Abstract
The interaction of photosynthesis and respiration has been studied in vivo under conditions of limited water supply and after consecutive rewatering. The role of the alternative (valt) and cytochrome (vcyt) pathways on drought stress-induced suppression of photosynthesis and during photosynthetic recovery was examined in the Nicotiana sylvestris wild type (WT) and the complex I-deficient CMSII mutant. Although photosynthetic traits, including net photosynthesis (AN), stomatal (gs) and mesophyll conductances (gm), as well as respiration (vcyt and valt) differed between well-watered CMSII and WT, similar reductions of AN, gs, and gm were observed during severe drought stress. However, total respiration (Vt) remained slightly higher in CMSII due to the still increased vcyt (to match ATP demand). valt and maximum carboxylation rates remained almost unaltered in both genotypes, while in CMSII, changes in photosynthetic light harvesting (i.e. Chl a/b ratio) were detected. In both genotypes, photosynthesis and respiration were restored after 2 d of rewatering, predominantly limited by a delayed stomatal response. Despite complex I dysfunction and hence altered redox balance, the CMSII mutant seems to be able to adjust its photosynthetic machinery during and after drought stress to reduce photo-oxidation and to maintain the cell redox state and the ATP level.
Keywords: Alternative oxidase (AOX), complex I dysfunction, drought stress, mesophyll conductance, photosynthesis, recovery
Introduction
Limited water availability impairs plant growth and is one of the main issues of future climate changes (Ciais et al., 2005; Loreto and Centritto, 2008). Thus, adaptation and survival strategies are demanded from plants to persist in their current habitats. As drought stress mainly affects the plant carbon balance, in particular, photosynthesis and respiration, adjustments at the leaf level are of primary importance, while long-term adjustments at the whole plant level may then follow (Chaves et al., 2003; Flexas et al., 2006).
Suppression of photosynthesis during drought stress due to the closure of stomata and the contribution of leaf-internal limitations to CO2 diffusion, particularly mesophyll conductance (gm), has been determined in numerous studies and plant species (Flexas et al., 2004, 2008; Niinemets et al., 2005; Warren and Adams, 2006). Although plant species respond differently to varying drought stress intensities, photosynthetic limitation is firstly and predominantly driven by stomata, in particular, by a decline in stomatal conductance. Further on, when stomatal conductance drops below a certain threshold (<50 mmol H2O m−2 s−1) limitations of non-stomatal processes become more important, in particular, decreased gm and impaired photo-biochemistry (Flexas and Medrano, 2002; Chaves et al., 2003; Flexas et al., 2004). Adjustment of leaf diffusion components for CO2 is one way for plants to cope with situations of limited water supply and concurrently to improve their water use efficiency. As well as leaf internal adjustments of diffusion components during drought stress, increased thermal dissipation of excess energy and other photo-protective processes (e.g. an enhanced xanthophyll cycle) may contribute to improved stress tolerance and adaptation (Demmig-Adams and Adams III, 1996; Havaux and Niyogi, 1999; Mittler, 2002).
In parallel to these changes inside the chloroplast, respiratory pathways in mitochondria might also be altered, because of their interaction with the photosynthetic pathway. The respiratory chain is thought to dissipate excess reductants originated from chloroplasts (Raghavendra and Padmasree, 2003). Moreover, the non-phosphorylating pathways, which involve the cyanide-resistant alternative oxidase (AOX) and the type II NAD(P)H dehydrogenases, are considered to be efficient dissipation systems for these reductants, because electron flow through these pathways is not limited by adenylate control (Noctor et al., 2007). Thus, the non-phosphorylating pathways may function as a mechanism of plant photo-protection, while the components of this mechanism have not been characterized in detail. Indeed, several studies have highlighted that different mutants with some impaired mitochondrial function also have a lower photosynthetic capacity (Juszczuk et al., 2007; Nunes-Nesi et al., 2007; Giraud et al., 2008), and this includes the CMSII mutant of Nicotiana sylvestris (Sabar et al., 2000; Priault et al., 2006a, b).
Several studies on the effect of severe drought stress on respiratory pathways have revealed contrasting results, as respiration remained unaltered in soybean (Ribas-Carbo et al., 2005b), increased in wheat (Bartoli et al., 2005), and decreased in bean and pepper (Gonzalez-Meler et al., 1997). However, changes in the in vivo activities of the cytochrome oxidase (COX) and AOX pathways, measured with the oxygen isotope fractionation technique that has been demonstrated to be the most reliable technique for the studies of electron partitioning between the two main respiratory pathways (Ribas-Carbo et al., 1995; Day et al., 1996), have been reported by Ribas-Carbo and colleagues (Flexas et al., 2005; Ribas-Carbo et al., 2005b). In their study on soybean (Ribas-Carbo et al., 2005b), a decrease in COX activity was detected in leaves during severe drought stress, while AOX activity increased. Whether, and to what extent, plant species-specific factors and/or experimental conditions affect in vivo respiratory pathways under drought stress awaits further studies.
To examine the effect of stress-induced changes on respiratory pathways, in particular with relation to photosynthesis, plants with modified AOX expression have been intensively studied (Noctor et al., 2007). Among these, the Nicotiana sylvestris cytoplasmic male-sterile CMSII mutant, which lacks a functional mitochondrial complex I (Gutierres et al., 1997) and possesses high amounts of AOX transcript and protein (Sabar et al., 2000), has received increased attention. The observed differences in activities of the photosynthetic and respiratory pathways as compared with wild-type plants have been proposed to result from alterations in the cellular redox balance (Dutilleul et al., 2003b; Priault et al., 2006a; Vidal et al., 2007) and/or limitations of CO2 diffusion inside the leaf (Priault et al., 2006b). Moreover, loss of complex I function might be compensated by enhanced non-phosphorylating NAD(P)H dehydrogenases, resulting in the maintenance of cell redox balance and cross-talk between mitochondria and chloroplasts (Dutilleul et al., 2003a, b; Vidal et al., 2007).
Thus, the tobacco CMSII mutant offers great possibilities to study the interaction between respiratory and photosynthetic pathways, in particular, under contrasting environmental conditions (e.g. high light, temperature, and drought stress). With regard to the increasing importance of understanding plant responses to drought stress, particularly within the context of climate changes, more studies are needed to examine the plant carbon balance, especially the relationship between the carboxylation and oxygenation processes. Due to the scarcity of in vivo studies on photosynthesis and respiration under drought stress (Gonzalez-Meler et al., 1997; Bartoli et al., 2005; Ribas-Carbo et al., 2005b; Giraud et al., 2008), more research is necessary to understand the interrelation between chloroplasts and mitochondria. Furthermore, the lack of knowledge regarding the underlying and limiting processes of photosynthetic recovery from drought stress awaits further attention, as the capability to recover from drought events ensures the survival and growth of plants in their habitats (Flexas et al., 2006; Galle et al., 2007; Galmes et al., 2007).
Apart from osmotic readjustments in Nicotiana sylvestris CMSII mutants (soluble sugars, proline) and wild-type plants under drought stress (R de Paepe, unpublished results), the role of respiratory pathways during drought stress induced the suppression of photosynthesis and, during photosynthetic recovery after rewatering, has been examined in more detail in the present study on CMSII and wild-type plants. The following questions have been addressed. (i) How does respiratory complex I deficiency and hence altered non-phosphorylating pathways, as related to the proposed function in the dissipation of excess energy from the chloroplasts, affect photosynthetic activity during drought stress? (ii) What are the impacts on photosynthetic recovery during rewatering, especially with regard to the possible functions of respiratory pathways? (iii) Is an efficient respiratory chain necessary for photosynthetic recovery after drought stress and what about other limiting factors?
Materials and methods
Plant material and growth conditions
Two genotypes of tobacco (Nicotiana sylvestris), wild-type (WT) and CMSII mutant (Gutierres et al., 1997), were grown in a growth chamber (photoperiod of 12 h) at approximately 800 μmol m−2 s−1 photon flux density (PPFD) and at 26/22 °C day/night temperature. Humidity was maintained around 40–50%. Prior to the experiments, plants were kept under well-watered conditions, adding nutrient solution (Hoagland) once a week. Experiments were started with 6-week-old and 11-week-old WT and CMSII plants, respectively. This difference in age was due to the retarded growth of CMSII and the need to perform the experiment with plants of a similar developmental stage, which was considered to be achieved when plants presented a similar size and number of leaves according to Priault et al. (2007). Throughout the experiments, the two youngest fully expanded leaves were used for gas-exchange and respiration measurements. Drought stress was imposed by withholding water until severe drought stress was reached. Severe drought stress was considered when the stomatal conductance for water vapour dropped below 50 mmol m−2 s−1, according to Medrano et al. (2002). Thereafter, plants were maintained at this intensity of drought stress for a few days by adding the amount of water they lost during the day. After this period of stress-acclimation plants were consecutively rewatered to field capacity and the recovery of photosynthetic traits was followed.
Plant water status
From the first day of withholding water until the first day of rewatering, plants were weighed at the end of the photoperiod and the total loss of water was recorded. When necessary plants were irrigated with the amount of water lost during the day.
In parallel with gas-exchange and respiration measurements (see below), a minimum of three leaf samples were collected around midday to determine the relative leaf water content (RWCm).
where FW, TW, and DW denote for weight of fresh, turgid, and dry leaf tissue, respectively. FW was determined immediately after sampling, while TW was obtained after incubating leaf discs in distilled water for 48 h in the dark at 4 °C. DW was determined after 72 h in a drying oven at c. 70 °C.
Leaf gas-exchange and chlorophyll a fluorescence
Throughout the experiments, maximum net CO2 assimilation (AN), stomatal conductance (gs) and chlorophyll a fluorescence were measured simultaneously with an open infrared gas-exchange analyser system (Li-6400; Li-Cor Inc., Lincoln, NE, USA) equipped with a leaf chamber fluorometer (Li-6400-40, Li-Cor Inc.). Under well-watered conditions (WW), on the first (S1) and last day (S4) of severe drought stress, as well as after 1 (R1), 2 (R2), and 5 d (R5) of rewatering, at least four measurements on the two youngest fully expanded leaves of WT and CMSII plants were performed under light-saturating PPFD of 1500 μmol m−2 s−1 (provided by the light source of the Li-6400 with 10% blue light). Leaves were always illuminated until AN and gs reached steady-state (after c. 20 min). The CO2 concentration in the Li-6400 leaf chamber (Ca) was set to 400 μmol CO2 mol−1 air, temperature was 25 °C and the relative humidity of the incoming air ranged between 40% and 50%. CO2 response curves (‘AN–Ci curves’) were performed in well-watered, non-watered, and rewatered plants by varying the CO2 concentration around leaves stepwise in the range of 50–1800 μmol CO2 mol−1 air. These leaves had been previously acclimated to saturating light conditions (c. 15–20 min at a PPFD of 1500 μmol m−2 s−1).
From the fluorescence measurements, the actual quantum efficiency of the photosystem II (PSII)-driven electron transport (ΦPSII) was determined according to Genty et al. (1989) as
where Fs is the steady-state fluorescence in the light (here PPFD 1500 μmol m−2 s−1) and Fm′ the maximum fluorescence obtained with a light-saturating pulse (∼8000 μmol m−2 s−1). As ΦPSII represents the number of electrons transferred per photon absorbed by PSII, the rate of electron transport (J) can be calculated as
where the term α includes the product of leaf absorptance and the partitioning of absorbed quanta between photosystems I and II. α was determined for each treatment from the slope of the relationship between ΦPSII and ΦCO2 (i.e. the quantum efficiency of gross CO2 fixation), which was obtained by varying light intensity under non-photorespiratory conditions in an atmosphere containing <1% O2 (Valentini et al., 1995).
From combined gas-exchange and chlorophyll a fluorescence measurements, the mesophyll conductance for CO2 (gm) was estimated according to Harley et al. (1992) as
where AN and Ci were obtained from gas-exchange measurements. A value of 37.4 μmol mol−1 for the CO2 compensation point under non-respiratory conditions (Γ*) was used after Bernacchi et al. (2002) as determined for the related species Nicotiana tabacum. Other Rubisco kinetics and their temperature dependencies were also taken from Bernacchi et al. (2002). Dark respiration (Rd; Vt) was determined at 25 °C with an isotope ratio mass spectrometer (IRMS) at 25 °C as described by Ribas-Carbo et al. (2005b). Calculated values of gm were used to convert A–Ci curves into A–Cc curves according to the following equation:
Maximum velocity of carboxylation (Vc,max) and maximum electron transport rate (Jmax) was derived from A–Cc curves according to Bernacchi et al. (2002).
Corrections for the leakage of CO2 into and out of the leaf chamber of the Li-6400 have been applied to all gas-exchange data, as described by Flexas et al. (2007). Due to low gs values under severe drought stress and a possibly increased contribution of conductance via the cuticle (gc), estimates of gs were corrected for gc as described elsewhere (Boyer et al., 1997). In short, for each experimental condition gas-exchange was measured across the leaf with its lower side sealed with lubricant and an impermeable plastic foil to hinder any gas exchange via its cuticle and stomata. The obtained gc was multiplied by 2 to account for the upper and lower sides of the leaf and Ci was recalculated based on the new gs values (gs–gc), using the equations provided by the manufacturer (LI-6400 manual version 5, Li-Cor Inc., Lincoln, Nebraska, USA).
Respiration measurements on intact tissue
Respiration in the light (Rl) and in the dark (Rdc) was determined in leaves of at least four well-watered CMSII and WT plants, using a Li-6400 gas-exchange system (Li-cor Inc. Lincoln, NE, USA). Rl was determined according to the ‘Laisk-method’ (Laisk, 1977), using the y-axis intersection of three AN–Ci curves (as described above) performed at three different light intensities (750, 250, and 75 μmol photons m−2 s−1). In both genotypes, the apparent CO2 compensation point (Ci*), as determined by the Laisk-method, ranged between 38.3 and 43.7 μmol mol−1. Rdc was measured at c. 400 μmol CO2 mol−1 air and 25 °C in attached leaves that were dark-adapted for more than 30 min.
After gas-exchange measurements (WW, S1, S4, R1, R2, R5) total dark respiration (Vt) and the activities of the cytochrome oxidase (COX) and the alternative oxidase (AOX) pathways were determined with a dual-inlet mass spectrometer system (Delta Plus, Thermo LCC, Bremen, Germany), as previously described (Gaston et al., 2003; Florez-Sarasa et al., 2007).
First, leaves were incubated in the dark for 30 min to avoid light-enhanced dark respiration and then used for respiration measurements. A 10 cm2 leaf disc was cut, weighed, and immediately placed in a closed 3 ml stainless-steel cuvette, which was maintained at a constant temperature of 25 °C, using a copper plate and a serpentine around the cuvette with a temperature-controlled water bath. The values of 34/32(18O2/16O2) and 32/28(16O2/28N2) mass ratios were obtained from a standard and the sample air with dual-inlet analysis and four or six replicate cycles for each respiration measurement. Calculations of the oxygen isotope fractionation were made as previously described (Ribas-Carbo et al., 2005a). The electron partitioning between the two pathways in the absence of inhibitors was calculated as described by Guy et al. (1989). The r2 value of all unconstrained linear regressions between –ln f and ln(R/Ro) with a minimum of five data points was at least 0.995, which has been considered as robust and well acceptable (Ribas-Carbo et al., 1997).
The electron partitioning through the AOX pathway (τa) was calculated as described in Guy et al. (1989) as follows:
where Δn, Δc, and Δa are the isotope fractionation in the absence of inhibitors, in the presence of SHAM, and in the presence of KCN, respectively. The Δc value of 21.3‰ obtained by Vidal et al. (2007) was used, as COX pathway discrimination has been shown to be constant (Ribas-Carbo et al., 2005a). A Δa value of 30.0‰ was determined experimentally. The individual activities of the COX (νcyt) and AOX (νalt) pathways were determined by multiplying the total oxygen uptake rate (Vt) and the partitioning to each pathway as follows:
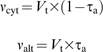 |
ATP production of WT and CMSII plants was calculated according to the model used by Vidal et al. (2007).
Pigment and protein analysis
Snap-frozen leaf discs (in liquid nitrogen) were collected daily after 8 h of light and stored at –80 °C until analysis.
Frozen leaf material was ground to fine powder and homogenized with ice-cold extraction buffer (0.5M TRIS, 10 mM EDTA, 1% Triton X-100, 5 mM DTT, and 0.25% protease-inhibitor cocktail). The leaf extract was then centrifuged at 12 500 rpm and 4 °C. The supernatant was transferred to new vials and kept on ice, while aliquots were used for the determination of total soluble protein content (TSP), photosynthetic pigment composition, and Western blot analysis.
For pigment analysis an aliquot of leaf extract was dissolved in 80% acetone, centrifuged at 12 500 rpm for 2 min and the absorbance of the supernatant was determined at 470, 646, and 663 nm in a spectrophotometer (DU 640, Beckmann Coulter Inc.). The content of chlorophyll a, b and carotenoids was then calculated according to Lichtenthaler (1987).
The TSP was determined with the Bio-Rad protein assay (Bio-Rad Laboratories, Inc.) according to the method of Bradford, using bovine serum albumin as a standard.
For SDS-PAGE, an aliquot of leaf extract was mixed 1:1 with SDS-sample buffer (0.5 M TRIS, 0.3% glycerol, 1.5% SDS, 0.15% bromophenol blue, and 5 μM DTT), after which samples were boiled in a water bath for c. 5 min and then kept at –20 °C for later analysis. Mixes of at least three samples (=plants) per day with equal amounts of soluble proteins were loaded per lane for SDS-PAGE, using a Mini-Protean electrophoresis system (Bio-Rad Laboratories, Inc.).
SDS-PAGE gels were blotted onto nitrocellulose membranes with the Mini-Protean system of Bio-Rad. Immunodetection of mitochondrial proteins (AOX and porin) via colorimetry was carried out with the BCIP/NBT alkaline phosphatase system according to the manufacturer's instructions (Sigma-Aldrich Co.). A 1/50 dilution of the monoclonal antibody against AOX (Elthon et al., 1989) and a 1/500 dilution of the monoclonal antibody against the voltage-dependent anion channel porin (PM035, from Dr Tom Elthon, Lincoln, NE, USA) were used as primary antibodies. Densitometry quantification of AOX and porin bands were made with TotalLab Software (Nonlinear Dynamics Ltd, UK).
Results
Changes of photosynthetic traits during drought stress and recovery
Relative leaf water content (RWCm) was reduced by 3% in WT and by 12% in CMSII, when the desired level of severe drought stress (S1) was reached (Table 1a, b), as indicated by a stomatal conductance for water vapour below 50 mmol m−2 s−1 (Medrano et al., 2002). After 4 d of severe drought stress (S4) RWCm dropped by more than 20% in both WT and CMSII,. However, RWCm was immediately restored in WT and CMSII after rewatering, indicating the rapid restoration of plant water status.
Table 1a.
Changes of photosynthetic parameters in well-watered (Control) and drought stressed (Stress) tobacco wild-type (WT) plants throughout the experiment
| WW | S1 | S4 | R1 | R2 | R5 | ||
| RWCm | 77.5±1.1 a | 75.1±3.1 a | 61.7±2.1 b | 78.9±1.1 a | – | 79.8±4.0 a | |
| Vc,max | Control | 166.0±28.5 a | – | 138.1±26.7 a | – | – | 124.0±10.4 ab |
| Stress | 159.7±12.5 a | 112.5±9.1 ab | 139.7±17.0 a | 160.9±5.1 a | 162.1±11.6 a | ||
| Jmax | Control | 200.3±19.1 a | – | 183.2±22.1 a | – | – | 170.1±10.4 a |
| Stress | 136.4±9.6 b | 104.4±10.0 b | 174.0±13.7 a | 203.3±7.7 a | 203.7±10.7 a | ||
| qP | Control | 0.58±0.02 a | 0.48±0.02 ab | 0.45±0.09 ab | 0.46±0.09 a | 0.42±0.09 ab | 0.42±0.08 b |
| Stress | 0.44±0.03 b | 0.17±0.06 c | 0.41±0.10 b | 0.57±0.02 a | 0.55±0.03 a | ||
| Cc(Vc,max//Jmax) | Control | 153.8±9.3 a | – | 155.7±1.4 a | – | – | 146.0±11.7 |
| Stress | 85.4±4.6 b | 72.1±6.6 b | 104.3±4.8 b | 124.3±8.7 ab | 149.0±5.9 a |
RWCm, Vc,max, Jmax, qP, and Cc denote for relative leaf water content at midday, maximum carboxylation rate, maximum photosynthetic electron transport rate, photochemical quenching of PSII, and chloroplast CO2 concentration at the turning point of Rubisco (Vc,max) and RuBP (Jmax) limitation to photosynthesis, respectively. Means and standard errors of at least four measurements are presented for well-watered condition (WW), first (S1), and last day (S4) of severe drought stress, as well as for the first (R1), second (R2), and fifth day (R5) of rewatering. For each parameter statistically significant differences to the control value (WW) are indicated by different letters (P <0.05).
Table 1b.
Changes of photosynthetic parameters in well-watered (Control) and drought-stressed (Stress) tobacco CMS mutants throughout the experiment; for details see legend Table 1a
| WW | S1 | S4 | R1 | R2 | R5 | ||
| RWCm | 81.6±2.9 a | 71.3±3.4 ab | 58.8±2.5 c | 76.5±4.1 ab | – | 87.4±1.3 a | |
| Vc,max | Control | 139.9±21.9 a | – | 124.0±13.7 a | – | – | 108.1±3.3 ab |
| Stress | 184.3±18.2 a | 110.7±9.5 a | 135.8±13.9 a | 150.8±11.4 a | 167.8±8.8 a | ||
| Jmax | Control | 166.4±27.3 b | – | 152.8±13.7 | – | – | 139.2±3.1 |
| Stress | 146.7±13.7 b | 92.8±8.6 c | 189.9±9.3 ab | 206.6±8.4 ab | 214.6±4.8 a | ||
| qP | Control | 0.47±0.07 a | 0.32±0.07 ab | 0.36±0.07 ab | 0.36±0.06 ab | 0.29±0.07 b | 0.29±0.07 b |
| Stress | 0.34±0.03 b | 0.08±0.01 c | 0.49±0.03 a | 0.44±0.09 a | 0.38±0.09 a | ||
| Cc (Vc,max//Jmax) | Control | 148.3±9.3 a | – | 154.0±10.0 a | – | – | 161.0±3.3 a |
| Stress | 75.8±3.8 b | 72.0±4.8 b | 142.1±10.7 a | 148.0±18.9 a | 140.7±1.9 a |
The maximum carboxylation rate (Vc,max) under well-watered conditions (Control) was slightly higher in WT than in CMS (Table 1a, b), which has been previously observed (Priault et al., 2006b) and might be related to a higher initial Rubisco activity (Priault et al., 2006a) or the number of catalytic sites (Dutilleul et al., 2003a). Despite a slight decrease of Vc,max with time in control plants, presumably due to leaf ageing, Vc,max remained almost unaltered in stressed plants.
As in Vc,max, alterations in Jmax were small in control plants of WT and CMSII during the experiment (Table 1a, b). However, unlike Vc,max, Jmax decreased by about 40% during severe drought stress (S4), but it was restored to control values within 1 d of rewatering (R1) and maintained this level thereafter.
In close relation with the course of Jmax, the photochemical quenching (qP; proportion of open PSII reaction centres) declined considerably with progression of drought stress, resulting in a reduction of more than 70% (Table 1a, b). Notably, the reduction of qP was more pronounced in CMSII under severe drought stress (S4; Table 1b). However, this reduction was completely reversed after rewatering (R1), resulting in somewhat higher qP values than the corresponding controls. In well-watered plants, qP remained almost unchanged, except for a slight decline with time (possible ‘ageing effect’).
The chloroplastic CO2 concentration (Cc) at the intercept of the photosynthetic limitation by Rubisco activity (Vc,max), and the regeneration of ribulose-1,6-bisphosphate (Jmax) deducted from AN–Cc curves, was similar for WT and CMSII under well-watered conditions (Table 1a, b). Moreover, in both genotypes similar changes were determined during drought stress, as Cc values decreased from about 150 μmol mol−1 to 72 μmol mol−1 (S4). During rewatering Cc values increased in both WT and CMSII, while restoration to control values was reached within 1 d of rewatering in CMSII and after 2 d in WT.
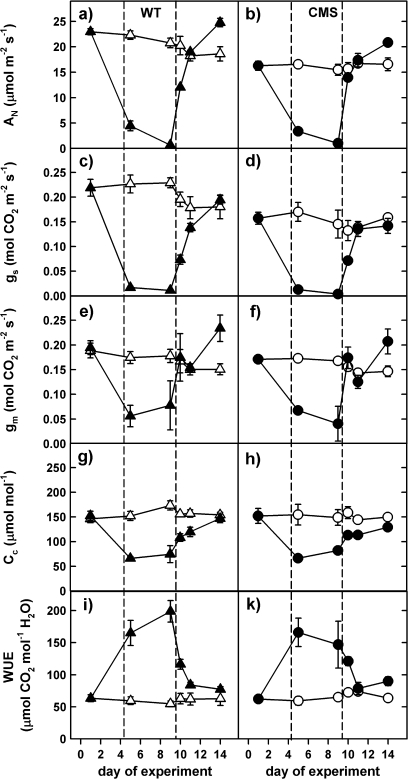
Net photosynthesis (AN) and stomatal conductance (gs) were 20–30% lower in CMSII than in WT under well-watered conditions (Fig. 1a–d; Control), while stomatal density was about 10% lower in CMSII than in WT (data not shown). In both genotypes, similar values of AN and gs were reached under severe drought stress. Thereafter, AN and gs were completely restored to control values after 2 d of rewatering. Interestingly, although AN was restored to control values by c. 90% in CMSII and by ‘only’ about 60% in WT plants after the first day of rewatering, the rate of photosynthetic recovery seemed to be very similar for both genotypes (Fig. 1a, b; compare absolute AN values of day 10). The restoration of gs was generally slower (lower rate) than that of AN in both WT and CMSII, particularly during the first day of rewatering, where they reached only 40–50% of the corresponding control values. These results clearly indicate a higher intrinsic water use efficiency (WUE) during the initial period of rewatering in WT and CMSII (Fig. 1i, k), while highest WUE (3–4-fold increase) could be observed during severe stress.
Fig. 1.
Changes of leaf gas-exchange parameters during drought stress and rewatering in WT (triangles) and CMSII (circles) plants. Net photosynthetic rates (AN), stomatal conductance (gs), mesophyll conductance (gm), chloroplastic CO2 concentration (Cc). and the intrinsic water use efficiency (WUE) of photosynthetic carbon fixation are presented for well-watered control (open symbols) and stressed (closed symbols) plants. Means and standard errors of at least four plants are shown. The vertical dashed lines indicate the beginning and end of the severe drought period.
Mesophyll conductance (gm) of WT and CMSII plants were very similar under well-watered conditions and followed a similar trend during drought stress and rewatering (Fig. 1e, f). During severe drought stress, gm dropped below 0.1 mol CO2 m−2 s−1 in WT and CMSII, while it was completely restored to control values within 1 d of rewatering suggesting a negligible restriction of leaf internal CO2 diffusion. Notably, in spite of maintained gs (Fig. 1c, d) gm increased after 5 d of rewatering (day 14; Fig. 1e, f). Changes in chloroplastic CO2 concentration (Cc) were similar in WT and CMSII plants during the experiment, which resulted in a decrease of about 50% during drought stress and a progressive restoration to control values during rewatering (Fig. 1g, h).
Besides the changes in Cc (Fig. 1g, h), the relationship of internal CO2 (Ci) to Cc remained almost unaltered throughout the experiment in stressed and control plants of both genotypes (data not shown).
Changes in respiratory traits during drought stress and recovery
In order to confirm that dark respiration (Rd, Vt) measured by the oxygen isotope technique (IRMS) can be used for calculating gm and the related parameters (i.e. Vc,max, Jmax), as well as to check whether it was representative of leaf respiration occurring during photosynthesis, respiration in the dark (Rdc) and the light (Rl) were also determined with a gas-exchange system (Table 2). Besides the precautions and problems with the Laisk method used for Rl estimation, in particular during situations of drought stress (Galmes et al., 2006), as well as the fundamental problem of measuring respiration in the light, the ratio between CMSII and WT values remained almost unaltered between 1.2 and 1.5.
Table 2.
Comparison of leaf respiration parameters in well-watered WT and CMSII plants, determined with the Li-6400 and IRMS system
| Respiration parameter | WT | CMSII | Ratio CMSII/WT |
| Rl (μmol CO2 m−2 s−1)a | 0.45±0.04 | 0.66±0.07 | 1.5* |
| Rdc (μmol CO2 m−2 s−1)a | 1.50±0.05 | 1.81±0.08 | 1.2* |
| Vt (Rd) (μmol O2 m−2 s−1) | 0.89±0.06 | 1.30±0.10 | 1.5* |
Means and standard errors of at least four plants are shown. Asterisks indicate significant differences (P ≤0.05) between WT and CMSII for each parameter. For details see Materials and methods.
These respiration parameters have been determined in leaves of another set of WT and CMSII plants, which were grown under similar conditions as the well-watered plants used for the Vt measurement.
Absolute values of respiration parameters ranged between 0.45 and 1.50 in WT and between 0.66 and 1.81 in CMSII, as determined by isotope (O2) and by gas-exchange (CO2) analysis. However, these differences only marginally affect calculations of gm, as changes of gm were smaller than 8% when using the most extreme values (0.45 and 1.81; data not shown). Thus, dark respiration values determined by oxygen isotope analysis were used for all calculations and as a tracer of leaf respiratory activity in each genotype.
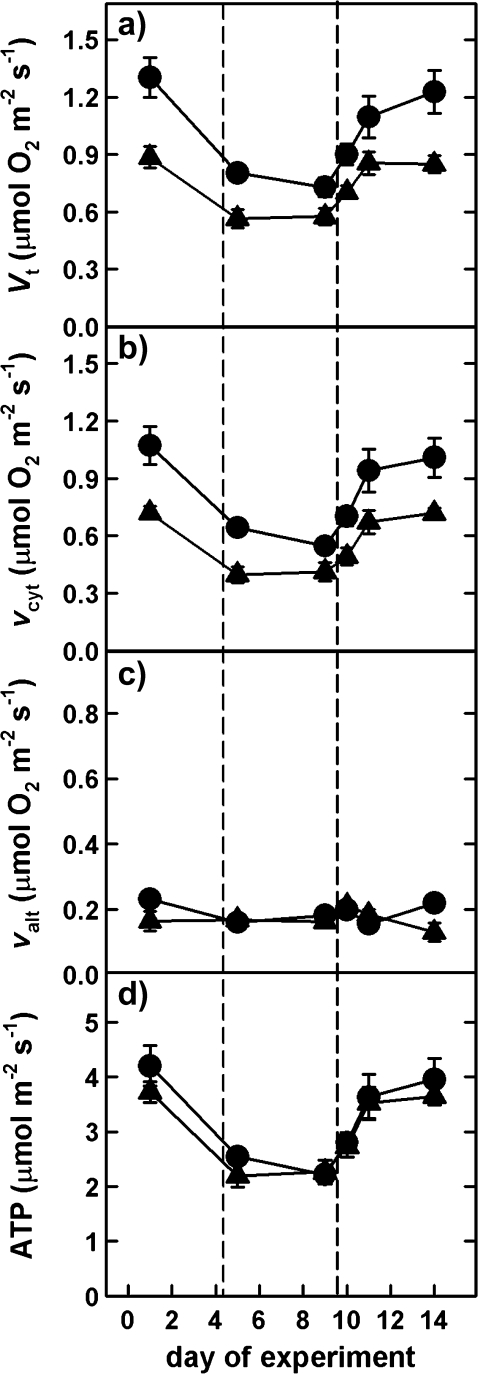
When comparing respiratory pathways in WT and CMSII, total respiration (Vt) was significantly higher in CMSII than in WT (Fig. 2a). The higher Vt in CMSII was mainly due to increased cytochrome oxidase activity (vcyt). The course of Vt, vcyt, and valt (alternative pathway) during the experiment was similar in both genotypes. The reduction of Vt during drought stress was predominantly due to reduced vcyt, while valt remained almost unaltered. However, when ATP production was modelled no significant differences between genotypes were detected, irrespective of being drought stressed or not (Fig. 2d).
Fig. 2.
Changes of total respiration (Vt), cytochrome (vcyt), and alternative (valt) pathway activities, as well as modelled mitochondrial ATP production during drought stress and rewatering in WT (triangles) and CMSII (circles) plants. Values of control plants remained unaltered throughout the experiment and are therefore not depicted. Means and standard errors of at least four plants are shown. The vertical dashed lines indicate the beginning and end of the severe drought period.
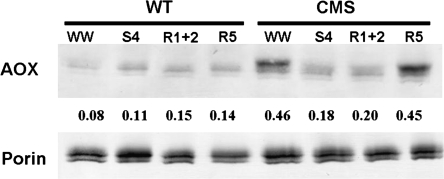
The AOX protein level was relatively constant in WT plants during the whole experimental period (Fig. 3), while a marked reduction of AOX protein was detected in CMSII plants during severe drought stress and in the initial phase of recovery (first and second days of rewatering). However, AOX protein was more abundant in CMSII under control conditions (well-watered) than in WT, as previously reported (Gutierres et al., 1997; Sabar et al., 2000; Priault et al., 2007). Changes in total mitochondrial protein content were negligible, as indicated by the anti-porin Western blot (Fig. 3). Control plants under well-watered conditions showed no change in AOX and in porins during the experimental period.
Fig. 3.
Western blot analysis of mitochondrial alternative oxidase (AOX) and porin protein in WT and CMSII during drought stress and rewatering. WW, S4, R1+2, and R5 denote for the start of the experiment (well-watered), end of severe drought stress, initial phase (first and second day), and terminal phase (fifth day) of rewatering, respectively. The values below the AOX bands represent the ratio of AOX and porin quantified by densitometry. Each lane was loaded with the same amount of protein of combined leaf extracts from four plants.
Changes of soluble leaf components related to photosynthesis during drought stress and recovery
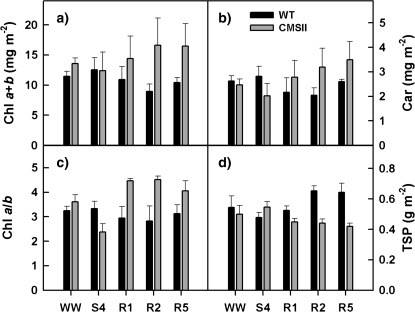
Changes in contents of chlorophylls (Chl a+b) and carotenoids (Car) were only small throughout the experiment, while they increased somewhat during rewatering in CMS, but not in WT (Fig. 4a, b). Furthermore, the ratio of chlorophyll a to b (Chl a/b) was increased after rewatering in CMSII, but not in WT, indicating changes within the photosynthetic apparatus (i.e. the light-harvesting complexes). On the other hand, total soluble protein content (TSP) was increased after rewatering in WT, whereas it slightly declined in CMSII. These changes also remained similar when Chl a+b, Car, and TSP are presented on a dry-weight basis (data not shown), although specific leaf area increased during drought and initial recovery (see Supplementary Fig. 1 at JXB online). Under well-watered conditions Chl a+b, Car, Chl a/b, and TSP remained unaltered throughout the experimental period in both WT and CMSII. WT and CMSII also did not differ in total lipid peroxidation (data not shown) neither under drought stress nor under recovery or well-watered conditions, suggesting no or only minor effects of general oxidative stress.
Fig. 4.
Changes of photosynthetic pigments and soluble proteins in WT (triangles) and CMSII (circles) during drought stress and rewatering. The contents of chlorophylls (Chl a+b), carotenoids (Car), and total soluble proteins (TSP), as well as the ratio of chlorophyll a to b (Chl a/b) are depicted. WW, S4, R1, R2 and R5 denote for the start of the experiment (well-watered), the end of severe drought stress (day 9), the first two days (day 10, 11), and the last day of rewatering (day 14), respectively. Means and standard errors of at least four plants are shown.
Discussion
Well-watered conditions
The differences in the photosynthetic and respiratory parameters in WT and CMSII mutants under well-watered conditions, which had been described earlier (Sabar et al., 2000; Dutilleul et al., 2003a; Priault et al., 2006a, b), were proposed to result from altered cross-talk between the respiratory and photosynthetic pathways (Sabar et al., 2000; Dutilleul et al., 2003a; Priault et al., 2006a), as well as from an impaired internal CO2 supply (i.e via mesophyll conductance, gm) (Priault et al., 2006b). Unlike the findings of Priault et al. (2006b), impaired CO2 supply due to decreased gm was not observed in CMSII plants in the present study, as gm values, and hence Cc, did not differ between WT and CMSII (Fig. 1e–h). The growing light intensity was more than two times higher in this study than in the study of Priault et al. (2006b), which may explain the different results of gm. As shown recently, quantity and intensity of incident light seems to affect gm (Gorton et al., 2003; Warren et al., 2007). Similar gm and Cc (and Vc,max) but different AN in WT and CMSII might be related to differences in activation state of Rubisco (or its amount) under the prevailing growth light conditions (800 μmol m−2 s−1), as previously shown for CMSII and WT plants growing under moderate light (350 μmol photons m−2 s−1) and after 3 h of high light treatment of 1000 μmol m−2 s−1 (Priault et al., 2006a). Moreover, lower stomatal density in CMSII than in WT leaves may also partially contribute to that discrepancy in AN and gs between both genotypes.
Drought-stress conditions
WT and CMSII plants were similarly affected by drought stress, as the values of AN and of gs reached almost zero during severe stress (day 9; Fig. 1), leading to a considerable drop in internal CO2 supply (gm and Cc). On the other hand, maximum carboxylation (Vc,max) was almost unaffected by drought stress, indicating preserved photosynthetic functioning and little metabolic limitations. However, reduced light capture via PSII antennas, as indicated by reduced ratios of Chl a/b (Fig. 4c) and fewer open PSII reaction centres (qP; Table 1b) were detected in CMSII mutants. Thus, the photosynthetic apparatus of CMSII seemed to be more affected by drought stress than WT.
With regard to respiration and irrespective of drought stress or not, CMSII plants always displayed a 20–30% higher respiration rate (Vt) than WT, due to increased vcyt (Fig. 2), adjusting ATP production to a similar level as in WT. In fact, calculated total ATP production was almost equal in CMSII and WT and showed a similar trend under drought stress in both genotypes (Fig. 2), suggesting a strict control of respiration by ATP demand. Interestingly, although AOX activity was maintained, AOX protein content was slightly decreased during severe stress and the initial phase of recovery in CMSII. Altered redox signalling in CMSII plants has been proposed to cause differential AOX expression patterns in CMSII plants (Dutilleul et al., 2003b; Vidal et al., 2007). Nevertheless, the AOX protein level and activity are not directly related, as already discussed earlier (Millenaar and Lambers, 2003), and indicated by different correlations of AOX protein level and activity under stress (Lennon et al., 1997; Ribas-Carbo et al., 2005b; Vidal et al., 2007). Moreover, it seems likely that the reduced level of AOX protein was still sufficient to maintain valt under both normal and stressful conditions. In general, the lack of mitochondrial complex I seemed to be compensated by a higher rate of respiration through vcyt, which further indicates the importance of mitochondrially synthesized ATP, particularly in situations of low photosynthetic carbon assimilation (i.e. during drought stress).
Recovery during rewatering
Leaf water status was restored immediately after rewatering in WT and CMSII plants, providing full water supply to the leaves. As gm was immediately restored to control values within the first day of rewatering, the limitation of photosynthetic recovery due to leaf internal resistances for CO2 diffusion (i.e. gm) can be discounted. Consequently, similar rates of photosynthetic recovery were obtained for WT and CMSII after rewatering, while complete restoration was reached after 2 d of rewatering. Photosynthetic recovery was mainly limited by stomata, as the restoration of gs was slightly delayed. As a consequence, increased WUE during drought stress also persisted during the rewatering phase in stressed WT and CMSII plants, indicating an improved carbon fixation per loss of water. The rise of gm at the end of the rewatering phase might also partly explain this persistence of increased WUE and the high AN.
Although the functioning of the photosynthetic apparatus during drought stress seemed to be more affected in CMSII than in WT, photosynthetic parameters like Jmax, qP, and Chl a/b were restored immediately after rewatering in the mutant, resulting in even higher ratios in the case of Chl a/b (presumably due to the increased number of light harvesting complexes and thus enhanced light capture for improved photosynthetic activity). Thus, the CMSII mutant seems to be highly flexible in adjusting its photosynthetic machinery during and after drought stress. Here, also metabolic changes might play a role, as the carbon/nitrogen balance is affected in CMSII (Dutilleul et al., 2003a, 2005).
Conclusions
With regard to the first two questions which have been raised in the introduction about the effect of complex I deficiency on photosynthetic activity during drought stress and recovery, photosynthesis was inhibited and restored in a similar manner in both CMSII mutant and WT. Adjustments of photosynthetic traits seemed to be involved, whereas, for example, light capture (i.e qP, Chl a/b) markedly changed in CMSII to minimize excess (reducing) energy. Furthermore, respiration (i.e. vcyt) always remained higher in CMSII than in WT, most probably to adjust ATP production to similar levels than in WT.
Therefore, and with regard to the third question about the necessity of an efficient respiratory chain for photosynthetic recovery, a more efficient respiration chain is not required, as the CMSII mutant is capable of adjusting the activities of the photosynthetic and respiratory pathways (similar ATP level as in WT) to ensure the restoration of photosynthesis as in the WT.
The strict control of respiration by mitochondrial ATP demand under drought stress and recovery can be assumed, which emphasizes the importance of ATP production being maintained by mitochondria under limited photosynthetic carbon assimilation (i.e. during drought stress). However, the enhanced activity of NAD(P)H dehydrogenases (Rasmusson et al., 1998), as well as an altered cell redox balance due to changes in antioxidant levels (Dutilleul et al., 2003b) or alterations in the nitrogen/carbon balance (Dutilleul et al., 2005) might play a role in the compensation of an impaired respiratory chain. Furthermore, the proposed role of valt in maintaining the redox balance during severe drought stress (Ribas-Carbo et al., 2005b) still remains unclear, as valt was not altered in both CMSII and WT. A possible explanation might be that the degree of oxidative stress was not severe enough to alter valt. Further studies are needed to explore the interactions of mitochondrial non-phosphorylating pathways with photosynthetic processes and cell homeostasis under limited water supply, as well as under other stressful conditions.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. 1. Changes of specific leaf are (SLA) during the experiment in WT and CMSII plants.
Supplementary Material
Acknowledgments
The authors are grateful to the Central Service of the UIB Palma (Spain) for providing the MS facilities and technical assistance. The group of Dr P Escribá, Laboratory of Molecular and Cellular Biomedicine, UIB Palma (Spain) is kindly acknowledged for densitometry quantification of western blots. This study was financed by the Spanish Ministry of Education and Research (Project BFU2008-1072-E/BFI), the French Centre National de la Rechreche Scientifique and the Université Paris 11. A Galle had a fellowship from the Swiss National Science Foundation. I Florez-Sarasa had a FPI scholarship from the Spanish Ministry of Science and Innovation.
References
- Bartoli CG, Gomez F, Gergoff G, Guiamet JJ, Puntarulo S. Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. Journal of Experimental Botany. 2005;56:1269–1276. doi: 10.1093/jxb/eri111. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology. 2002;130:1992–1998. doi: 10.1104/pp.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology. 1997;114:185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Ciais P, Reichstein M, Viovy N, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT. The cyanide-resistant oxidase: to inhibit or not to inhibit, that is the question. Plant Physiology. 1996;110:1–2. doi: 10.1104/pp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science. 1996;1:21–26. [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, de Paepe R, Foyer CH, Noctor G. Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiology. 2003a;131:264–275. doi: 10.1104/pp.011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul C, Garmier M, Noctor G, Mathieu C, Chetrit P, Foyer CH, de Paepe R. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. The Plant Cell. 2003b;15:1212–1226. doi: 10.1105/tpc.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul C, Lelarge C, Prioul JL, de Paepe R, Foyer CH, Noctor G. Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiology. 2005;139:64–78. doi: 10.1104/pp.105.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiology. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology. 2004;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Flexas J, Galmes J, Ribas-Carbo M, Medrano H. The effects of drought in plant respiration. In: Lambers H, Ribas-Carbo M, editors. Plant respiration: from cell to ecosystem. Advances in photosynthesis and respiration series. Vol. 18. Dordrecht: Kluwer Academic Publishers; 2005. pp. 85–94. [Google Scholar]
- Flexas J, Bota J, Galmes J, Medrano H, Ribas-Carbo M. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiologia Plantarum. 2006;127:343–352. [Google Scholar]
- Flexas J, Diaz-Espejo A, Berry J, Cifre J, Galmes J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Analysis of leakage in IRGA's leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany. 2007;58:1533–1543. doi: 10.1093/jxb/erm027. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa ID, Bouma TJ, Medrano H, Azcon-Bieto J, Ribas-Carbo M. Contribution of the cytochrome and alternative pathways to growth respiration and maintenance respiration in Arabidopsis thaliana. Physiologia Plantarum. 2007;129:143–151. [Google Scholar]
- Galle A, Haldimann P, Feller U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytologist. 2007;174:799–810. doi: 10.1111/j.1469-8137.2007.02047.x. [DOI] [PubMed] [Google Scholar]
- Galmes J, Medrano H, Flexas J. Acclimation of Rubisco specificity factor to drought in tobacco: discrepancies between in vitro and in vivo estimations. Journal of Experimental Botany. 2006;57:3659–3667. doi: 10.1093/jxb/erl113. [DOI] [PubMed] [Google Scholar]
- Galmes J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Gaston S, Ribas-Carbo M, Busquets S, Berry JA, Zabalza A, Royuela M. Changes in mitochondrial electron partitioning in response to herbicides inhibiting branched-chain amino acid biosynthesis in soybean. Plant Physiology. 2003;133:1351–1359. doi: 10.1104/pp.103.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Giraud E, Ho LHM, Clifton R, et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiology. 2008;147:595–610. doi: 10.1104/pp.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Matamala R, Penuelas J. Effects of prolonged drought stress and nitrogen deficiency on the respiratory O2 uptake of bean and pepper leaves. Photosynthetica. 1997;34:505–512. [Google Scholar]
- Gorton HL, Herbert SK, Vogelmann TC. Photoacoustic analysis indicates that chloroplast movement does not alter liquid-phase CO2 diffusion in leaves of Alocasia brisbanensis. Plant Physiology. 2003;132:1529–1539. doi: 10.1104/pp.102.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierres S, Sabar M, Lelandais C, Chetrit P, Diolez P, Degand H, Boutry M, Vedel F, deKouchkovsky Y, de Paepe R. Lack of mitochondrial and nuclear-encoded subunits of complex I and alteration of the respiratory chain in Nicotiana sylvestris mitochondrial deletion mutants. Proceedings of the National Academy of Sciences, USA. 1997;94:3436–3441. doi: 10.1073/pnas.94.7.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta. 1989;177:483–491. doi: 10.1007/BF00392616. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Dimarco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proceedings of the National Academy of Sciences, USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczuk IM, Flexas J, Szal B, Dabrowska Z, Ribas-Carbo M, Rychter AM. Effect of mitochondrial genome rearrangement on respiratory activity, photosynthesis, photorespiration and energy status of MSC16 cucumber (Cucumis sativus) mutant. Physiologia Plantarum. 2007;131:527–541. doi: 10.1111/j.1399-3054.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- Laisk A. Kinetics of photosynthesis and photorespiration in C3 plants. Nauka, Moscow. 1977 [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effects of salicylic acid and tobacco mosaic virus Infection on the alternative oxidase of tobacco. Plant Physiology. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Loreto F, Centritto M. Leaf carbon assimilation in a water-limited world. Plant Biosystems. 2008;142:154–161. [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulias J, Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Lambers H. The alternative oxidase: in vivo regulation and function. Plant Biology. 2003;5:2–15. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Cescatti A, Rodeghiero M, Tosens T. Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant, Cell and Environment. 2005;28:1552–1566. [Google Scholar]
- Noctor G, de Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends in Plant Science. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR. Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. The Plant Journal. 2007;50:1093–1106. doi: 10.1111/j.1365-313X.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- Priault P, Fresneau C, Noctor G, de Paepe R, Cornic G, Streb P. The mitochondrial CMSII mutation of Nicotiana sylvestris impairs adjustment of photosynthetic carbon assimilation to higher growth irradiance. Journal of Experimental Botany. 2006a;57:2075–2085. doi: 10.1093/jxb/erj161. [DOI] [PubMed] [Google Scholar]
- Priault P, Tcherkez G, Cornic G, de Paepe R, Naik R, Ghashghaie J, Streb P. The lack of mitochondrial complex I in a CMSII mutant of Nicotiana sylvestris increases photorespiration through an increased internal resistance to CO2 diffusion. Journal of Experimental Botany. 2006b;57:3195–3207. doi: 10.1093/jxb/erl083. [DOI] [PubMed] [Google Scholar]
- Priault P, Vidal G, de Paepe R, Ribas-Carbo M. Leaf age-related changes in respiratory pathways are dependent on complex I activity in Nicotiana sylvestris. Physiologia Plantarum. 2007;129:152–162. [Google Scholar]
- Raghavendra AS, Padmasree K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends in Plant Science. 2003;8:546–553. doi: 10.1016/j.tplants.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Heiser V, Zabaleta E, Brennicke A, Grohmann L. Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochimica et Biophysica Acta-Bioenergetics. 1998;1364:101–111. doi: 10.1016/s0005-2728(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant-mitochondria. Plant Physiology. 1995;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN. The regulation of electron partitioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiology. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Robinson S, Giles L. The application of the oxygen-isotope technique to assess respiratory pathway partitioning. In: Lambers H, Ribas-Carbo M, editors. Plant respiration: from cell to ecosystem. Advances in photosynthesis and respiration series. Vol. 18. Dordrecht, The Netherlands: Springer; 2005a. pp. 31–42. [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, Lambers H, Medrano H, Berry JA, Flexas J. Effects of water stress on respiration in soybean leaves. Plant Physiology. 2005b;139:466–473. doi: 10.1104/pp.105.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabar M, de Paepe R, de Kouchkovsky Y. Complex I impairment, respiratory compensations, and photosynthetic decrease in nuclear and mitochondrial male sterile mutants of Nicotiana sylvestris. Plant Physiology. 2000;124:1239–1249. doi: 10.1104/pp.124.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini R, Epron D, Deangelis P, Matteucci G, Dreyer E. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water-supply. Plant, Cell and Environment. 1995;18:631–640. [Google Scholar]
- Vidal G, Ribas-Carbo M, Garmier M, Dubertret G, Rasmusson AG, Mathieu C, Foyer CH, de Paepe R. Lack of respiratory chain complex I impairs alternative oxidase engagement and modulates redox signaling during elicitor-induced cell death in tobacco. The Plant Cell. 2007;19:640–655. doi: 10.1105/tpc.106.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR, Adams MA. Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant, Cell and Environment. 2006;29:192–201. doi: 10.1111/j.1365-3040.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- Warren CR, Low M, Matyssek R, Tausz M. Internal conductance to CO2 transfer of adult Fagus sylvatica: variation between sun and shade leaves and due to free-air ozone fumigation. Environmental and Experimental Botany. 2007;59:130–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.