Abstract
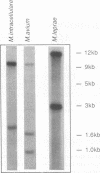
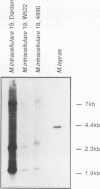
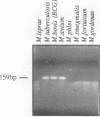
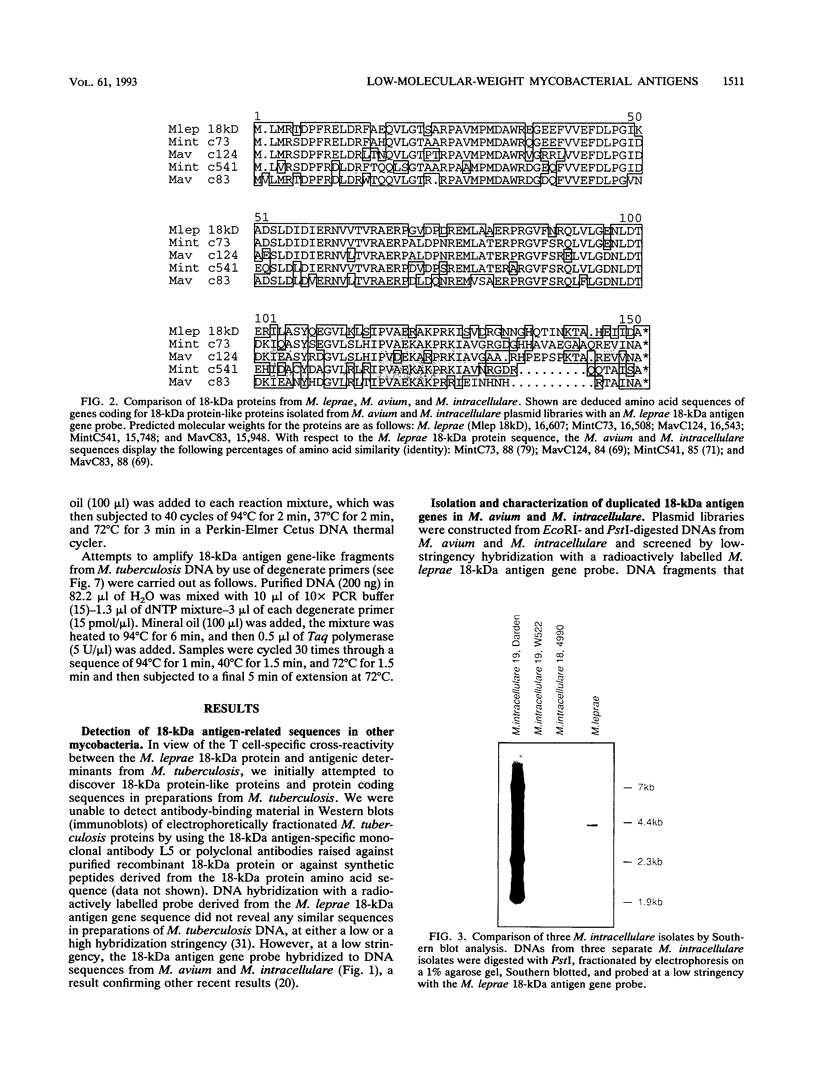
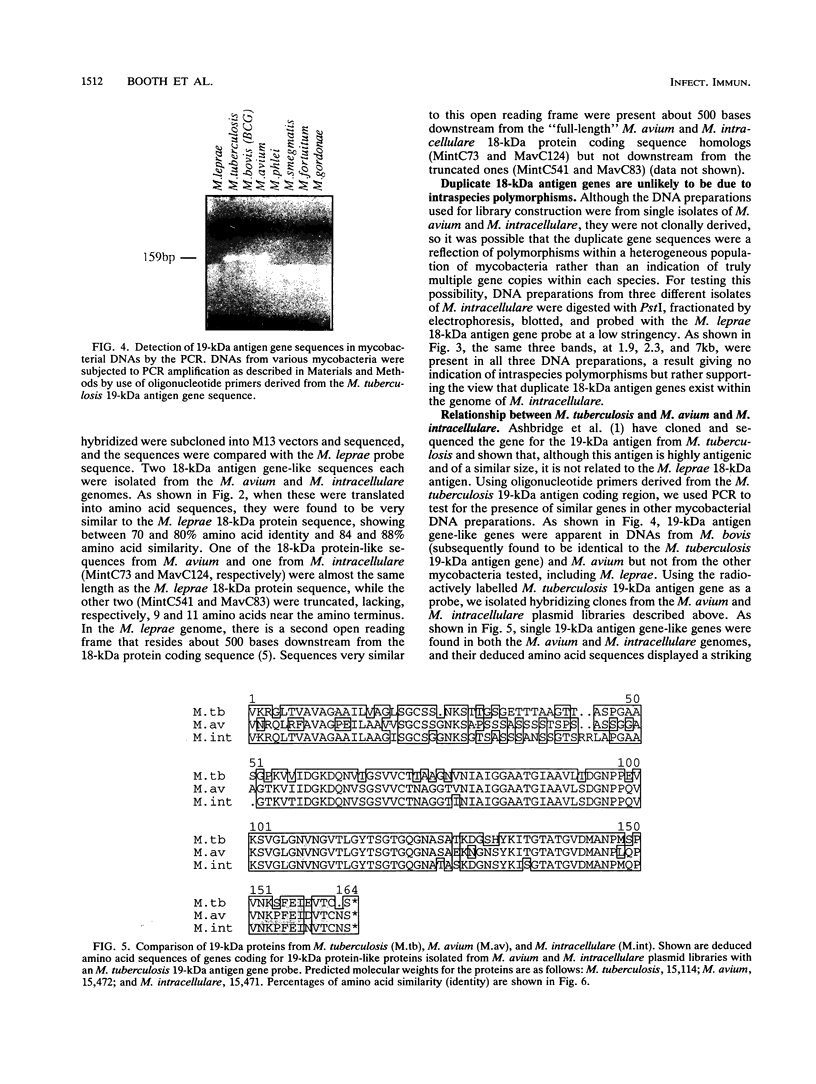
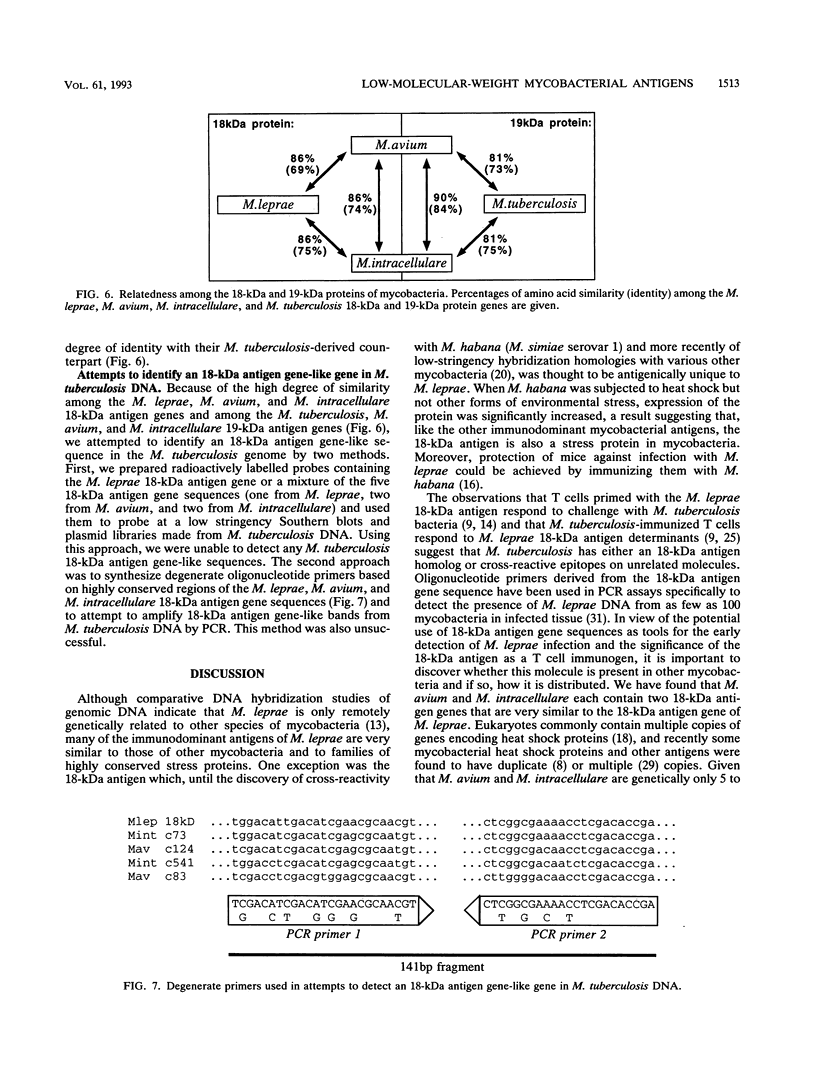
Most of the antigens of Mycobacterium leprae and M. tuberculosis that have been identified are members of stress protein families, which are highly conserved throughout many diverse species. Of the M. leprae and M. tuberculosis antigens identified by monoclonal antibodies, all except the 18-kDa M. leprae antigen and the 19-kDa M. tuberculosis antigen are strongly cross-reaction between these two species and are coded within very similar genes. Studies of T cell reactivity against mycobacterial antigens have indicated that M. tuberculosis bears epitopes that are cross-reactive with the M. leprae 18-kDa antigen, but attempts to identify an 18-kDa antigen-like protein or protein coding sequence in M. tuberculosis have been unsuccessful. We have used a combination of low-stringency DNA hybridization and polymerase chain reaction techniques to identify, isolate, and sequence genes from M. avium and M. intracellulare that are very similar to the 18-kDa antigen gene of M. leprae and others that are homologs of the 19-kDa antigen gene of M. tuberculosis. Unlike M. leprae, which contains a single 18-kDa antigen gene, M. avium and M. intracellulare each have two 18-kDa antigen coding sequences. Although the M. leprae, M. avium, and M. intracellulare 18-kDa antigen genes are all very similar to one another, as are the M. tuberculosis, M. avium, and M. intracellulare 19-kDa antigen genes, we have been unable to detect any 18-kDa antigen-like coding sequences in DNA from M. tuberculosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashbridge K. R., Prestidge R. L., Booth R. J., Watson J. D. The mapping of an antibody-binding region on the Mycobacterium tuberculosis 19 kilodalton antigen. J Immunol. 1990 Apr 15;144(8):3137–3142. [PubMed] [Google Scholar]
- Baess I. Deoxyribonucleic acid relatedness among species of slowly-growing mycobacteria. Acta Pathol Microbiol Scand B. 1979 Aug;87(4):221–226. doi: 10.1111/j.1699-0463.1979.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol. 1989 Apr;135(4):931–939. doi: 10.1099/00221287-135-4-931. [DOI] [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Stoker N. G., Lee S. P., Jackson M., Grant K. A., Jouy N. F., Lucas S. B., Hasan R., Hussain R., McAdam K. P. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989 Jul;57(7):1979–1983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Bäckström B. T., Prestidge R. L., Love S. G., Harding D. R., Watson J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae. Similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J Immunol. 1991 Mar 15;146(6):1934–1940. [PubMed] [Google Scholar]
- Filley E., Abou-Zeid C., Waters M., Rook G. The use of antigen-bearing nitrocellulose particles derived from Western blots to study proliferative responses to 27 antigenic fractions from Mycobacterium leprae in patients and controls. Immunology. 1989 May;67(1):75–80. [PMC free article] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskinsky C. M., Jacobs W. R., Jr, Clark-Curtiss J. E., Bloom B. R. Genetic relationships among Mycobacterium leprae, Mycobacterium tuberculosis, and candidate leprosy vaccine strains determined by DNA hybridization: identification of an M. leprae-specific repetitive sequence. Infect Immun. 1989 May;57(5):1535–1541. doi: 10.1128/iai.57.5.1535-1541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lamb F. I., Singh N. B., Colston M. J. The specific 18-kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as a heat-shock protein. J Immunol. 1990 Mar 1;144(5):1922–1925. [PubMed] [Google Scholar]
- Lee B. Y., Hefta S. A., Brennan P. J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992 May;60(5):2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Moudgil K. D., Williams D. L., Gillis T. P. DNA hybridization analysis of mycobacterial DNA using the 18-kDa protein gene of Mycobacterium leprae. FEMS Microbiol Immunol. 1992 Feb;4(3):165–174. doi: 10.1111/j.1574-6968.1992.tb04983.x. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Nerland A. H., Mustafa A. S., Sweetser D., Godal T., Young R. A. A protein antigen of Mycobacterium leprae is related to a family of small heat shock proteins. J Bacteriol. 1988 Dec;170(12):5919–5921. doi: 10.1128/jb.170.12.5919-5921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Shinnick T. M., Mustafa A. S., Lundin K. E., Godal T., Nerland A. H. Heterogeneity among human T cell clones recognizing an HLA-DR4,Dw4-restricted epitope from the 18-kDa antigen of Mycobacterium leprae defined by synthetic peptides. J Immunol. 1990 Feb 15;144(4):1478–1483. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke de Wit T. F., Bekelie S., Osland A., Miko T. L., Hermans P. W., van Soolingen D., Drijfhout J. W., Schöningh R., Janson A. A., Thole J. E. Mycobacteria contain two groEL genes: the second Mycobacterium leprae groEL gene is arranged in an operon with groES. Mol Microbiol. 1992 Jul;6(14):1995–2007. doi: 10.1111/j.1365-2958.1992.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Roche P. W., Theuvenet W. J., Britton W. J. Cellular immune responses to mycobacterial heat shock proteins in Nepali leprosy patients and controls. Int J Lepr Other Mycobact Dis. 1992 Mar;60(1):36–43. [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Leprosy: understanding protective immunity. Immunol Today. 1989 Jul;10(7):218–221. doi: 10.1016/0167-5699(89)90253-3. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Gillis T. P., Booth R. J., Looker D., Watson J. D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J Infect Dis. 1990 Jul;162(1):193–200. doi: 10.1093/infdis/162.1.193. [DOI] [PubMed] [Google Scholar]
- Young D. B. Stress-induced proteins and the immune response to leprosy. Microbiol Sci. 1988 May;5(5):143–146. [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]