Abstract
Objectives
It was the aim of this study to examine the influence of the antiviral medication oseltamivir on stroke and transient ischemic attack (TIA) in adults diagnosed with influenza.
Methods
This retrospective cohort study used medical and pharmaceutical claims data from May 2000 to September 2006 from an administrative claims database. Episodes of stroke/TIA in the 6 months after influenza in adults (aged ≥18 years) prescribed oseltamivir within 1 day before or 2 days after influenza diagnosis (oseltamivir cohort), and adults prescribed no antivirals (comparison cohort) were compared using multivariate analyses adjusted for demographic and clinical risk factors.
Results
The oseltamivir cohort comprised 49,238 patients and the comparison cohort 102,692 patients. Oseltamivir was associated with a 28% reduction in risk of stroke/TIA in the 6 months after influenza [hazard ratio (HR) 0.72; 95% CI 0.62–0.82] and with significant reductions after 1 and 3 months. In patients <65 years of age, there was a 34% risk reduction with oseltamivir after 6 months (HR 0.66; 95% CI 0.56–0.77) and also significant reductions after 1 and 3 months. In those aged ≥65 years, there was a 51% reduction in risk after 1 month (HR 0.49; 95% CI 0.27–0.91).
Conclusions
Prescription of oseltamivir for influenza is associated with a reduced risk of stroke/TIA.
Key Words: Cardiovascular epidemiology, Cardiovascular risk factors, Influenza infection, Ischemic heart disease, Stroke
Introduction
Acute infections such as influenza have been associated with an increased risk of cardiovascular disease, including ischemic stroke. Higher rates of cardiovascular events in winter and/or coincident with the influenza season have been reported [1, 2, 3, 4, 5]. Influenza has been estimated to cause as many as 90,000 cardiac deaths per annum by triggering acute coronary events [1]. However, the true burden of influenza-related cardiovascular morbidity and mortality is generally neglected and under-reported since influenza is not recognized and acknowledged as a contributing factor while reporting the events [1].
Influenza vaccination is the most effective and widely accepted method for influenza prevention. Previous studies have shown that influenza vaccination can reduce the risk of cardiovascular events including myocardial infarction and stroke [1, 6, 7, 8, 9, 10, 11, 12]. However, influenza vaccination is not always fully effective [13], especially when there is a strain mismatch and, in the elderly, due to lower antigenic response. In addition, rates of vaccine uptake are far below optimum, including in subjects at high risk of cardiovascular diseases [8, 13, 14]. Therefore, many individuals remain at risk of influenza infection and its complications.
Once an individual is infected with influenza, antiviral agents, such as the neuraminidase inhibitor oseltamivir, are effective in treating the symptoms of influenza and reducing the duration of illness [15, 16, 17, 18]. Previous studies have hypothesized that oseltamivir may be associated with a reduced risk of hospitalization in patients with influenza and a reduced risk of death in hospitalized patients [18, 19, 20].
We hypothesized that the risk of stroke or transient ischemic attack (TIA) after influenza infection may be reduced by oseltamivir treatment. Therefore, we conducted a retrospective cohort study in a large group of subjects to examine the rates of stroke and TIA in adults prescribed oseltamivir for influenza.
Subjects and Methods
Study Design and Patients
In this retrospective cohort study from May 2000 to September 2006, outcomes in the 6 months following influenza diagnosis in adults (aged ≥18 years) were compared for patients prescribed oseltamivir and those who did not receive any antiviral medication.
Anonymous, patient-level medical and pharmaceutical claims data were obtained from a managed-care database from a large insurer with >10 million covered lives. Data were primarily from health maintenance organization plans, with other types of plans included, such as Medicare Risk Plans (when data were complete for patients aged ≥65 years). Patients with International Classification of Diseases, 9th Revision, Clinical Modifications (ICD-9-CM) diagnosis codes for influenza, 487.0, 487.1 or 487.8 on outpatient claims were identified. The date of the first claim for influenza was used as the study index date for each patient. Patients’ demographic and medical history data and history of risk factors for stroke during the 12 months before the index date were collected.
Patients were excluded from the study if they were not continuously insured for 12 months before influenza diagnosis. Patients with inpatient hospital, inpatient psychiatric facility or emergency room influenza claims were excluded as full drug data may not have been reported. Patients with only laboratory claims were excluded due to the possibility of ‘rule-out’ diagnoses. Only the first claim for influenza for each patient during the study period was included.
Patients were included in the oseltamivir cohort if they had a claim for oseltamivir within 1 day before or 2 days after the index date and no other antiviral drug claim within ±6 months of that date, and in the comparison cohort, if they had no claims for antiviral medication (i.e. oseltamivir, zanamivir, amantadine or rimantadine) within ±6 months of the index date. Patients prescribed oseltamivir for prophylaxis were not included in this study.
Outcomes
The overall incidence rates of stroke or TIA in the 6 months following the index date were evaluated. Patients who subsequently died as a result of a stroke or TIA were included in the analysis. Events were identified from claims with the ICD-9-CM codes listed in table 1 for at least 1 inpatient, outpatient or emergency room visit, but not from diagnosis codes found on laboratory orders alone.
Table 1.
Diagnosis codes for stroke-related outcomes
| Diagnosis |
ICD-9-CM code |
|---|---|
| Hemorrhagic stroke | |
| Subarachnoid hemorrhage | 430 |
| Intracerebral hemorrhage | 431 |
| Other and unspecified intracranial hemorrhage | 432.xx |
| Thromboembolic stroke | |
| Occlusion and stenosis of precerebral arteries | 433.xx |
| Occlusion of cerebral arteries | 434.xx |
| Transient cerebral ischemia | 435.xx |
| Other | |
| Acute but ill-defined cardiovascular disease | 436 |
Statistical Analyses
Analyses were conducted for all patients in the 2 cohorts and also for the 2 cohorts further subdivided into patients <65 and ≥65 years of age. Demographic and medical history characteristics were compared between the 2 cohorts using χ2, Fisher's exact or 2-sided Wilcoxon rank-sum tests, and histories of stroke risk factors using χ2 tests. Rates of stroke or TIA were calculated as overall incidence rates with 95% confidence intervals (CIs) and as incidence rate ratios (IRRs), also with 95% CIs.
As the 2 cohorts were not fully matched, multivariate analyses (Cox proportional hazard regression models) adjusted for demographic and clinical risk factors (table 2) were used to examine the influence of oseltamivir on the outcomes. Results were expressed as hazard ratios (HRs) with 95% CIs. To decide whether or not to include a variable as a predictor in the multivariate model, stepwise regression with an α of 0.05 was used, with the variable of interest – a claim for oseltamivir – forced into the model. If variables were found to be non-significant in the stepwise regression analysis, they were not utilized in the multivariate model.
Table 2.
Patients' demographic characteristics, medical histories and stroke risk factors in the 12 months before influenza
| Oseltamivir (n = 49,238) |
Comparison (no antiviral) (n = 102,692) |
p value |
|||
|---|---|---|---|---|---|
| Age | Mean ± SD, years | 39.94 ± 12.69 | 42.38 ± 14.79 | ||
| <65 years | 48,048 (97.58) | 95,300 (92.80) | } | <0.0001 | |
| ≥65 years |
1,190 (2.42) |
7,392 (7.20) |
|||
| Gender | Female | 26,250 (53.31) | 59,011 (57.46) | ||
| Male | 22,979 (46.67) | 43,661 (42.52) | <0.0001 | ||
| Not known |
9 (0.02) |
20 (0.02) |
|||
| Comorbidities1 | History of stroke/cerebrovascular disease | 262 (0.53) | 1,382 (1.35) | <0.0001 | |
| Hypertension | 5,820 (11.82) | 16,088 (15.67) | <0.0001 | ||
| Diabetes mellitus | 2,093 (4.25) | 6,212 (6.05) | <0.0001 | ||
| Hyperlipidemia | 4,850 (9.85) | 11,463 (11.16) | <0.0001 | ||
| Obesity | 3,670 (7.45) | 9,119 (8.88) | <0.0001 | ||
| Emphysema/COPD | 344 (0.70) | 2,090 (2.04) | <0.0001 | ||
| Sickle cell anemia | 2 (0.00) | 16 (0.02) | NS | ||
| Atrial fibrillation | 209 (0.42) | 1,164 (1.13) | <0.0001 | ||
| AMI | 98 (0.20) | 402 (0.39) | <0.0001 | ||
| Other ischemic heart disease | 1,063 (2.16) | 3,835 (3.73) | <0.0001 | ||
| Other heart disease/conditions | 634 (1.29) | 3,072 (2.99) | <0.0001 | ||
| Cancer | 783 (1.59) | 2,544 (2.48) | <0.0001 | ||
| Cerebrovascular revascularization | 10 (0.02) | 42 (0.04) | 0.0423 | ||
| Coronary revascularization | 134 (0.27) | 371 (0.36) | 0.0047 | ||
| Pneumonia (index date/previous 30 days) | 86 (0.17) | 1,710 (1.67) | <0.0001 | ||
| Chronic renal disease/failure | 232 (0.47) | 1,407 (1.37) | <0.0001 | ||
| Peripheral vascular disease | 213 (0.43) | 1,097 (1.07) | <0.0001 | ||
| Dementia | 58 (0.12) | 410 (0.40) | <0.0001 | ||
| Rheumatoid arthritis | 215 (0.44) | 659 (0.64) | <0.0001 | ||
| Systemic lupus erythematosus | 98 (0.20) | 226 (0.22) | NS | ||
| Arteritis/polyarteritis | 23 (0.05) | 108 (0.11) | 0.0003 | ||
| Inflammatory diseases of the CNS | 22 (0.04) | 88 (0.09) | 0.0054 | ||
| Epilepsy | 83 (0.17) | 294 (0.29) | <0.0001 | ||
| Migraine |
1,014 (2.06) |
2,270 (2.21) |
NS |
||
| Treatments in the preceding 12 months | Influenza vaccination | 1,987 (4.04) | 8,577 (8.35) | <0.0001 NS | |
| Other vaccinations | 1,980 (4.02) | 4,137 (4.03) | |||
| Antibiotic use (index date/previous 9 days) | 8,869 (18.01) | 27,689 (26.96) | <0.0001 | ||
| Statin use | 4,776 (9.70) | 10,084 (9.82) | NS | ||
| Estrogen/progestin2 | 10,190 (20.70) | 19,639 (19.12) | <0.0001 | ||
| β-Blocker use | 3,422 (6.95) | 8,396 (8.18) | <0.0001 | ||
| Clopidogrel |
382 (0.78) |
1,073 (1.04) |
<0.0001 |
||
| Influenza diagnoses3 | 0 | 48,698 (98.90) | 99,808 (97.19) | ||
| 1 | 514 (1.04) | 2,259 (2.20) | } | <0.0001 | |
| ≥2 |
26 (0.05) |
625 (0.61) |
|||
| Antibiotic prescriptions4 | 0 | 25,831 (52.46) | 57,378 (55.87) | ||
| 1 | 11,676 (23.71) | 22,155 (21.57) | } | <0.0001 | |
| ≥2 |
11,731 (23.83) |
23,159 (22.55) |
|||
| Treatment received on diagnosis date or during the 6-month period prior to or after influenza diagnosis | |||||
| Ribavirin | 19 (0.04) | 73 (0.07) | 0.016 | ||
Figures in parentheses are percentages. COPD = Chronic obstructive pulmonary disease; NS = not significant (p > 0.05); CNS = central nervous system.
Risk factors recorded in the preceding 12 months.
Proportion of users among women: 38.8% oseltamivir, 33.33% comparison cohort.
In the previous 12 months (>30 days apart), excluding index diagnosis.
In the previous 12 months.
Sensitivity Analyses
Sensitivity analyses were used to evaluate the impact of a history of acute myocardial infarction (AMI), which is often associated with increased stroke risk. Stroke was characterized as either hemorrhagic or thromboembolic (table 1). Descriptive analyses assessing the differences in incidence of these different types of stroke outcomes were performed. The effect of influenza diagnosis during the influenza season (set at October 1 to March 31) versus outside of the influenza season was also evaluated.
Propensity score analysis can reduce bias due to non-randomization in this type of study. A logistic regression propensity model was estimated for the propensity for being prescribed oseltamivir versus not being prescribed oseltamivir. Stepwise logistic regression was used to test all variables for inclusion. The propensity score, based on regression adjustment [21], was added as one of the independent variables to the ‘all patients, all ages, all seasons’ and ‘all patients, all ages, influenza season’ models in this sensitivity analysis.
Results
Study Population Characteristics
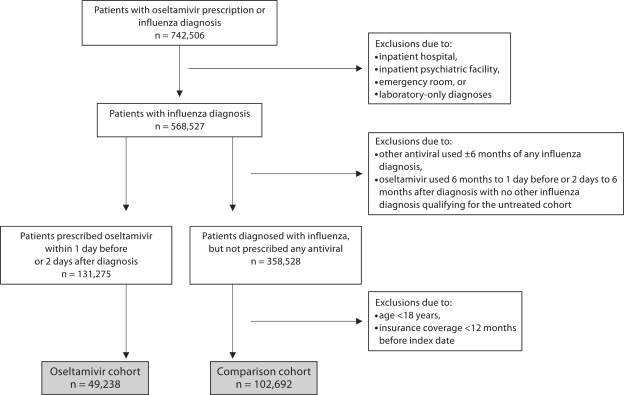
The numbers of patients included in the study and reasons for exclusion are shown in figure 1. Patient demographics, medical histories and risk factors for stroke, as recorded in the 12 months prior to influenza diagnosis, are shown in table 2. The mean age of patients in the oseltamivir cohort was slightly lower, with a higher proportion of patients in the comparison group <65 years of age. The comparison cohort also included a higher proportion of female patients.
Fig. 1.
Oseltamivir and comparison cohort generation.
Significantly higher proportions of patients in the comparison cohort had a pre-existing comorbidity (except sickle cell anemia, lupus and migraine; table 2). The proportion of patients with a history of AMI was low in both cohorts, and was lowest in the oseltamivir cohort, as was the proportion of patients who had been prescribed β-blockers and clopidogrel. Statin use was similar in the 2 cohorts (both approximately 10% of patients) and, as might be expected, strongly correlated with a history of hyperlipidemia (data not shown). A higher proportion of patients in the oseltamivir cohort had been prescribed antibiotics in the 12 months before influenza diagnosis, although a higher percentage in the comparison cohort had an antibiotic prescribed at diagnosis or during the preceding 9 days.
Clinical Outcomes
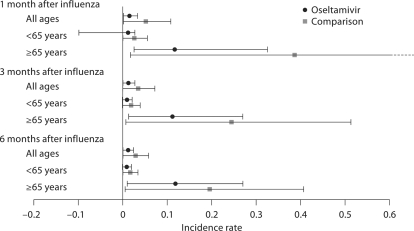
Rates of stroke or TIA in the 2 cohorts are shown in figure 2. According to these unadjusted data, patients prescribed oseltamivir had a significantly lower risk of stroke or TIA compared with those not prescribed an antiviral. Overall, and in those aged ≥65 years, IRRs were lowest in the first month after diagnosis (table 3).
Fig. 2.
Incidence rates of stroke and TIA in the oseltamivir and comparison groups, with 95% CIs. Broken horizontal line indicates upper 95% CI >0.6.
Table 3.
Incidence rates and IRRs for stroke or TIA after influenza diagnosis
| Age group | Oseltamivir |
Comparison (no antiviral) |
||||
|---|---|---|---|---|---|---|
| incidence rate1 |
95% CI |
incidence rate1 |
95% CI |
IRR |
95% CI2 |
|
| 1 Month after influenza | ||||||
| All ages | 0.015 | 0.013–0.019 | 0.052 | 0.050–0.057 | 0.286 | 0.213–0.377 |
| Age <65 years | 0.012 | 0.011–0.016 | 0.026 | 0.025–0.030 | 0.472 | 0.336–0.651 |
| Age ≥65 years | 0.117 | 0.091–0.209 | 0.387 | 0.369–0.441 | 0.301 | 0.148–0.550 |
| Flu season | 0.015 | 0.014–0.019 | 0.044 | 0.042–0.049 | 0.341 | 0.252–0.455 |
| 2001 flu season | 0.010 | 0.006–0.022 | 0.042 | 0.038–0.054 | 0.230 | 0.072–0.571 |
| 2002 flu season | 0.022 | 0.016–0.048 | 0.033 | 0.029–0.046 | 0.675 | 0.231–1.636 |
| 2003 flu season | 0.017 | 0.013–0.028 | 0.037 | 0.034–0.048 | 0.448 | 0.231–0.815 |
| 2004 flu season | 0.018 | 0.015–0.027 | 0.054 | 0.050–0.067 | 0.324 | 0.194–0.521 |
| 2005 flu season | 0.011 | 0.009–0.020 | 0.052 | 0.047–0.068 | 0.213 | 0.097–0.423 |
| Off season |
0.007 |
0.002–0.037 |
0.081 |
0.076–0.095 |
0.083 |
0.002–0.471 |
| 3 Months after influenza | ||||||
| All ages | 0.013 | 0.012–0.015 | 0.035 | 0.034–0.038 | 0.366 | 0.305–0.436 |
| Age <65 years | 0.010 | 0.010–0.012 | 0.019 | 0.019–0.021 | 0.535 | 0.433–0.658 |
| Age ≥65 years | 0.112 | 0.098–0.159 | 0.245 | 0.237–0.270 | 0.459 | 0.308–0.662 |
| Flu season | 0.013 | 0.012–0.016 | 0.032 | 0.031–0.034 | 0.420 | 0.348–0.504 |
| 2001 flu season | 0.012 | 0.010–0.018 | 0.030 | 0.028–0.036 | 0.387 | 0.222–0.639 |
| 2002 flu season | 0.019 | 0.015–0.031 | 0.025 | 0.023–0.031 | 0.748 | 0.398–1.318 |
| 2003 flu season | 0.014 | 0.012–0.019 | 0.029 | 0.027–0.035 | 0.465 | 0.306–0.690 |
| 2004 flu season | 0.014 | 0.013–0.019 | 0.037 | 0.036–0.043 | 0.387 | 0.280–0.527 |
| 2005 flu season | 0.010 | 0.009–0.015 | 0.033 | 0.031–0.040 | 0.316 | 0.200–0.485 |
| Off season |
0.002 |
0.001–0.013 |
0.049 |
0.047–0.056 |
0.047 |
0.001–0.264 |
| 6 Months after influenza | ||||||
| All ages | 0.012 | 0.011–0.013 | 0.029 | 0.028–0.030 | 0.413 | 0.360–0.471 |
| Age <65 years | 0.009 | 0.009–0.011 | 0.017 | 0.016–0.018 | 0.559 | 0.476–0.655 |
| Age ≥65 years | 0.119 | 0.108–0.152 | 0.196 | 0.190–0.212 | 0.607 | 0.460–0.787 |
| Flu season | 0.012 | 0.012–0.014 | 0.026 | 0.026–0.028 | 0.458 | 0.379–0.525 |
| 2001 flu season | 0.012 | 0.010–0.016 | 0.026 | 0.024–0.029 | 0.455 | 0.308–0.655 |
| 2002 flu season | 0.014 | 0.012–0.021 | 0.023 | 0.021–0.027 | 0.619 | 0.375–0.980 |
| 2003 flu season | 0.013 | 0.012–0.017 | 0.026 | 0.025–0.030 | 0.521 | 0.384–0.697 |
| 2004 flu season | 0.013 | 0.012–0.016 | 0.030 | 0.029–0.033 | 0.444 | 0.349–0.562 |
| 2005 flu season | 0.009 | 0.008–0.012 | 0.026 | 0.025–0.031 | 0.341 | 0.240–0.477 |
| Off season | 0.007 | 0.005–0.016 | 0.039 | 0.037–0.043 | 0.192 | 0.070–0.423 |
Incidence rate = rate of events per patient year.
Fisher's exact test.
All the variables in table 2 were tested in the multivariate Cox models for their influence on the risk of stroke or TIA following influenza. All the factors found to have an effect (i.e. with an α <0.05, except for oseltamivir vs. no antiviral treatment) and subsequently included in the multivariate analyses are listed in table 4. A range of variables were found to be positively associated with risk of stroke or TIA, including age, cancer, diabetes, history of stroke or cerebrovascular disease, and migraine, as well as prescription of statin or antihypertensive therapy and hospitalization for any cause. A negative association was found for recent antibiotic treatment.
Table 4.
Demographic, medical history and stroke risk factor variables associated with the risk of stroke or TIA and included in the multivariate models
| AMI included as a covariate | All ages (n = 151,930) |
Age <65 years (n = 143,348) |
Age ≥65 years (n = 8,582) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month |
3 months |
6 months |
1 month |
3 months |
6 months |
1 month |
3 months |
6 months |
||||||||||
| yes |
no |
yes |
no |
yes |
no |
yes |
no |
yes |
no |
yes |
no |
yes |
no |
yes |
no |
yes |
no |
|
| Oseltamivir treatment status1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Demographic characteristics | ||||||||||||||||||
| Age at index2 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Gender3 |
− |
− |
||||||||||||||||
| Risk factors in the 12 months before influenza1 | ||||||||||||||||||
| AMI | − | − | − | − | + | − | − | − | − | |||||||||
| Atrial fibrillation | + | + | ||||||||||||||||
| Cancer | + | + | + | + | + | + | + | + | + | + | ||||||||
| Coronary revascularization | − | − | − | − | ||||||||||||||
| Diabetes mellitus | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Emphysema/COPD | + | + | + | + | ||||||||||||||
| Epilepsy | + | + | ||||||||||||||||
| Stroke and cerebrovascular disease | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Hypertension | + | + | + | + | + | + | ||||||||||||
| Inflammatory CNS disease | + | + | + | + | ||||||||||||||
| Migraine | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Obesity | + | + | + | + | + | + | ||||||||||||
| Other heart disease/conditions | + | + | + | + | ||||||||||||||
| Other ischemic heart disease | + | + | + | + | ||||||||||||||
| Peripheral vascular disease | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Pneumonia (index date/previous 30 days) | + | + | ||||||||||||||||
| Rheumatoid arthritis | + | + | + | + | + | + | ||||||||||||
| Systemic lupus erythematosus |
+ |
+ |
+ |
+ |
+ |
+ |
||||||||||||
| Treatment in the 12 months before influenza | ||||||||||||||||||
| Statin1 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Antihypertensive therapy1 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Antibiotics within 9 days1 | − | − | − | − | − | − | − | − | ||||||||||
| Antibiotic prescriptions4 | ||||||||||||||||||
| 1 | − | − | ||||||||||||||||
| ≥2 |
+ |
+ |
||||||||||||||||
| Hospitalizations (any type)4 | ||||||||||||||||||
| 1 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| ≥2 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||
| Emergency room visits4 | ||||||||||||||||||
| 1 | + | + | ||||||||||||||||
| ≥2 |
− |
− |
||||||||||||||||
| Outpatient visits5 | ||||||||||||||||||
| None | + | + | ||||||||||||||||
| 1 | + | + | ||||||||||||||||
Variables with a stepwise regression with α < 0.05 and with a prescription of oseltamivir versus no antiviral prescription are included; 12-month history of AMI was used as a covariate. COPD = Chronic obstructive pulmonary disease; CNS = central nervous system; – = negatively associated with stroke risk; + = positively associated with stroke risk.
Binary variables.
Continuous variable.
Data for females used as reference variable.
Value of 0 used as reference value.
Value of ≥2 used as reference value.
Following adjustment for the various factors, oseltamivir prescription was shown to be associated with significant reductions in the risk of stroke or TIA (table 5). There were risk reductions of 44, 36 and 28% for the overall age group at 1, 3 and 6 months after diagnosis, respectively. In patients <65 years of age, the corresponding risk reductions were 41, 37 and 34%, respectively. In the ≥65 years age group, the risk reduction was only significant for the first month after diagnosis (51% reduction).
Table 5.
Multivariate analysis of the relative risk of stroke or TIA after influenza diagnosis in patients prescribed oseltamivir versus no antiviral treatment
| Model type | 1 month |
3 months |
6 months |
|||
|---|---|---|---|---|---|---|
| HR |
p value1 |
HR |
p value1 |
HR |
p value1 |
|
| All patients | ||||||
| All ages (n = 151,930) | 0.562 (0.422–0.748) | <0.0001 | 0.639 (0.532–0.767) | <0.0001 | 0.717 (0.624–0.823) | <0.0001 |
| Age <65 years (n = 143,348) | 0.586 (0.425–0.809) | 0.0011 | 0.633 (0.514–0.781) | <0.0001 | 0.658 (0.561–0.772) | <0.0001 |
| Age ≥65 years (n = 8,582) |
0.492 (0.266–0.908) |
0.0233 |
0.719 (0.496–1.043) |
NS |
0.919 (0.705–1.198) |
NS |
| Diagnosis during or outside influenza season (all ages) | ||||||
| During season (n = 129,253) | 0.655 (0.486–0.884) | 0.0056 | 0.710 (0.587–0.858) | 0.0004 | 0.754 (0.653–0.871) | 0.0001 |
| Out of season (n = 22,677) | 0.176 (0.025–1.267) | NS | 0.098 (0.014–0.703) | 0.0208 | 0.370 (0.164–0.833) | 0.0164 |
Figures in parentheses are 95% CIs. History of AMI was a statistically significant (negative) covariate in the 1- and 6-month models. It was not significant and therefore not included in the 3-month model. When AMI was not included as a covariate in the models, the results were very similar: HR 0.560 (95% CI 0.421–0.746) for the 1-month model and HR 0.715 (95% CI 0.623–0.821) for the 6-month model. NS = Not significant (p = 0.05).
Wald χ2 test.
Sensitivity Analyses
A history of AMI as a covariate had no significant impact on the findings of this study (table 5).
Differences in the incidence of the different types of stroke outcomes were evaluated. The incidence rate of hemorrhagic strokes was lower in the oseltamivir group than in the comparison group during all follow-up periods, although this difference was statistically significant only for 3 and 6 months of follow-up. After 6 months of follow-up, the incidence rate of hemorrhagic stroke was 0.001 in the oseltamivir group and 0.002 in the comparison group (IRR 0.461, 95% CI 0.269–0.756). Subarachnoid hemorrhage made up only 15% (n = 3) of the hemorrhagic stroke events in the oseltamivir group and 20% (n = 18) in the comparison group. Results were similar to overall hemorrhagic stroke when subarachnoid hemorrhagic events were excluded. Patients treated with oseltamivir had significantly lower incidence rates of thromboembolic strokes than in the comparison group in all follow-up periods.
Propensity scores were added as independent variables to the ‘all patients, all ages, all seasons’ and ‘all patients, all ages, influenza season’ models. The propensity scores were not statistically significant in either of these models and did not substantially alter the results.
The findings during and outside the influenza season were largely confirmatory of the overall findings, except that oseltamivir was not associated with a risk reduction in the first month after influenza diagnosis outside the influenza season (table 5).
Discussion
In this study, adults prescribed oseltamivir had a significantly reduced risk of stroke or TIA in the 6 months following influenza diagnosis compared with those not prescribed antiviral therapy.
A large body of evidence from ex vivo and in vitroanimal and human studies has demonstrated that influenza results in increased local expression of proinflammatory cytokines, increased platelet aggregation, endothelial dysfunction, loss of the protective properties of high-density lipoprotein and elevation of systemic markers of inflammation [1, 2, 22, 23, 24, 25]. All these changes have the potential to either directly or indirectly stimulate thrombogenesis and exacerbate inflammation of atherosclerotic plaques, which, along with increased hemodynamic stress, could increase the risk of stroke.
Oseltamivir treatment in clinical trials lowered viral titer, the number of patients reporting fever and the duration of fever, and other symptoms compared with placebo [16, 17, 26]. In addition, in a study of experimental human influenza A, patients treated with oseltamivir had lower levels of the proinflammatory cytokines interferon-γ, interleukin-6 and tumor necrosis factor-α in nasal washings, compared with the placebo group. In the placebo group, levels of these cytokines were 2- to 4-fold above baseline [26]. Given the potential proinflammatory and prothrombotic consequences of influenza, it might be anticipated that a treatment that reduces levels of proinflammatory cytokines, inflammation, viral load and duration of illness would have a positive impact on the risk of thrombotic events such as stroke after influenza infection. This is reflected in the findings of our study.
Oseltamivir was associated with a protective effect on stroke and TIA in adults <65 years of age for up to 6 months after influenza diagnosis, and in the first month after influenza in those aged ≥65 years. However, rates of stroke or TIA were approximately 10 times lower in patients <65 years of age. This difference in rates of stroke or TIA is in accordance with other studies showing that prevalence of stroke increases with increasing age [27, 28].
Previous studies have demonstrated that the highest risk of cardiovascular events occurs in the first month after influenza infection and that the incidence of cardiovascular events declines gradually afterwards [3]. Accordingly, we found that oseltamivir treatment exerts its greatest level of protection in the first month after influenza infection and the extent of protection decreases over the next few months.
Age-related variations in the occurrence of different types of stroke, such as the significant risk reduction within 1 month after influenza diagnosis, but not later, in older patients, could have a number of causes. For example, there is evidence that strokes due to thromboembolic events could account for a higher proportion of strokes in older patients [28]. It could be argued that any treatment that reduces the risk of thromboembolism might thus have a greater immediate impact on stroke risk in the ≥65 years group. In addition, the lower IRRs observed overall in the first month after influenza could also be a reflection of variation in the types of stroke with time following influenza.
The sensitivity analysis showed that a history of AMI, which is widely considered to be a risk factor for stroke, had no impact on the effect of oseltamivir in reducing the risk of stroke or TIA.
The risk reductions observed with oseltamivir during influenza seasons were consistent with the overall findings of the study, which might be expected as patients diagnosed during influenza seasons accounted for approximately 85% of the study participants. The number of patients diagnosed outside the influenza season was thus relatively small, which may have hampered accurate statistical analysis. However, it could also be speculated that as influenza diagnoses were not confirmed by influenza tests, some of the patients diagnosed outside the influenza season could have had influenza-like illnesses other than influenza, in which case oseltamivir would not have been an effective treatment.
Several limitations in the design of this study should be acknowledged. Firstly, the database utilized included only patients covered by specific forms of health insurance and may not have been representative of the entire United States population. Secondly, the ICD-9-CM code for influenza was assigned from physicians’ diagnoses, without a requirement for either near-patient or laboratory testing for influenza virus. However, as many physicians use clinical diagnosis alone for influenza, this was considered adequate and may, in fact, better reflect real-life situations in physicians’ clinics. Our database captured a rather low number of elderly subjects by design and this has limited our ability to generalize our findings to subjects ≥65 years old. Finally, patients were not randomized to the oseltamivir and comparison cohorts. However, multivariate models were used to adjust for variations between the cohorts. Additionally, a propensity score analysis found that the incidence of stroke was not affected by the propensity to receive oseltamivir, indicating that there was no substantial confounding by indication.
Conclusion
The results of this retrospective cohort study provide the first evidence that, when prescribed on first presentation of clinically diagnosed influenza, oseltamivir is associated with a reduced risk of stroke or TIA compared with no antiviral treatment. These results, if confirmed by randomized studies, offer a novel approach to prevention of stroke and TIA.
While efforts should be taken to increase the influenza vaccination rate in high-risk individuals, clinicians should be aware of the effectiveness of neuraminidase inhibitors in reducing burden of influenza and its complications when patients become infected due to lack or failure of influenza vaccination. Finally, an influenza pandemic is a likely event in the near future and it has been observed that during previous pandemics, the majority of mortality has been due to cardiovascular events triggered by influenza [29, 30]. Antivirals can potentially provide additional protection in these situations by their ability to prevent post-influenza cardiovascular events.
Acknowledgements
This study was funded by Roche. The authors would like to thank Hsing-Ting Yu, MPH, and Yan Xiong, MS, of Cerner LifeSciences, Beverly Hills, Calif., USA, for programming and statistical analysis, and Annie Rowe, PhD, of Envision Pharma, Horsham, UK, for editorial assistance.
References
- 1.Madjid M, Naghavi M, Litovsky S, Casscells SW. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 2003;108:2730–2736. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 2.Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J. 2004;31:4–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M, Miller CC, Zarubaev VV, Marinich IG, Kiselev OI, Lobzin YV, Filippov AE, Casscells SW., 3rd Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28:1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5.Field TS, Zhu H, Tarrant M, Mitchell JR, Hill MD. Relationship between supra-annual trends in influenza rates and stroke occurrence. Neuroepidemiology. 2004;23:228–235. doi: 10.1159/000079948. [DOI] [PubMed] [Google Scholar]
- 6.Lavallée P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. Association between influenza vaccination and reduced risk of brain infarction. Stroke. 2002;33:513–518. doi: 10.1161/hs0202.102328. [DOI] [PubMed] [Google Scholar]
- 7.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–1506. doi: 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M, Awan I, Ali M, Frazier L, Casscells W. Influenza and atherosclerosis: vaccination for cardiovascular disease prevention. Expert Opin Biol Ther. 2005;5:91–96. doi: 10.1517/14712598.5.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, Dunbar S, Krumholz HM, American Heart Association, American College of Cardiology Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation. 2006;114:1549–1553. doi: 10.1161/CIRCULATIONAHA.106.178242. [DOI] [PubMed] [Google Scholar]
- 10.Naghavi M, Barlas Z, Siadaty S, Naguib S, Madjid M, Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation. 2000;102:3039–3045. doi: 10.1161/01.cir.102.25.3039. [DOI] [PubMed] [Google Scholar]
- 11.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) study. Circulation. 2002;105:2143–2147. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 12.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 13.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. [PubMed] [Google Scholar]
- 14.Singleton JA, Wortley P, Lu PJ. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J. 2004;31:22–27. [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326:1235. doi: 10.1136/bmj.326.7401.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, Rode A, Kinnersley N, Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 17.Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 19.Nordstrom BL, Sung I, Suter P, Szneke P. Risk of pneumonia and other complications of influenza-like illness in patients treated with oseltamivir. Curr Med Res Opin. 2005;21:761–768. doi: 10.1185/030079905x46214. [DOI] [PubMed] [Google Scholar]
- 20.McGeer A. Oseltamivir was safe and effective for prophylaxis of influenza in the frail elderly. ACP J Club. 2002;136:24. [PubMed] [Google Scholar]
- 21.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 23.Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, Quismorio FP, Jr, Weiner JM, Fisher M. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–2011. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 24.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 25.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, Straus SE. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 27.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 28.Gillum RF. Cerebrovascular disease morbidity in the United States, 1970–1983. Age, sex, region, and vascular surgery. Stroke. 1986;17:656–661. doi: 10.1161/01.str.17.4.656. [DOI] [PubMed] [Google Scholar]
- 29.Madjid M, Casscells SW. Of birds and men: cardiologists’ role in influenza pandemics. Lancet. 2004;364:1309. doi: 10.1016/S0140-6736(04)17176-6. [DOI] [PubMed] [Google Scholar]
- 30.Madjid M, Luepker RV, Greenlund KJ, Taubert KA, Roy MJ, Robertson RM. ACCF/AHA/CDC conference report on emerging infectious diseases and biological terrorism threats. Task force IV: cardiovascular effects of emerging infectious diseases and biological terrorism threats: basic, clinical, and population science research and training needs. J Am Coll Cardiol. 2007;49:1407–1412. doi: 10.1016/j.jacc.2007.01.022. [DOI] [PubMed] [Google Scholar]