Abstract
A growing number of studies have investigated how motivation interacts with particular cognitive functions, including attention, working memory, and other executive functions. In these studies, the emphasis has been on understanding how motivation impacts brain regions that contribute to improving behavioral performance. Less is understood about how positive incentives may actually impair behavioral performance. Here, we were interested in investigating a situation in which reward would be potentially deleterious to behavioral performance. Specifically, we hypothesized that rewarding participants for correct going would impair stopping performance. Critically, we hypothesized that the effects on inhibition would be specific, namely, not simply attributable to a speeding-up of reaction time during go trials. To investigate the interaction between inhibition and motivation, participants performed a stop-signal task during two conditions, namely, during a neutral, control condition and during a rewarded condition during which they were rewarded for correct go performance. Behaviorally, participants exhibited longer stop-signal reaction times during the reward relative to the control condition, indicating that it was harder to inhibit their responses during the former condition. Neuroimaging findings revealed that a host of brain regions were involved in stop-signal inhibition, as indexed via the contrast of successful and unsuccessful stop trials. Critically, a subset of these regions, which included the right inferior frontal gyrus, the left precentral gyrus, and bilateral putamen, exhibited significant inhibition by condition interactions, demonstrating that cognitive and motivational signals interact in the brain during inhibitory control.
Human behavioral flexibility depends on a set of so-called executive control functions that are engaged when non-habitual behaviors are required. Human behavior is also shaped by motivational factors, which are closely tied to reward and punishment. A growing number of studies have investigated how motivation interacts with particular cognitive functions, including attention, working memory, and other executive functions (Engelmann et al., 2009; Locke and Braver, 2008; Mohanty et al., 2008; Small et al., 2005). In these studies, the emphasis has been on understanding how motivation impacts brain regions and contributes to improving behavioral performance (Braver et al., 2007; Pessoa, 2009). Less is understood about how positive incentives may actually impair cognitive performance. Such knowledge is of importance, however, because diminished behavioral control is a central feature of many clinical and non-clinical groups, including impulsive individuals, ADHD, OCD, and drug abuse populations (Chambers et al., 2009; Li and Sinha, 2008). In particular, the latter appears to be linked to alterations in processes that are involved in optimizing behavioral responses (Garavan and Stout, 2005).
Inhibiting a prepotent response has been investigated both behaviorally, with monkey physiology, and with human ERPs and fMRI by using go/no-go and stop-signal tasks. Inhibition is believed to involve “control regions” in prefrontal cortex (Aron et al., 2007b; Chambers et al., 2009). In particular, the inferior frontal cortex (IFC), especially on the right hemisphere, is thought to be centrally involved in this function (Aron et al., 2003). Other frontal regions, including the pre-supplementary motor area (pre-SMA), anterior cingulate cortex (ACC), superior/medial frontal gyrus, and precentral gyrus, also have been implicated in response inhibition (Floden and Stuss, 2006; Li et al., 2006; Nachev et al., 2007; Picton et al., 2007). Subcortical regions, including the caudate, putamen, and the subthalamic nucleus, appear to be important, too (Aron et al., 2007a; Aron and Poldrack, 2006; Li et al., 2008).
Response inhibition is known to be compromised in, for instance, chronic cocaine users (Hester and Garavan, 2004) and impulsive individuals (Logan et al., 1997), consistent with the notion that it interacts with motivational factors. However, the explicit effect of motivation on inhibition remains largely unexplored and little is known about its neural basis. Here, we were interested in investigating a situation in which reward would be potentially deleterious to behavioral performance. Specifically, we hypothesized that rewarding participants for correct going would impair stopping performance. Critically, we hypothesized that the effects on inhibition would be specific, namely, not simply attributable to a speeding-up of reaction time during go trials. To probe the neural correlates of the interaction between inhibition and motivation, participants performed a stop-signal task under two conditions during functional magnetic resonance imaging (fMRI). During the reward condition, participants were rewarded for correct go performance; no explicit incentive was associated with stopping performance. During the control condition, no incentives were administered. We anticipated that the stop-signal reaction time (SSRT), a measure of the time course of inhibition, would be increased during the reward relative to the control condition. Critically, we expected that this behavioral interaction would be paralleled by changes in evoked responses across brain regions involved in response inhibition. In particular, we predicted that differential responses evoked to successful and unsuccessful stop trials would be reduced during the rewarded vs. control condition, consistent with the notion that stop-task inhibition was harder during the former condition.
Methods
Subjects
Thirty-five volunteers (22 ± 3 years old; 19 females) participated in the study, which was approved by the Institutional Review Board of Indiana University, Bloomington. Subjects were recruited based on responses to flyers posted on different message boards at the Bloomington campus. All subjects were in good health with no past history of psychiatric or neurological disease as assessed by a brief neuropsychiatric interview (MINI) (Sheehan et al., 1998). All participants had normal or corrected-to-normal vision and were free of medications. All participants gave informed written consent. One participant's data were removed from the analysis because of unusually poor performance on go trials (70% correct).
Stimuli and behavioral task
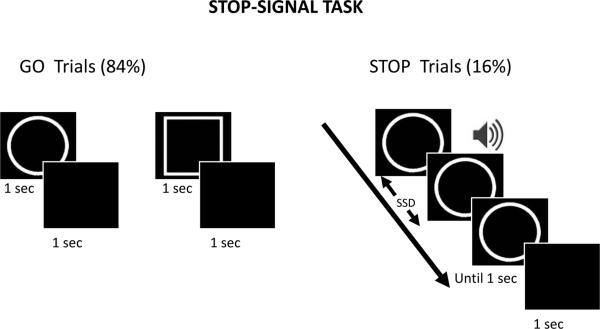
Response inhibition, which requires cancelling an intended action, has been investigated both behaviorally, with monkey physiology, and with human ERPs and fMRI by using go/no-go (Casey et al., 1997; Eimer, 1993; Kalaska and Crammond, 1995) and stop-signal (Aron et al., 2007b; Boucher et al., 2007; Logan, 1994; Logan and Cowan, 1984) tasks. We employed a stop-signal paradigm to investigate the effects of motivation on response inhibition (Fig. 1). We used a simple choice-reaction time task, which included both go and stop trials. Each go trial started with the presentation of a simple shape-stimulus and participants were asked to indicate “circle” or “square” via button-press on an MR-compatible response box by using the index or middle finger of their right hand. Participants were instructed to respond as soon as possible during the presentation of the shape stimulus (trials with reaction time longer than 1 s were treated as incorrect trials). Following the visual stimulus, subjects viewed a blank screen for 1000 ms. Stop trials were identical to go trials, except that a brief tone (duration 300 ms) was played after a variable stop-signal delay (SSD) relative to the onset of the go stimulus, which indicated that participants should withhold their response. The SSD was adjusted dynamically throughout the experiment, such that if the participant successfully inhibited their response on a stop trial, the SSD was increased by 50 ms on a subsequent stop trial, and if the participant failed to inhibit their response, the SSD was reduced by 50 ms on a subsequent stop trial (Logan et al., 1997; Rubia et al., 2003). This staircasing procedure ensured that the participants were successfully inhibiting their response on approximately 50% of the stop trials. Participants were instructed to respond as quickly and accurately as possible and were asked to inhibit their response upon hearing a tone that followed the initial shape stimulus. They were also told that sometimes it might not be possible to successfully inhibit their response and that, in such cases, they should simply continue performing the task. Overall, the importance of going and stopping was stressed equally. Participants performed a short practice run (approximately four minutes) during the initial anatomical scan (see below) to familiarize themselves with the task.
Figure 1.
Stop-signal task paradigm. During go trials (84%) subjects responded to the go signal (circle or square?), whereas during stop trials (16%) they were instructed to withhold the response upon hearing an auditory stop signal. The stop signal followed the go stimulus after a variable-length delay (stop signal delay: SSD), which was updated based on a staircase procedure that maintained behavioral performance at approximately 50% correct.
Each participant performed four runs of the stop-signal task under the control (no-reward) condition and another four runs under the reward condition in an alternating fashion (the order was counterbalanced across participants). The type of run was indicated at the beginning of the run via the presentation of an instruction screen. Separate staircases were used to adjust the SSD during control and reward runs. The initial value of the SSD was set to 250 ms in both staircases. Each run comprised a total of 150 trials, out of which there were 126 (84%) go trials and 24 (16%) stop trials. Go and stop trials contained circle and square shape stimuli in equal proportion.
Reward and control runs contained the same proportion of go and stop trials except that participants had a chance of winning money based on their performance on go trials during reward runs. Specifically, participants were told the following: “During the reward runs of the experiment, each correct answer on go trials will have a potential of winning $1. At the end of each reward run, the program will pick 5 go trials at random and based on your performance on those trials you will be rewarded. The amount awarded will be displayed after each reward run. Overall, you will have the chance of winning an extra $20 based on your performance in the four reward runs”. They were also clearly informed that there was no reward associated with stop trials in this experiment. At the end of the experiment, each participant's base pay ($25) was potentially increased as outlined above and the total amount was paid immediately in cash.
MR data acquisition
MR data were collected using a 3 Tesla Siemens TRIO scanner (Siemens Medical Systems, Erlangen, Germany). Each scanning session began with a high-resolution MPRAGE anatomical scan (TR = 1900 ms, TE = 4.15 ms, TI = 1100 ms, 1 mm isotropic voxels, 256 mm field of view). Subsequently, in each functional run, 153 EPI volumes were acquired with a TR of 2000 and TE of 25 ms. Each volume consisted of 34 axial-slices with a thickness of 3.8 mm and an in-plane resolution of 3.8 × 3.8 mm (240 mm field of view).
Behavioral data analysis
The SSD was adjusted dynamically to yield an inhibition success rate of approximately 50%. The stop-signal reaction time, which provides an estimate of the “inhibitory reaction time”, was calculated by subtracting the average SSD from the median RT during correct go trials, following the race model (Logan and Cowan, 1984). The calculation of SSRT was performed separately for control and reward runs.
General fMRI data analysis
Pre-processing of the data was done using tools from the AFNI software package (Cox, 1996) (http://afni.nimh.nih.gov/afni). The first 3 volumes of each functional run were discarded to account for equilibration effects. The remaining volumes were slice-time corrected using Fourier interpolation such that all slices were realigned to the first slice to account for the timing offset between slices (via program 3dTshift). Six-parameter rigid-body motion correction within and across runs was performed using Fourier interpolation (Cox and Jesmanowicz, 1999) such that all volumes were spatially registered to the volume acquired closest in time to the particular subject's high-resolution anatomy (via program 3dvolreg). To normalize the functional data to Talairach space (Talairach and Tournoux, 1988), initially each subject's high-resolution MPRAGE anatomical volume was spatially registered to the TT_N27 template (in Talairach space) using a 12-parameter affine transformation (Jenkinson and Smith, 2001); the same transformation was applied to the functional data (via program @auto_tlrc). All volumes were spatially smoothed using a Gaussian filter with a full-width at half maximum of 7.6 mm (i.e., two times the voxel dimension; via program 3dmerge). Finally, the signal intensity of each voxel was scaled to a mean of 100.
Voxelwise analysis
Each participant's fMRI data were analyzed by deconvolving the responses of three main event types with shifted delta (i.e., stick) functions (starting at the onset of the shape stimulus): successful stop trials (succ), unsuccessful stop trials (unsucc), and a nuisance event type that included all incorrect go trials. Constant, linear, and quadratic terms were included for each run separately (as covariates of no interest) to model the baseline and drifts of the MR signal. Correct go trials were not modeled explicitly and constituted the implicit baseline in the model. This type of baseline condition has been used successfully in several fMRI studies of the stop-signal task (Chamberlain et al., 2009; Rubia et al., 2003; Rubia et al., 2007). Therefore, all parameters estimates reported in this study are with respect to correct go trials as a baseline. Because a hemodynamic shape was not assumed in our analysis, as an index of response strength, we employed the maximum of the estimated responses at times 4 and 6 s following trial onset.
A central goal of the voxelwise analyses was to determine regions of interest (see below). However, for completeness, a voxelwise analysis was also performed and followed the same 2 inhibition (succ, unsucc) × 2 condition (reward, control) repeated-measures ANOVA that was employed in the region of interest analysis.
Region of Interest analysis
To maximize statistical power, we focused our analysis on a set of regions of interest (ROIs) that were robustly activated by our task. ROIs were defined based on the main effect of inhibition (i.e., succ vs. unsucc trials pooled over control and reward conditions) at a p value of .05, corrected for multiple comparisons according to a false discovery rate procedure (Genovese et al., 2002). We adopted this selection criterion to determine ROIs because it was statistically independent of the central goal of our analysis, namely, to probe inhibition by condition interactions (see below) (Kriegeskorte et al., 2009; Vul et al., 2009). Individual ROIs were drawn using a sphere of 5-mm radius centered at peak voxel of each cluster (at the group level). A representative time series for the ROI was then defined by averaging across all the voxels.
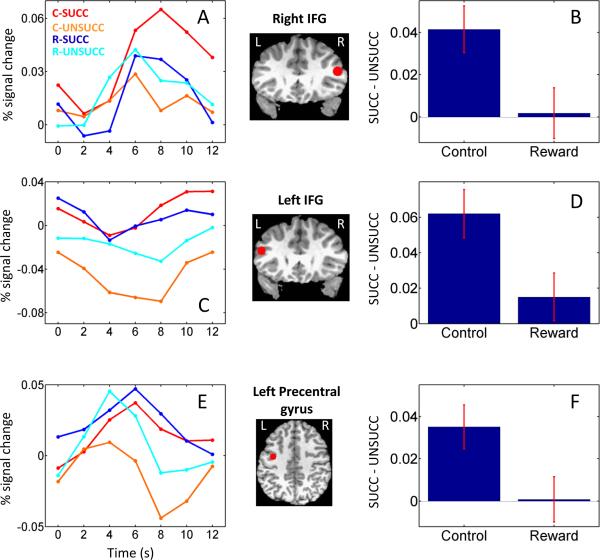
As in the voxelwise analysis, as an index of response strength, we employed the maximum of the estimated responses at times 4 and 6 s following trial onset. This procedure was applied for all ROIs, except the right inferior frontal gyrus, because the responses in this region appeared to be slightly shifted in time (see Fig. 2A). For this region, the maximum of the estimated responses at times 6 and 8 s was employed as the index of response strength. Note that this procedure did not change our results as employing the maximum of the estimated responses at times 4 and 6 s following trial onset also revealed an interaction between inhibition and motivation (p < .05; see below).
Figure 2.
Average deconvolved responses (relative to correct go trials) as a function of trial type in the right inferior frontal gyrus (R IFG; A), left inferior frontal gyrus (L IFG; C), and left precentral sulcus (E). Average stop-trial differential activation as a function of condition in the right IFG (B), left IFG (D), and left precentral sulcus (F).The brain insets indicate the position of the ROIs. Error bars indicate standard within-subject errors (Loftus and Masson, 1994). C: control; R: reward; succ: successful stop trial; unsucc: unsuccessful stop trial.
Our main objective was to assess how the neural correlates of response inhibition were modulated by motivation. As others have suggested, to investigate the neural correlates of response inhibition in the stop-signal task, we contrasted succ and unsucc trials; e.g., (Li et al., 2006; Rubia et al., 2003). Accordingly, for each ROI, response strength was submitted to a 2 inhibition (succ, unsucc) × 2 condition (reward, control) repeated-measures ANOVA. In Table 2, we report regions exhibiting an inhibition by condition interaction. To potentially maximize the behavioral effect of motivation, the control and reward conditions were administered in separate scanning runs. In addition, go trials were employed as an implicit baseline condition in our estimation procedure. For these reasons, our study was not optimally suited to determine the main effect of motivation per se (Engelmann et al., 2009; Locke and Braver, 2008); we thus do not report this main effect.
Table 2.
ROI analysis (peak Talairach coordinates and F values)
| Location | x | y | z | COND × INHIB, F(1,33) | |
|---|---|---|---|---|---|
| SUCC > UNSUCC | |||||
| Parietal | |||||
| Intraparietal sulcus | R | 24 | −56 | 55 | 5.25* |
| L | −26 | −51 | 54 | 5.01* | |
| Inferior Parietal lobe | R | 35 | −41 | 47 | 4.69* |
| Frontal | |||||
| Frontal Eye Field | R | 28 | −13 | 51 | 0.82 |
| L | −24 | −12 | 51 | 0.97 | |
| Precentral gyrus | L | −38 | −7 | 41 | 9.71*** |
| Middle Frontal gyrus | L | −28 | 1 | 49 | 2.44 |
| Inferior Frontal gyrus | R | 47 | 26 | 19 | 7.0** |
| L | −45 | 23 | 23 | 7.09** | |
| Superior Frontal gyrus | L | −13 | 31 | 46 | 0.11 |
| Subcortical | |||||
| Putamen | R | 21 | 4 | 3 | 5.56* |
| L | −21 | 4 | 3 | 4.83* | |
| Caudate | R | 11 | 6 | 1 | 0.02 |
| L | −8 | 6 | 1 | 0.77 | |
| UNSUCC > SUCC | |||||
| Central sulcus | L | −36 | −27 | 51 | 0.2 |
| Posterior Cingulate cortex | L | 2 | −25 | 32 | 2.01 |
| Anterior Insula | L | −35 | 18 | 9 | 0.12 |
| Anterior Cingulate | R/L | 1 | 23 | 31 | 0.03 |
Bold font: statistically significant results:
p < 0.05
p < 0.01
p < 0.005
SUCC: successful inhibition; UNSUCC: unsuccessful inhibition.
Results
Behavioral results
Behavioral results are summarized in Table 1. A small improvement in behavioral accuracy was observed for go performance. Go error rate was 2.3% during the control condition and 1.5% during the reward condition (p < .01; Wilcoxon signed rank test). However, no significant differences were observed in the case of reaction time (control: 487.1 ms; reward: 484.0 ms; t(33) = .71, p = .48). As expected, because of the staircasing procedure, stop performance was approximately 50% correct during both conditions (control: 50.5%; reward: 49.1%; t(33) = .83, p = .41). Critically, SSRT was longer during the reward (213.2 ms) relative to the control condition (192.6 ms) [t(33) = 2.78, p < 0.01], revealing that it was harder to inhibit the behavioral response during the former condition. Finally, during both conditions, the reaction time of unsucc trials was faster than those of correct go trials (control: t(33) = 2.75, p < .01; reward: t(33) = 6.63, p < .001), in line with predictions of the race model (Logan and Cowan, 1984).
Table 1.
Behavioral performance: mean results
| Control | Reward | |
|---|---|---|
| Go RT (ms)a | 487.1 ± 18.6 | 484.0 ± 19.12 |
| Inhibition rate (%) | 50.5 ± 0.6 | 49.1 ± 0.8 |
| SSD (ms) | 294.5 ± 25.8 | 270.7 ± 26.1* |
| SSRT (ms) | 192.6 ± 11.0 | 213.2 ± 10.9* |
| UNSUCC RT (ms) | 461.0 ± 17.4 | 471.7 ± 17.7 |
| Go error Rate (%) | 2.3 ± 0.3 | 1.5 ± 0.2* |
values reported are the mean of individual median RTs.
p < 0.01. SSD: stop-signal delay; SSRT: stop-signal reaction time; UNSUCC: unsuccessful stop trial
fMRI results
To maximize statistical power, interactions between motivation and inhibition were probed across a set of ROIs that were strongly engaged during response inhibition (Table 2). ROI signals were tested via a 2 inhibition (succ, unsucc) by 2 condition (control, reward) repeated-measures ANOVA. Consistent with a growing body of literature, in a voxel-based analysis, a main effect of inhibition was observed throughout parietal and frontal regions, in addition to subcortical ones (Table 2). These regions included the parietal cortex, precentral gyrus, ACC, superior frontal gyrus, middle frontal gyrus (MFG), inferior frontal gyrus (IFG), caudate, and putamen. In most of these regions, responses observed during succ trials were stronger than unsucc trials. Regions with the reverse pattern included the left anterior insula, posterior cingulate cortex, and ACC.
Significant inhibition by condition interactions were observed in frontal cortex in bilateral IFG and left precentral gyrus. For instance, in the right IFG (Fig. 2A, B), succ trials evoked stronger responses relative to unsucc trials, but whereas this difference was sizeable during control trials, it was greatly reduced during the reward condition. It is also of interest that, for unsucc trials, evoked responses appeared to more steeply decline after the peak (at 6 s), while this was not the case for succ trials (especially during the control condition). In addition, the peak of the estimated response during succ trials in the control condition was slightly shifted in time, similar to that of a previous report (Rubia et al., 2003). Although an inhibition by condition interaction was also observed in the left IFG, a very different pattern of results was observed (Fig. 2C, D). Finally, the pattern observed in the left precentral gyrus was similar to that observed in the right IFG (Fig. 2E, F). Although robust responses were observed during the reward condition, comparable responses were obtained for succ and unsucc trials. During the control condition, however, succ responses were considerably larger than unsucc responses.
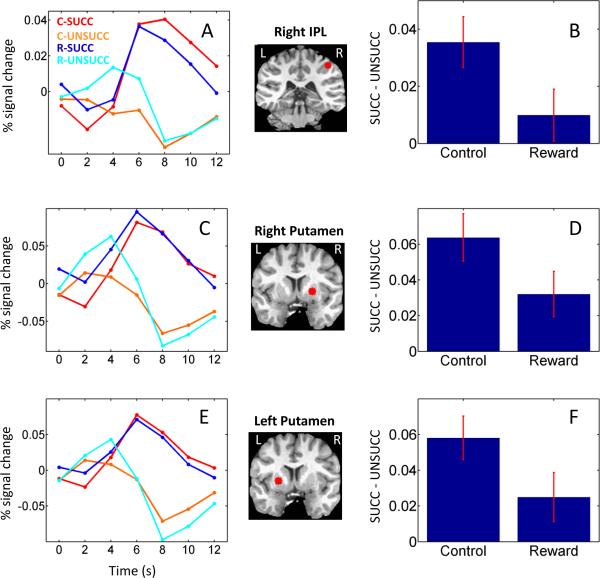
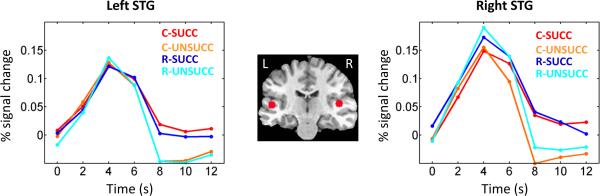
We also observed significant inhibition by condition interactions at parietal sites, including the right inferior parietal lobe (IPL; Fig. 3A, B) and bilateral intraparietal sulcus (IPS). In these regions, the difference between succ and unsucc trials was greater during the control relative to the reward condition. An additional inhibition by condition interaction was observed in the right (Fig. 3C, D) and left putamen (Fig. 3E, F). Finally, although the focus of our investigation involved cortical and sub-cortical regions that are believed to be involved in response inhibition, as a control analysis, we evaluated the data in three sensory ROIs that evoked robust responses to the auditory and visual stimuli employed in the task, namely, right and left superior temporal gyrus (STG) and early visual cortex. Across the three ROIs, no main effect of inhibition or inhibition by condition interaction was detected (Fig. 4 shows responses in STG).
Figure 3.
Average deconvolved responses (relative to correct go trials) as a function of trial type in the right inferior parietal lobe (R IPL; A), right putamen (C), and left putamen (E). Average stop-trial differential activation as a function of condition in the right IPL (B), right putamen (D), and left putamen (F). The brain insets indicate the position of the ROIs. Error bars indicate standard within-subject errors (Loftus and Masson, 1994). C: control; R: reward; succ: successful stop trial; unsucc: unsuccessful stop trial;
Figure 4.
Average deconvolved responses (relative to correct go trials) as a function of trial type in the left (A) and right (B) superior temporal gyrus (STG). No significant differences were observed. The brain insets indicate the position of the ROIs. C: control; R: reward; succ: successful stop trial; unsucc: unsuccessful stop trial.
For completeness, we also report the results of whole-brain analyses separately for control and reward conditions, as well as inhibition by condition interaction results (Table 3). Overall, stop-signal inhibition effects were relatively weak during the reward condition, which resulted in inhibition by condition interactions across several frontal and subcortical regions believed to be involved in response inhibition (note that these activations did not survive, however, correction for multiple comparisons). Note that the F values reported in Table 3 (voxelwise analysis) are somewhat larger than those observed in Table 2 (ROI analysis). There are at least two reasons why the statistical values differ. For one, the ROI analysis employed a representative time series that was obtained by averaging all of the voxels inside the ROI (5-mm sphere) – which likely decreases some of the highest contributions from “peak voxels”. In contrast, the voxelwise analysis reports “peak” F values. A related factor is that we performed our ROI inferences via a two-step procedure, by first defining the ROI based on the effect of inhibition and then testing for interaction effects on the resulting representative time series. This kind of ROI selection was used to prevent any circularity in the results of the ROI analysis that can arise from selection procedures (Kriegeskorte et al., 2009; Vul et al., 2009). Therefore, the two procedures of assessing statistical interactions (ROI and voxelwise) between reward and inhibition do not map to each other in a simple direct manner.
Table 3.
Voxelwise analysis (peak Talairach coordinates, t, and F values)
| INHIBITION (Control) | INHIBITION (Reward) | COND × INHIB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | x | y | z | t(33) | x | y | z | t(33) | x | y | z | F(1,33) | |
| SUCC > UNSUCC | |||||||||||||
| Parietal | |||||||||||||
| Intraparietal sulcus | R | 26 | −55 | 53 | 3.96* | 23 | −56 | 56 | 1.69 | 29 | −59 | 47 | 11.82U |
| L | −19 | −60 | 48 | 4.15* | 28 | −56 | 53 | 1.54 | −30 | −56 | 54 | 5.05 | |
| Inferior Parietal lobe | R | 36 | −38 | 38 | 4.25* | 31 | −39 | 45 | 1.45 | 29 | −39 | 53 | 12.47U |
| Frontal | |||||||||||||
| Frontal Eye Field | R | 25 | −15 | 50 | 3.87* | 29 | −10 | 47 | 3.05 | 23 | −13 | 53 | 1.26 |
| L | −22 | −15 | 52 | 4.19* | −21 | −16 | 50 | 3.1 | 25 | 13 | 55 | 2.6 | |
| Precentral gyrus | L | −34 | −10 | 41 | 3.60* | −41 | −5 | 41 | 1.03 | −41 | −9 | 41 | 14.05U |
| Middle Frontal gyrus | L | −27 | 1 | 50 | 4.81* | −28 | 1 | 52 | 2.44 | −28 | 0 | 49 | 3.35 |
| Inferior Frontal gyrus | R | 49 | 25 | 17 | 4.48* | 47 | 23 | 20 | 1.47 | 50 | 31 | 18 | 14.89U |
| L | −47 | 23 | 25 | 4.19* | −43 | 23 | 20 | 1.78 | −49 | 20 | 26 | 11.27U | |
| Superior Frontal gyrus | L | −9 | 27 | 49 | 3.61* | −19 | 22 | 53 | 2.76 | −13 | 31 | 46 | 0.94 |
| Subcortical | |||||||||||||
| Putamen | R | 21 | 6 | 0 | 5.18* | 26 | 3 | 2 | 3.14 | 23 | 2 | 2 | 10.31U |
| L | −21 | 4 | 9 | 5.68* | −21 | 4 | 3 | 1.75 | −18 | 4 | 7 | 9.38U | |
| Caudate | R | 11 | 5 | 2 | 2.86 | 11 | 5 | 3 | 2.82 | 11 | 6 | 1 | 0.4 |
| L | −12 | 6 | 2 | 2.89 | −8 | 8 | 2 | 3.52* | −9 | 6 | 7 | 2.57 | |
| UNSUCC > SUCC | |||||||||||||
| Central sulcus | L | −37 | −25 | 51 | 3.97* | 37 | 25 | 51 | 4.26* | −37 | −27 | 54 | 0.16 |
| Posterior Cingulate cortex | L | 0 | −25 | 31 | 4.97* | 1 | −22 | 35 | 2.41 | 2 | −23 | 28 | 2.73 |
| Anterior Insula | L | −35 | 13 | 11 | 3.33 | −35 | 11 | 5 | 3.64* | −28 | 18 | 10 | 1.05 |
| Anterior Cingulate cortex | R/L | 1 | 17 | 35 | 4.53* | 1 | 17 | 34 | 3.94* | 1 | 26 | 34 | 0.28 |
Bold font: statistically significant results:
p < 0.05 (False Discovery Rate corrected)
p < 0.005 (uncorrected).
SUCC: successful inhibition; UNSUCC: unsuccessful inhibition.
Discussion
In this paper, we investigated the interaction between inhibition and motivation. To do so, participants performed a stop-signal task during two conditions, namely, during a neutral, control condition and during a motivated condition during which they were rewarded for correct go performance. Behaviorally, participants exhibited longer SSRTs during the reward relative to the control condition, indicating that it was harder to inhibit their responses during the former condition. Our neuroimaging findings revealed that a host of brain regions were involved in stop-signal inhibition, as indexed via a contrast of successful (succ) and unsuccessful (unsucc) stop trials. Critically, a subset of these regions exhibited significant inhibition by condition interactions, demonstrating that cognitive and motivational signals interact in the brain during signal-task inhibitory control.
Behavioral findings
The stop-signal task has been widely utilized in the study of response inhibition. A strength of this paradigm is that it allows the estimation of the “inhibitory reaction time” (SSRT), which is by definition, unobserved. The SSRT has been characterized in several clinical and non-clinical populations, including impulsive individuals (Logan et al., 1997), cocaine dependents (Colzato et al., 2007; Fillmore and Rush, 2002; Li et al., 2007), and ADHD (Alderson et al., 2007) and schizophrenia populations (Enticott et al., 2008), among others. The SSRT is remarkably stable across individuals and studies and typically averages in the range of 200-300 ms.
In the present experiment, participants exhibited longer SSRT during the rewarded (213 ms) relative to the control (193 ms) condition. At the same time, no difference between go RT was observed as a function of experimental condition, demonstrating the specificity of the impact of the motivational manipulation. Taken together, these results reveal that, in our task, motivation impaired inhibitory processing rather than facilitated the execution of prepotent responses; see also (Fillmore and Vogel-Sprott, 2000). In other words, during the reward condition, participants encountered difficulty inhibiting prepotent responses not because these responses were unusually fast, but instead because their inhibitory responses, which countermanded their go responses, were significantly slowed down.
Neuroimaging findings
Previous neuroimaging studies of response inhibition have identified a network of brain regions believed to be engaged by inhibitory processes. Important nodes are thought to include the superior frontal gyrus (Li et al., 2006) and the IFC, possibly extending into the anterior insula (Aron, 2007). Lesion and transcranial magnetic stimulation studies converge on the suggestion that the right IFC is a critical locus in inhibitory control (Aron et al., 2003; Chambers et al., 2006). Although, in general, neuroimaging studies report a set of parietal, frontal, and subcortical sites believed to be involved in response inhibition, the emphasis on specific brain regions has depended on the type of index used to identify the neural correlates of inhibition. In particular, two strategies can be highlighted. One approach has contrasted succ trials to go trials (Aron and Poldrack, 2006; Xue et al., 2008), whereas a second strategy has contrasted succ vs. unsucc trials (Li et al., 2006; Rubia et al., 2003). While both approaches have contributed to our understanding of the neural basis of executive control during stop-signal inhibition, we opted for the latter strategy as both trial types are matched along several important dimensions of interest. In particular, the contrast of succ and go trials is unmatched for stimulus frequency and potential “oddball” effects. The rationale for contrasting succ trials to go trials is, at times, that the outcome on a stop trial (successful or unsuccessful) depends mainly on the speed of the go process; e.g., (Aron and Poldrack, 2006; Leung and Cai, 2007). However, according to the race model (Logan and Cowan, 1984), the outcome of a stop trial depends on the relative finishing times of both the go and stop processes. Accordingly, even during trials with no change in the speed of the go process, variability in stop processing will lead to, at times, the go process winning the race if it finishes before the stop process. Furthermore, when the go process is faster (as in unsucc trials), it may lead to weaker stop responses because central resources may be shared between go and stop processes (Boehler et al., 2009). Note that the latter point does not violate the independence assumption of the race model, because the race model is not a process model. In other words, it only describes the finishing times of go and stop processes and does not specify the exact nature of the underlying mechanisms (Logan, 1994; Verbruggen and Logan, 2008). Nevertheless, we do not claim that the contrast of succ vs. unsucc trials perfectly isolates purely inhibitory processes, as differences in attention may also contribute to the associated differential responses (see below).
An important challenge when studying the contributions of motivation to evoked brain responses is to disentangle specific effects of motivation from relatively unspecific effects of arousal or effort. Accordingly, in the present experiment, stop trials occurred infrequently within a rapid stream of go trials, which were blocked according to condition. In this manner, any changes to the go process were explicitly incorporated into the baseline, as stop-task inhibition was indexed via the contrast of succ and unsucc trials. This contrast revealed a network of brain regions that largely overlapped with that reported in other studies. In particular, the right IFG exhibited stronger evoked responses during succ vs. unsucc trials, a result that has been observed in several (Chamberlain et al., 2009; Li et al., 2006; Rubia et al., 2003; Rubia et al., 2007), but not all (Aron and Poldrack, 2006), studies in the literature. As stated, the succ vs. unsucc contrast used in the present study may not entirely isolate inhibitory processes engaged by the stop-signal task. In particular, this contrast may include potential differences in attention, as “low” attentional engagement may be associated with unsuccessful inhibition while “high” attentional engagement may be associated with successful inhibition. Consistent with this notion, a recent MEG study by Boehler et al. (2009) revealed that fluctuations of sensory processing linked to both go and stop stimuli impact inhibitory performance during the stop-signal task. Furthermore, given the role of the right IFC in attentional mechanisms (Corbetta and Shulman, 2002), the succ vs. unsucc contrast may also engage this region during the stop-signal task for reasons that are more attentional than inhibitory; similar considerations apply to other regions of the “attentional network”.
Because longer SSRTs were observed during the rewarded condition, based on findings reporting reduced prefrontal engagement in inhibitory control in ADHD (Aron et al., 2007b; Dimoska et al., 2003; Rubia et al., 1999; Tamm et al., 2004), schizophrenia (Rubia et al., 2001), and cocaine dependence (Li et al., 2007), we expected reduced inhibitory-related responses in brain regions involved in inhibition during the motivation condition of our task relative to control. Consistent with this notion, succ vs. unsucc differential responses observed in the right IFG were reduced during rewarded trials.
Although the right IFC is more consistently reported to be involved in response inhibition, the IFC on the left hemisphere has been reported to exhibit differential succ vs. unsucc responses (Li et al., 2006; Rubia et al., 2007) and has been found to be important for response inhibition based on a recent lesion study (Swick et al., 2008). In the present study, a different interaction pattern of results was observed in the left IFG. Relative to the go baseline, stop-related responses were negative. Interestingly, during the control condition, succ trials evoked a larger response (i.e., less negative) than unsucc trials, and this difference decreased during the motivated condition. It thus appears that the left IFG is engaged by both go and stop processes and that motivation reduces differential responses.
Other brain regions that exhibited significant stop-signal inhibition by motivation interactions included the left precentral gyrus, regions in parietal cortex, and bilateral putamen. The precentral gyrus in involved in carrying out stimulus-response associations (Brass et al., 2009) and has been suggested to be a key “inhibitory motor area” (Li et al., 2006). Here, we show that whereas a robust differential response succ vs. unsucc was observed during the control condition, this difference was reduced during the reward condition. Although neuroimaging studies of both go/no-go and stop-signal tasks have identified sites in parietal cortex, their precise role in inhibition is less well understood. For instance, Li et al. (2006) did not observe differential activation in parietal cortex when succ and unsucc trials were contrasted. In the present study, the right IPL exhibited stronger responses for succ vs. unsucc trials, as well as a significant inhibition by motivation interaction (the latter was observed in the IPS, too). Given the important contribution of the parietal cortex to attentional processes (Corbetta and Shulman, 2002; Kastner and Ungerleider, 2000), it is possible that, during the reward condition, participants paid increased attention to the go stimulus. Fewer attentional resources may have then been available to process the stop stimulus, thereby impairing overall inhibitory performance – leading to an increase in SSRT (Pessoa, 2009). This interpretation is consistent with the MEG study cited above in which successful inhibition was accompanied by attenuated sensory processing of the go stimulus (Boehler et al. 2009) and, more generally, with the notion that go and stop may share processing resources – which in the present case may have been redistributed between go and stop processes given the motivational manipulation. Finally, the putamen has been implicated both in response inhibition (Chambers et al., 2009; Eagle and Robbins, 2003) and motivation (Schultz, 2000). The present findings provide further support not only for its role in these two processes but, critically, for the putamen's involvement during the interaction of cognitive inhibitory operations and motivational processes.
The pattern of main effects and interactions we observed in this study was specific to cortical and subcortical regions previously implicated in response inhibition and were not observed in additional ROIs that were likely engaged by the sensory stimuli (visual and auditory) employed in this task. These ROIs were engaged to a similar extent during all trial types.
In the present study, like others in the past, we employed the contrast of succ vs. unsucc trials to probe the neural correlates of stop-signal response inhibition. As reviewed above, an inhibition by condition statistical interaction was observed in several brain regions, a pattern of results that was paralleled by an increase in SSRT during the reward condition. Inspection of the results in Figure 2 illustrates that differential succ vs. unsucc responses were in some cases sharply reduced during the reward condition (e.g., for the right IFG). Despite this pattern of fMRI results, the staircase procedure was still able to maintain successful performance at about 50% correct – albeit with a slowed SSRT. Therefore, if the contrast of succ vs. unsucc trials is to be considered a valid index of response inhibition, collectively, our results suggest that response inhibition should not be viewed as the product of only regions exhibiting inhibition by condition interactions (e.g., right IFG), but also regions that exhibited a main effect of inhibition but no significant statistical interaction (e.g., caudate). In other words, the concerted activation across a network of regions, some of which were affected by the motivational manipulation employed here, is likely needed for the generation of the countermanding motor commands required during stop-signal response inhibition.
Although our study clearly revealed an interaction between rewarding go behavior and stop-task inhibition in several cortical and subcortical regions previously implicated in response inhibition, we were not able to investigate these interactions at a more mechanistic level because the go trials were used as an implicit baseline. In other words, the current study identified some of the sites whereby motivation and stop-task inhibition interact, but left unanswered important questions. For instance, the sites identified here may receive inputs from other regions that integrate motivation and inhibition, a possibility that should be addressed in future studies. In this context, techniques such as dynamic causal modeling (Friston et al., 2003) and other forms of computational modeling may provide invaluable tools to probe these questions at a more mechanistic level.
A growing literature has documented that impairments in response inhibition in clinical populations is linked to reduced activation in prefrontal cortex. For instance, adolescents with ADHD exhibited reduced activation in the IFC during an inhibitory task (Rubia et al., 2005). We showed here that a similar pattern of results is observed when participants are rewarded for their go performance during a stop-signal task. Our results thus demonstrate that, under specific circumstances, positive incentives are capable of impairing behavioral performance. Future studies are needed to probe how motivational manipulations of the type employed here affect inhibitory circuits in clinical populations that are known to exhibit compromised behavioral control.
Acknowledgements
We thank the anonymous reviewers for valuable feedback and Andrew Bauer for assistance with figures. Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589) and the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007a;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DW, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience. 2007b;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Munte TF, Krebs RM, Heinze HJ, Schoenfeld MA, Hopf JM. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cerebral Cortex. 2009;19:134–145. doi: 10.1093/cercor/bhn063. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychological Reviews. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Brass M, Wenke D, Spengler S, Waszak F. Neural correlates of overcoming interference from instructed and implemented stimulus-response associations. Journal of Neuroscience. 2009;29:1766–1772. doi: 10.1523/JNEUROSCI.5259-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford University Press; Oxford: 2007. pp. 76–106. [Google Scholar]
- Casey BJ, Trainor R, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Campo ND, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, et al. Atomoxetine Modulates Right Inferior Frontal Activation During Inhibitory Control: A Pharmacological Functional Magnetic Resonance Imaging Study. Biological Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive “brake failure” following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. Public Library of Science ONE. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox R, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biological Psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behavioral Neuroscience. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju EC, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Ogloff JR, Bradshaw JL. Response inhibition and impulsivity in schizophrenia. Psychiatry Research. 2008;157:251–254. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. Journal of Studies on Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cerebral Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. Journal of Neuroscience. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural Correlates of Impulse Control During Stop Signal Inhibition in Cocaine-Dependent Men. Neuropsychopharmacology. 2007;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience & Biobehavioral Reviews. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee T-W. Sub-cortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive Affective Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The Spatial Attention Network Interacts with Limbic and Monoaminergic Systems to Modulate Motivation-Induced Attention Shifts. Cerebral Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36(Suppl 2):T155–163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive function? Trends in Cognitive Sciences. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore ET. Hypofrontality in Attention Deficit Hyperactivity Disorder during higher order motor control: a study using fMRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex. 2005;15:1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BioMed Central Neuroscience. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxis atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives in Psychological Science. 2009;4 doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cerebral Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]