgG reactivity following vaccination with HspE7
Mean HPV16 E7 reactivity is shown as MFI (median fluorescence intensity) as determined by the Luminex assay over time. The arrows indicate the vaccination time points.
Influence of Hsp-E7 vaccination on other HPV related antibodies
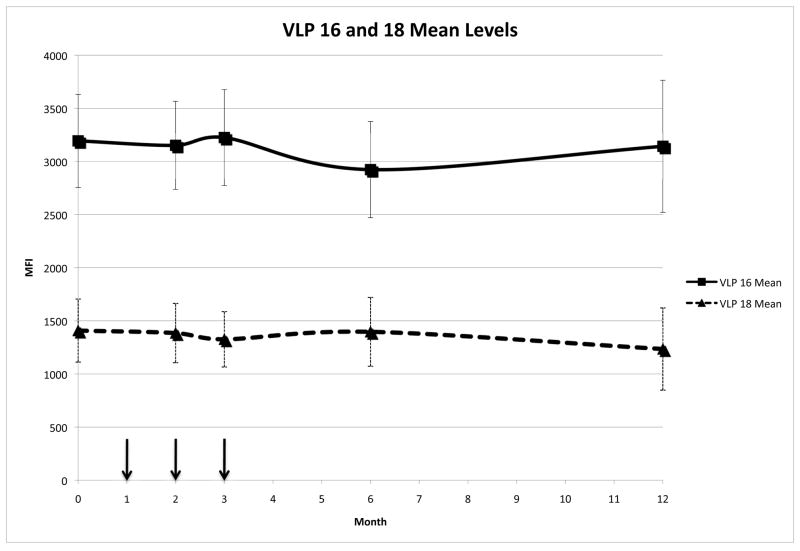
The mean serum IgG levels against HPV16 and HPV18 VLPs did not change over the course of the study (p=0.60 and p=0.72, respectively) suggesting that the observed increase in HPV16 E7 IgG levels was specific for HPV16 E7 (Figure 3). 73% of the women who were positive for HPV16 DNA were also HPV16 VLP seropositive. This data is consistent with a previous report using an HPV16 VLP ELISA where 77% of women with CIN were both DNA and serology positive [20].
Figure 3.
IgG reactivity following vaccination with HspE7
Mean HPV16 and HPV 18 VLP reactivities are shown as MFI (median fluorescence intensity) as determined by the Luminex assay over time. The arrows indicate the vaccination time points.
Influence of HPV type on anti-HPV16 E7 IgG levels
Since the HspE7 therapeutic vaccine utilizes the complete HPV16 E7 protein, we expected HPV16 DNA positive (33/57) women to have higher mean E7 antibody levels compared to HPV16 DNA negative women. In this analysis HPV16 DNA positive women (PCR positive at one or more time points during the course of the study [12]) had higher mean HPV16 E7 IgG levels (1047.42±102.57) compared to HPV16 DNA negative women (870.11±122.61), but the difference did not reach significance (p=0.27).
HPV16 E7 IgG levels and clinical response
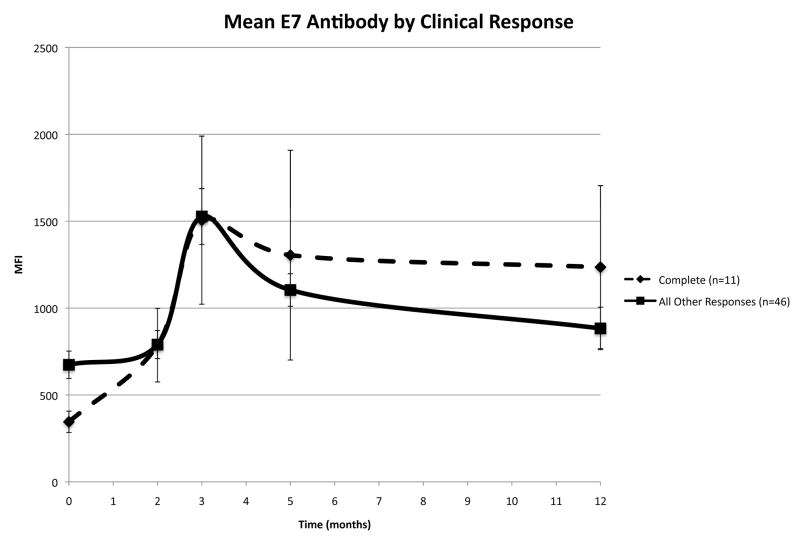
Vaccination resulted in a statistically significant change in anti-HPV16 E7 IgG levels independent of the clinical outcome (p=0.04). There appears to be a positive therapeutic effect of increased mean HPV16 E7 specific MFI with better clinical outcome. Women with a complete response had the highest mean HPV16 E7 specific MFI (1006.54±253.87), while women with stable or progressive disease had the lowest MFI (943.82±162.01). Partial responders had mean E7 IgG levels of 972.91±90.43. In addition, complete responders had the largest increase in E7 IgG levels from baseline to exit (M12) (mean change in IgG levels, complete: 834.94±508.2 versus all other responses: 242.75±99.65). To better understand how HPV16E7 IgG levels correlated with clinical response, we compared the IgG levels in women who had a complete clinical response (n=11) versus all women without a complete response (n=46) (Figure 4). At baseline, women who had a complete response (345.38±61.39) had significantly lower MFI values than other response groups (673.83±78.82; p<0.001). Also, at one year (M12) the complete responders (1235.74±468.98) had significantly higher (883.09±122.20; p<0.003) MFI levels. Finally, whereas the complete responders had a significantly higher response at exit compared to baseline (p<0.001), the IgG levels of those women without a complete response returned to baseline values at one year (M12) (p=0.907).
Figure 4.
IgG reactivity against HPV16 E7 stratified by clinical response.
Mean reactivity, for complete responders and all other responders, are shown as MFI (mean fluorescence intensity) over time.
Influence of prior LEEP on Ab levels
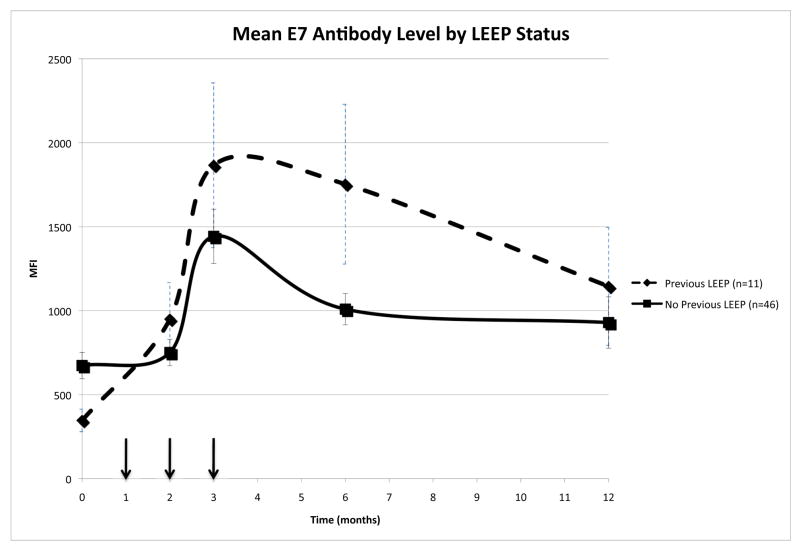
Nineteen percent of the patients (n=11) had been previously treated for cervical neoplasia by LEEP. These women were 2.7 times more likely to completely respond following vaccination compared to women who had not had a prior LEEP [12]. We observed that prior LEEP status affected HPV16 E7 IgG levels over the course of the study when comparing patients with and without a prior LEEP (p=0.01; figure 5). In previously LEEPed women high mean IgG levels were associated with clinical outcome to therapeutic vaccination (p=0.02). Thus, in this study women who previously underwent a LEEP and had high mean IgG levels were more likely to have a CRpath. This suggests that the LEEP procedure may have ‘primed’ these women, resulting in a higher IgG response following vaccination. It was also noted that women who underwent a previous LEEP procedure and were HPV16 DNA positive had lower IgG levels compared to their HPV16 DNA negative counterparts (HPV16(+) 1060.91±151.26; HPV16(-) 1661.97±1264.38; p=0.322).
Figure 5.
IgG reactivity against HPV16 E7 stratified by LEEP status.
Mean reactivities are shown as MFI (median fluorescence intensity) over time. The arrows indicate the vaccination time points.
Discussion
Recent in vitro and in vivo work has shown that exposure of Hsp-fusion proteins to dendritic cells results in cross-presentation of the antigen to CD8+ T cells and activation of dendritic cells [21, 22], resulting in potent antigen-specific CTL induction. We recently reported on the results of a phase 2 clinical trial using one of these Hsp-fusion vaccines, HspE7. The data from this trial suggested that HspE7 demonstrated activity in women with CIN III [12]. Another phase II efficacy study looking at a similar population showed that lesion regression was correlated with a class II restricted immune response [23]. Previous work with Hsp proteins has shown that these fusion proteins can elicit both humoral and cellular immune responses to the coupled antigen [24]. Based on these observations, we asked whether we could detect a B-cell response to HspE7 and whether this response predicted and/or was associated with clinical outcome.
In the present study, we show that HspE7 vaccination of women with CIN III results in a modest increase in HPV16 E7 specific serum IgG levels, and that these increased levels are maintained for at least 12 months. Although not statistically significant, we observed a positive therapeutic effect of increased mean HPV16 E7 specific antibody levels following vaccination with better clinical outcome. Women with a complete response had the highest mean HPV16 E7 specific MFI, while women with stable or progressive disease had the lowest MFI. Also, complete responders had the largest increase in E7 IgG levels from baseline to exit.
An interesting, yet unexpected observation in our previous report was that women with CIS who underwent a prior LEEP were 2.7 times more likely to have a CRpath after HspE7 vaccination [12]. In the present study, we noted that the combination of a woman’s prior LEEP status and mean IgG levels was associated with clinical response (p=0.02). Specifically, those women who had a CRpath had the highest serum E7 IgG levels.
It has been shown that patients with CIN III switch from expressing a primarily Th1 cytokine profile to a primarily Th2 cytokine profile. The expression of IL-4 and IL-10 by cervical lesions creates an anti-inflammatory, tumor-promoting environment [25, 26]. Increased levels of IL-10 have been shown to be correlated with the creation of CD4+CD25+ regulatory T-cells [27]. These T-regs are increased in patients with cervical neoplasia and have been shown to suppress HPV specific immunity [28]. Visser and colleagues showed that in vitro depletion of CD25+ cells resulted in an increased IFN-γ T cell response against HPV16 E7. Interestingly, local invasive procedures significantly enhance HPV16 E7 specific IFN-γ responses. The greatest enhancement was seen in women who had repeated surgeries [29]. It is possible that the repeated insult to the cervix creates an environment that is more susceptible to the mounting of an anti-HPV immune response. Interestingly, in our dataset, 70% of the women who had a CRpath displayed signs of cervical inflammation [12]. In addition, all previously LEEPed women who had a CRpath following vaccination also showed signs of cervical inflammation.
Therefore, we hypothesize that the unexpected success of the HspE7 vaccine in women with history of a prior LEEP [12] is the result of a ‘prepared’ state of the cervix due to the repeated insult. The observations that Pap smears [30] and LEEP procedures [31] increase local inflammatory cytokine responses raise the possibility of a return towards a Th1 cytokine environment which is more conducive to support HPV16 E7 specific CD8 T-cells to remove the tumor cells.
Since E7 is expressed in the nucleus of infected cells, it is unlikely that anti-E7 IgG levels will have a protective function. It is more likely that the IgG detected in the present study are the by-product of a successful cellular immune response in which CD8+ T-cells lyse the virally infected keratinocytes resulting in the release of E7 fragments in the local epithelial and stromal microenvironment. The subsequent cascading cytokine and chemokine responses in addition to recognition of these fragments by B-cells could then induce the higher production of E7 specific IgG.
To confirm these results a larger study is required, nevertheless the present study suggests that anti-HPV16 E7 IgG levels could be used to predict the clinical outcome in women with recurring disease following E7-specific therapeutic vaccination. It has been suggested that successful therapeutic vaccines targeting high-grade CIN lesions will have to overcome the immunosuppressive state of the patient. If the hypothetical ‘prepared state’ of the cervix explains the increased efficiency of the HspE7-based therapeutic vaccine in a subset of participants, it would be interesting to see whether the induction of local inflammation (e.g., by application of toll-like receptor agonists, pretreatment biopsy, or local intra-lesional vaccination) at the time of vaccination could increase the efficacy of therapeutic vaccines targeting HPV-associated neoplasia.
Supplementary Material
Acknowledgments
The authors acknowledge Drs. W. Shek, R. Dhawan and M. Wunderlich of the Charles River Laboratories, Joseph S. Harrison and the members of the Burk lab for helpful discussions. This study was supported in part by NCI/CTEP grant number N01-CM62204 and through the New York Cancer Consortium which is funded through NCI N01 CM17103.
Footnotes
Conflict of Interest Statement
MHE has advised Nventa, the company producing HspE7, but does not receive an honorarium. The other authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 2.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FUTURE II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196:1438–46. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 4.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 5.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 6.Skinner EN, Gehrig PA, Van LL. High-grade squamous intraepithelial lesions: abbreviating posttreatment surveillance. Obstet Gynecol. 2004;103:488–492. doi: 10.1097/01.AOG.0000114983.11562.08. [DOI] [PubMed] [Google Scholar]
- 7.Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer. 2008;113:9. doi: 10.1002/cncr.23747. [DOI] [PubMed] [Google Scholar]
- 8.Sadler L, Saftlas A. Cervical surgery and preterm birth. J Perinat Med. 2007;35:5–9. doi: 10.1515/JPM.2007.001. [DOI] [PubMed] [Google Scholar]
- 9.Griep AE, Herber R, Jeon S, Lohse JK, Dubielzig RR, Lambert PF. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J Virol. 1993;67:1373–1384. doi: 10.1128/jvi.67.3.1373-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govan VA. Strategies for human papillomavirus therapeutic vaccines and other therapies based on the E6 and E7 oncogenes. Ann N Y Acad Sci. 2005;1056:328–343. doi: 10.1196/annals.1352.016. [DOI] [PubMed] [Google Scholar]
- 11.Kadish AS, Einstein MH. Vaccine strategies for human papillomavirus-associated cancers. Curr Opin Oncol. 2005;17:456–461. doi: 10.1097/01.cco.0000174038.92526.29. [DOI] [PubMed] [Google Scholar]
- 12.Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, Streicher H, Goldberg GL, Runowicz CD. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol Oncol. 2007;106:453–460. doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–225. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy MP, White WI, Palmer-Hill F, Koenig S, Suzich JA. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J Virol. 1998;72:32–41. doi: 10.1128/jvi.72.1.32-41.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Studentsov YY, Schiffman M, Strickler HD, Ho GY, Pang YY, Schiller J, Herrero R, Burk RD. Enhanced enzyme-linked immunosorbent assay for detection of antibodies to virus-like particles of human papillomavirus. J Clin Microbiol. 2002;40:1755–1760. doi: 10.1128/JCM.40.5.1755-1760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- 17.Studentsov YY, Ho GY, Marks MA, Bierman R, Burk RD. Polymer-based enzyme-linked immunosorbent assay using human papillomavirus type 16 (HPV16) virus-like particles detects HPV16 clade-specific serologic responses. J Clin Microbiol. 2003;41:2827–2834. doi: 10.1128/JCM.41.7.2827-2834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris DG, Litaker M. Interobserver agreement for colposcopy quality control using digitized colposcopic images during the ALTS trial. J Low Genit Tract Dis. 2005;9:29–35. doi: 10.1097/00128360-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Brewer CA, Wilczynski SP, Kurosaki T, Daood R, Berman ML. Colposcopic regression patterns in high-grade cervical intraepithelial neoplasia. Obstet Gynecol. 1997;90:617–621. doi: 10.1016/s0029-7844(97)00343-8. [DOI] [PubMed] [Google Scholar]
- 20.Einstein MH, Studentsov YY, Ho GY, Fazzari M, Marks M, Kadish AS, Goldberg GL, Runowicz CD, Burk RD. Combined human papillomavirus DNA and human papillomavirus-like particle serologic assay to identify women at risk for high-grade cervical intraepithelial neoplasia. Int J Cancer. 2007;120:55–59. doi: 10.1002/ijc.22176. [DOI] [PubMed] [Google Scholar]
- 21.Palliser D, Ploegh H, Boes M. Myeloid differentiation factor 88 is required for cross-priming in vivo. J Immunol. 2004;172:3415–3421. doi: 10.4049/jimmunol.172.6.3415. [DOI] [PubMed] [Google Scholar]
- 22.Cho BK, Palliser D, Guillen E, Wisniewski J, Young RA, Chen J, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263–272. doi: 10.1016/s1074-7613(00)80179-x. [DOI] [PubMed] [Google Scholar]
- 23.Roman LD, Wilczynski S, Muderspach LI, Burnett AF, O’Meara A, Brinkman JA, Kast WM, Facio G, Felix JC, Aldana M, Weber JS. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2007;106:558–566. doi: 10.1016/j.ygyno.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- 25.Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, Helmerhorst TJ. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58:1096–1100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bais AG, Beckmann I, Ewing PC, Eijkemans MJ, Meijer CJ, Snijders PJ, Helmerhorst TJ. Cytokine release in HR-HPV(+) women without and with cervical dysplasia (CIN II and III) or carcinoma, compared with HR-HPV(−) controls. Mediators Inflamm. 2007;2007:24147. doi: 10.1155/2007/24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhairavabhotla RK, Verm V, Tongaonkar H, Shastri S, Dinshaw K, Chiplunkar S. Role of IL-10 in immune suppression in cervical cancer. Indian J Biochem Biophys. 2007;44:350–356. [PubMed] [Google Scholar]
- 28.Visser J, Nijman HW, Hoogenboom BN, Jager P, van BD, Schuuring E, Abdulahad W, Miedema F, van der Zee AG, Daemen T. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol. 2007;150:199–209. doi: 10.1111/j.1365-2249.2007.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser J, van BD, Hoogeboom BN, Reesink N, Klip H, Schuuring E, Nijhuis E, Pawlita M, Bungener L, de Vries-Idema J, Nijman H, Miedema F, Daemen T, van der ZA. Enhancement of human papilloma virus type 16 E7 specific T cell responses by local invasive procedures in patients with (pre)malignant cervical neoplasia. Int J Cancer. 2006;118:2529–2537. doi: 10.1002/ijc.21673. [DOI] [PubMed] [Google Scholar]
- 30.Passmore JA, Morroni C, Shapiro S, Williamson AL, Hoffman M. Papanicolaou smears and cervical inflammatory cytokine responses. J Inflamm(Lond) 2007;4:8. doi: 10.1186/1476-9255-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Subbarao S, Wright TC, Jr, Evans-Strickfaden T, Ellerbrock TV, Lennox JL, Butera ST, Hart CE. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix. J Infect Dis. 2000;181:1950–1956. doi: 10.1086/315514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.