Abstract
Background
Carbonic anhydrase inhibitors (CAI) reduce proximal reabsorption, activating tubuloglomerular feedback (TGF) and reducing glomerular filtration rate (GFR). Adenosine A1 receptors (A1R) mediate the TGF response and stimulate proximal reabsorption.
Methods
Clearance and micropuncture studies were performed in Wistar rats to determine whether blockade of A1R (KW3902 0.3 mg/kg i.v.) would prevent CAI (benzolamide 5 mg/kg i.v.) from lowering GFR, whether CAI and KW3902 exert additive effects on sodium excretion, and to what extent such interactions depend on events in the glomerulus, proximal tubule, or distal nephron.
Results
KW3902 raised GFR and prevented CAI from lowering GFR. KW3902 and CAI caused additive diuresis and natriuresis. KW3902 and CAI increased lithium clearance, but their effects were redundant. CAI increased the dependence of proximal reabsorption on active chloride transport. KW3902, alone, did likewise, but to a lesser extent than CAI. Adding KW3902 to CAI lessened the shift toward active chloride transport.
Conclusions
The data reveal that A1R mediate glomerular vascular resistance whether or not TGF is activated, that additive effects of CAI and KW3902 on salt excretion occur, in part, because KW3902 inhibits reabsorption downstream from the macula densa, and that KW3902 likely inhibits proximal reabsorption by interfering with apical sodium-hydrogen exchange.
Key Words: Tubuloglomerular feedback, Adenosine receptor, Carbonic anhydrase, Glomerular filtration rate, Proximal reabsorption, Renal blood flow
Introduction
Adenosine is an important regulator of glomerular and tubular function [1]. A diuretic and saliuretic response to adenosine receptor blockade was reported in the 1980s [2]. This finding has been reproduced in rats [3], dogs [4], mice [5], and humans [6]. As is reflected in the above citations, it is known that these effects are mediated through A1 subtype of adenosine receptors (A1R), which are expressed in the pre-glomerular arterioles and at several sites along the nephron [7]. A1R blockade was shown to suppress proximal reabsorption, as assessed by renal micropuncture [8] and lithium clearance in the rat [9]. In addition to direct tubular effects, adenosine affects renin release, and studies in mice lacking A1R [10,11,12] and particularly micropuncture experiments with clamping of A1R activity [13] demonstrated that adenosine via activation of A1R mediates the tubuloglomerular feedback (TGF) response. The TGF establishes an inverse relationship between the salt concentration at the macula densa and the filtration rate of the same nephron thus coordinating tubular reabsorption and glomerular filtration.
Another class of proximal diuretics are the carbonic anhydrase inhibitors (CAI), which interfere with transport of NaHCO3 and can reduce net proximal reabsorption by 50% [14, 15]. In the course of reducing proximal reabsorption, CAI also activate TGF, which causes GFR and renal blood flow to decline [15]. This negative impact of CAI on glomerular filtration, and therefore in diuretic activity, may explain why CAI never became first-line therapy for edematous conditions, notwithstanding initial enthusiasm in the 1950s [16].
Based on the foregoing pharmacology, the present studies were conducted to determine whether concomitant A1R blockade would prevent the CAI, benzolamide (BNZ), from causing renal vasoconstriction, thereby making BNZ a more suitable and effective diuretic, and to test for qualitative differences in the effects of A1R antagonist and BNZ on proximal reabsorption.
Materials and Methods
Animals
Animal experiments described herein were conducted in accordance with the NIH Guide for the Care and Use of Animals in Research. The protocolswere reviewed and approved by the Institutional Animal Careand Use Committee of the VA San Diego Healthcare System. Adult male Wistar rats (Simonsen Laboratories, Gilroy, Calif., USA) weighing 300–350 g were maintained on standard rat chow and tap water until the day of the experiments. Experiments included either whole-kidney measurements or in vivo micropuncture.
Surgical Preparation
Whole Kidney. Rats were anesthetized with thiobutabarbital (Inactin®, 100 mg/kg i.p.; Research Biochemicals International, Natick, Mass., USA) and placed on a thermostatically controlled surgical table to maintain body temperature at 37°C. Catheters were placed in the trachea (PE-240) and in the left jugular vein (PE-50) for infusion of saline, vehicle and drug. The right femoral artery was catheterized (PE-50) for collection of blood and for continuous measurement of mean arterial pressure (MAP). Quantitative urinary collection was by a catheter (PE-50) inserted into the bladder. The left renal artery was separated from renal vein through a midline incision of the abdomen plus a flank incision. The left kidney blood flow (RBF, ml/min) was monitored by a perivascular ultrasonic transit time flow probe (Transonics T206, Ithaca, N.Y., USA) connected to a computer for continuous recording. Two-kidney glomerular filtration rate (GFR, ml/min) was measured by urinary clearance of [3H]-inulin administered in Ringer's saline (5 μCi/ml at 1.5 ml/h). Delivery of sodium to the macula densa was measured by clearance of lithium which was administered as a 4-mg i.p. bolus of LiCl given at the onset of anesthesia and followed by a continuous infusion (2.1 mg/h, i.v.). After the surgical preparation, the animals were allowed 120 min to stabilize.
Micropuncture. Thiobutabarbital anesthesia, placement of vascular and bladder catheters, and monitoring were as described above for the whole-kidney experiments. In addition, the left kidney was prepared for micropuncture by exposure through a left flank incision, placement of ureteral catheter (PE-50) immobilization in a Lucite cup, and superfusion with warm saline according to an established protocol [14]. Animals were maintained in a hydropenic condition by infusing Ringer's saline at 2 ml/h. [3H]-inulin was added to the Ringer's saline at 80 μCi/ml as a marker of glomerular filtration. After preparation was complete, 1 h was allowed for stabilization prior to beginning micropuncture.
Experimental Procedures
Whole Kidney. Data were obtained from each animal during each of two experimental periods. Control data were obtained during the first period. Then animals were administered bolus injections of either the CAI, BNZ (5 mg/kg i.v., n = 6), A1R blocker, 8-(noradamantan-3-yl)-1,3-dipropylxanthine (KW3902 (KW); 0.3 mg/kg i.v., n = 7), or BNZ and KW3902 (n = 7). KW3902 was delivered in a proprietary lipid emulsion [17]. BNZ was administered in isotonic sodium bicarbonate. Animals not receiving KW3902 or BNZ received the appropriate volume(s) of vehicle(s) as placebo. Data collection for the second period was begun 5 min after the drugs were delivered.
Each period lasted 40 min during which time urine was collected to determine urine flow rate, sodium excretion (UNaV), lithium excretion (ULiV), and GFR, while RBF was continuously recorded to determine its average. Blood samples were taken at the beginning and end of each period to obtain the plasma inulin and lithium concentrations necessary for clearance calculations. During the second period, additional Ringer's saline was infused to offset the increase in urine volume.
Lithium clearance was used as a marker of salt delivery to the macula densa region of the nephron. Lithium is filtered at the glomerulus and reabsorbed by the proximal tubule and Henle's loop in rough proportion to sodium [18,19,20].
Micropuncture. The purpose of these experiments was not to confirm that KW3902 and BNZ inhibit proximal reabsorption, but to determine whether they do so by different mechanisms. The strategy for this was to make timed tubular fluid collections from random points along the proximal tubule, measure the volume and tubular fluid [3H]-inulin concentration to determine reabsorption of water up to that point, and use differences in the tubular fluid to plasma chloride concentration ratio as an index of the dependence on active transport of bicarbonate versus chloride as the driving force for water reabsorption. More details are provided in the Results section. Several such tubular fluid collections were made from each of 13 animals during each of 2 or 3 experimental periods. The first period served as control. Prior to the second period, animals received either a bolus injection of KW3902 (0.3 mg/kg i.v.) or bolus (5 mg/kg i.v.) followed by continuous infusion (5 mg/kg/h i.v.) of BNZ. Prior to the third period, animals already receiving BNZ were administered KW3902. All data for an experimental period were collected within 60 min of the start of that period. Quantitative collections were made into glass micropipettes of 9–11 μm diameter at the tip. To initiate collection, a small amount of mineral oil stained with Sudan black was injected into the tubule from the collection pipette, suction was applied momentarily to break surface tension at the pipette tip, then maintained only as needed to keep the oil block from migrating downstream. The volume of collectate was determined by transfer to a hollow-bore pipette of known diameter and measuring the length of the fluid column. Approximately 1 nl of each collection was then removed to determine its chloride concentration (samples were measured in duplicate) and the remainder counted for [3H]. The total CPM in each collection was adjusted for the amount of fluid removed to measure chloride.
Analytical and Data Analysis
Urine sodium and plasma chloride concentrations were measured using the Synchron LX20 Pro (Beckman-Coulter, Inc., Fullerton, Calif., USA). Urine lithium concentrations were measured using atomic emission spectroscopy (Quest Diagnostics, Willow Grove, Pa., USA). Plasma lithium concentrations were measured by spectrophotometric method according to the manufacturer's instructions (Thermo Electron, Louisville, Colo., USA). Tubular fluid chloride concentration was determined by the electrometric titration method of Ramsey et al. [21] as modified by Windhager and Giebisch [22] and as previously utilized by our laboratory [23]. Urinary flow rate was determined gravimetrically. GFR and single-nephron GFR (SNGFR) were determined by [3H]-inulin clearance in urine and tubular fluid, respectively. Concentration of [3H]-inulin in plasma, tubular collections, and urine was measured by liquid-phase scintillation counting.
Statistical comparisons were performed by analysis of variance with repeated measures, when appropriate. Analysis of variance, multivariate and linear regressions were done using proprietary software (Systat, SPSS Inc., Evanston, Ill., USA). Statistical significance was set at p < 0.05. Statistical tests on lithium clearance and fractional excretion of lithium were done on log-transformed data. This was necessary because a large coefficient of variation for normally distributed plasma lithium skewed the distributions toward high values for those parameters built from ratios with plasma lithium in the denominator.
Drugs and Solutions
BNZ was a gift from Dr. Thomas Maren. KW3902 was provided by NovaCardia, Inc. (San Diego, Calif., USA).
Results
Whole-Kidney Experiments
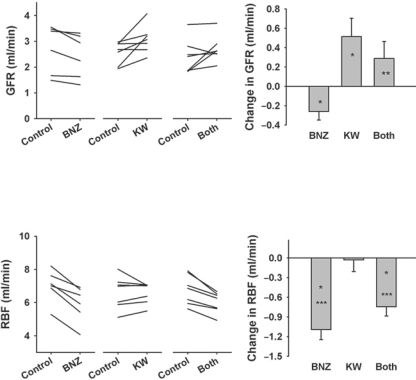
Renal Hemodynamic Effects (fig. 1). Neither BNZ or KW3902 affected blood pressure. KW3902 caused a minor increase in heart rate (table 1). BNZ caused GFR to decline (2.70 ± 0.38 to 2.44 ± 0.34 ml/min, p < 0.05), while KW3902 caused GFR to increase (2.56 ± 0.16 to 3.08 ± 0.20 ml/min, p < 0.05). When both drugs were given together, there was a non-significant tendency for GFR to increase (2.41 ± 0.25 to 2.70 ± 0.19 ml/min, NS). BNZ also caused RBF to decline (7.02 ± 0.40 to 5.93 ± 0.44 ml/min, p = 0.001). KW3902 did not affect RBF (6.61 ± 0.37 to 6.58 ± 0.24 ml/min, NS) over the clearance period, although it caused a momentary increase in RBF not observed with placebo. When both drugs were given together, RBF declined (6.76 ± 0.34 to 6.02 ± 0.24 ml/min, p = 0.002). Overall, BNZ and KW3902 had opposite effects on renal hemodynamics and their effects were additive.
Fig. 1.
Effect of BNZ (n = 6), KW3902 (n = 7) or both (n = 7) on GFR and RBF. Left panel: Individual experiments. Right panel: Changes from baseline after drug(s) were given. Data are means ± SEM. Significant differences are indicated as ∗ p < 0.05 vs. baseline, ∗∗ p < 0.05 vs. BNZ alone, and ∗∗∗ p < 0.05 vs. KW3902 alone.
Table 1.
MAP and heart rate before and after administration of BNZ, KW3902, or both
| MAP, mm Hg |
Heart rate, bpm |
|||||
|---|---|---|---|---|---|---|
| BNZ | KW | both | BNZ | KW | both | |
| Control | 97 ± 6 | 99 ± 4 | 98 ± 3 | 385 ± 22 | 368 ± 18 | 364 ± 10 |
| Drug | 92 ± 5 | 100 ± 3 | 95 ± 3 | 380 ± 16 | 386 ± 21∗ | 371 ± 13 |
Values expressed as means ± SE.
p = 0.005.
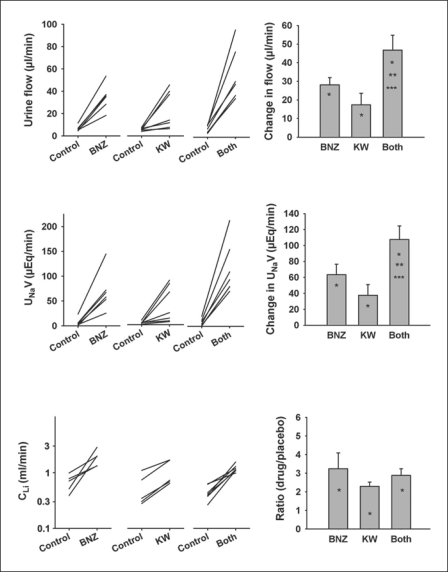
Urine Flow Rate, Sodium Excretion and Lithium Clearance (fig. 2). Both KW3902 (5.9 ± 0.4 to 23.3 ± 6.4 μl/min, p = 0.03), and BNZ (6.7 ± 1.0 to 34.7 ± 4.7 μl/min, p = 0.001) caused the urine flow rate to increase with their diuretic effects being additive (7.2 ± 1.3 to 54.0 ± 8.8 μl/min for the two given together, p = 0.001). Both KW3902 (0.39 ± 0.09 to 4.14 ± 1.4 μEq/min, p = 0.03) and BNZ (0.68 ± 0.34 to 7.01 ± 1.64 μEq/min, p = 0.005) caused significant increases in net urinary sodium excretion. The natriuretic effects of KW3902 and BNZ were additive (0.72 ± 0.27 to 11.45 ± 1.96 μEq/min for the two drugs given together, p = 0.005). Additive effects on the fractional excretion of sodium also occurred as manifest by normalizing sodium excretion to GFR (in μEq/ml): KW3902 (0.15 ± 0.04 to 1.354 ± 0.46); BNZ (0.22 ± 0.09 to 3.00 ± 0.58), and KW3902 + BNZ (0.29 ± 0.10 to 4.40 ± 0.84); all p < 0.05. Lithium clearance (CLi) increased in response to KW3902 and/or BNZ. However, unlike their impact on sodium excretion, the effects of BNZ and KW3902 on CLi were not additive.
Fig. 2.
Effect of BNZ, KW3902 or both on urinary flow rate, UNaV and CLi. Left panel: Individual experiments. Right panel: Changes from baseline after drug(s) were given. Data are means ± SEM. Significant differences are indicated as ∗ p < 0.05 vs. baseline, ∗∗ p < 0.05 vs. BNZ alone, and ∗∗∗ p < 0.05 vs. KW3902 alone.
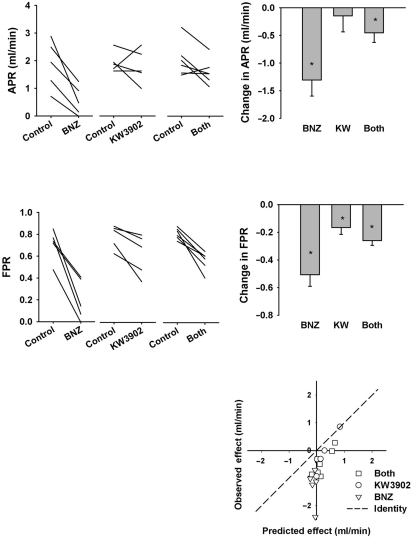
Proximal Reabsorption (fig. 3). For present purposes, net proximal reabsorption was taken to be the difference between GFR and CLi and fractional proximal reabsorption was taken as the difference between unity and the fractional excretion of lithium (FELi). Glomerulotubular balance (GTB) confers a strong dependence of net reabsorption on GFR. Hence, confounding changes in GFR must be accounted for when testing for direct effects of a drug on tubular reabsorption. The combination of BNZ and KW3902 increased GFR and reduced net proximal reabsorption. Therefore, it is safe to conclude, without further analysis, that the combination directly inhibited proximal reabsorption. BNZ, alone, reduced net reabsorption, but also reduced GFR. An inhibitory effect of BNZ on proximal reabsorption is confirmed by a decline in fractional proximal (lithium) reabsorption because simultaneous declines in GFR and fractional reabsorption cannot be explained by GTB. The proximal tubular effect of KW3902 was confounded by its impact on GFR such that KW3902 increased CLi without reducing net proximal reabsorption (of lithium). Indeed, KW3902 significantly increased FELi such that KW3902 appeared to be a proximal diuretic when GFR was treated as a traditional covariate. However, implicit in the null hypothesis for analysis of covariance is an assumption equating GTB with constant fractional reabsorption. Logical reasoning can invalidate this assumption since fractional reabsorption must approach unity at low GFR and zero at infinite GFR. To confirm that KW3902 was a proximal diuretic it is necessary to compare the observed decrease in fractional reabsorption to the expected decrease linked to GTB. This was done by assuming that the fractional reabsorption overestimates, by 25%, the efficiency of GTB, as previously shown by micropuncture [24]. By this more stringent criterion, KW3902 still appeared to be a proximal diuretic in all but one animal (fig. 3, bottom panel).
Fig. 3.
Effect of BNZ, KW3902 or both on net proximal reabsorption (GFR-CLi) and fractional proximal reabsorption of lithium (1-FELi). Left panel: Individual experiments. Right panel: Changes from baseline after drug(s) were given. Significant differences are indicated as ∗ p < 0.05 vs. baseline. Bottom panel: Observed changes in proximal reabsorption compared to prediction based on GFR and normal glomerulotubular balance. Symbols below the line of identity connote a primary decrease in proximal reabsorption.
Micropuncture Results
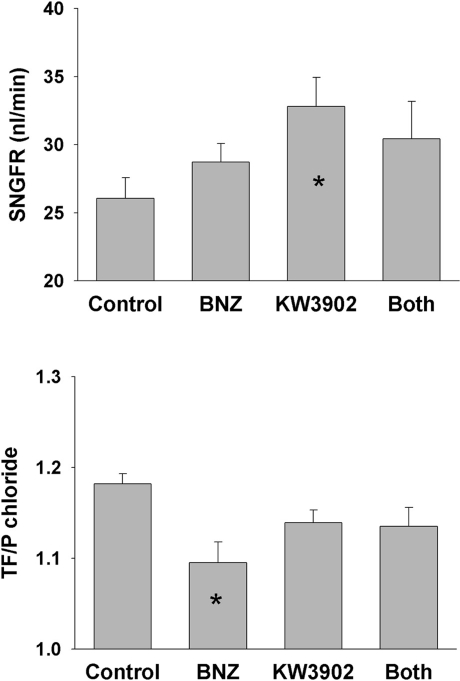
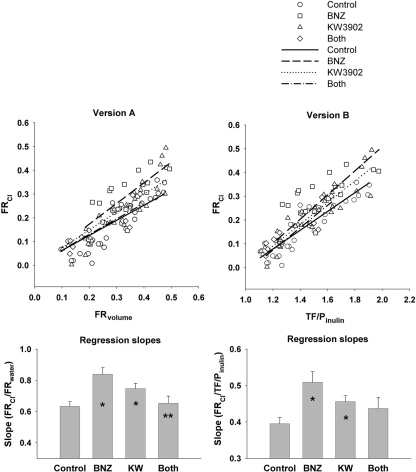
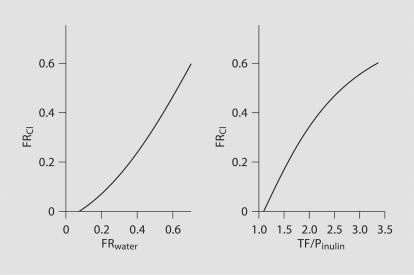
Analysis was performed on 137 tubular fluid collections with apparent tubular fluid/plasma inulin (TF/Pinulin) ranging from 0.93 to 3.32 and apparent TF/P chloride from 0.91 to 1.34. KW3902 caused SNGFR to increase by 25% over control (p = 0.021 after Tukey adjustment for multiple comparisons), while BNZ had no apparent effect on SNGFR (fig. 4). The experiments were designed to tap a wide range of fractional reabsorption rather than to confirm that KW3902 or BNZ are proximal diuretics. Nonetheless, a diuretic effect was confirmed for BNZ by two-way analysis of variance (p = 0.01), although no pairwise inter-group comparison remained statistically significant after Tukey correction. TF/P chloride was significantly greater than unity in all groups, greatest in controls, least in BNZ, and intermediate in KW3902 and BNZ + KW3902 (fig. 4). By two-way analysis of variance, the overall effect of BNZ was significant (p = 0.008) as was the tendency for addition of KW3902 to lessen the impact of BNZ (p = 0.014). A standard general multivariate linear model (GML) was applied to test for differences in the mechanisms whereby BNZ and KW3902 affect proximal reabsorption. The basic notion is that there are two major mechanisms that drive proximal reabsorption. One of these two mechanisms begins with active reabsorption of bicarbonate, which lowers mixing entropy to drive passive chloride reabsorption [25]. Evidence for the primacy of this mechanism is that it causes TF/P chloride to rise above unity. The second mechanism lumps together active reabsorption of chloride by various apical anion exchangers [26]. This mechanism operates without raising the TF/P chloride. While both mechanisms affect chloride reabsorption, a diuretic that selectively inhibits bicarbonate reabsorption will increase the total amount of chloride transport necessary to reabsorb a given amount of the filtered water. Hence, GML was used to test for effects of BNZ and KW3902 on the degree to which proximal reabsorption depends on chloride (fig. 5). Two versions of the model were tested. In both versions fractional chloride reabsorption (FRCl) was the dependent variable while KW3902 and BNZ were categorical factors. In one version of the model (version A), fractional reabsorption of volume (FRvolume) was treated as a continuous independent variable and in the other version (version B) this role was assigned to TF/Pinulin. The reason to test both versions is that they will introduce opposing biases when the distributions of values for the independent variable are skewed differently between the groups. For example, version A is prone to progressively overstate the dependence on chloride at high reabsorptions, whereas version B is prone to progressively understate the dependence on chloride at high reabsorptions. These nonlinearities arise from solute-solvent coupling, the predilection for bicarbonate reabsorption to precede chloride reabsorption in the S1 segment, and because the derivative of chloride reabsorption with respect to TF/Pinulin comes to vary as the inverse square of TF/Pinulin as the bicarbonate declines. These nonlinearities are visible in figure 6, which was generated by algebraic rearrangement of the transport profile for an idealized proximal tubule adapted from a medical textbook [27] and the original work of Rector [25]. Furthermore, to bring the data into compliance with the assumptions of GML, the model was restricted to data where 1 < TF/Pinulin < 2, which gave comparable distributions for the independent variables between the groups. Hence, 91 of the 137 tubular fluid collections were included in this analysis.
Fig. 4.
Effect of BNZ, KW3902 or both on SNGFR and TF/P chloride. ∗ p < 0.05 vs. control by Tukey adjustment for multiple group comparisons.
Fig. 5.
Dependency of water reabsorption on chloride reabsorption in controls (n = 31), BNZ-treated rats (n = 21) and KW3902- treated rats (n = 29). Both (n = 10) indicates BNZ administration followed by KW3902. Relations (slopes) of fractional chloride reabsorption (FRCl) to fractional reabsorption of volume (FRvolume) (version A) or to tubular fluid:plasma inulin ratio (TF/Pinulin) (version B) were derived by linear regression. A higher regression slope signifies increased dependence of overall reabsorption on active Cl− transport relative to HCO3− transport. ∗ p<0.05 vs. control, ∗∗ p < 0.05 vs. BNZ. See text for details.
Fig. 6.
FR Cl as a function of FR water or TF/P inulin for an idealized proximal tubule. Curves were generated by algebraic rearrangement of a figure published in several textbooks based on original work by Rector [25]. The point is to show that the two linear statistical models presently employed are subject to opposing biases that can be discounted if both models yield the same result.
Both versions of the model gave similar results, albeit at different levels of certainty (see fig. 5): BNZ caused a major increase in the dependence of overall reabsorption on chloride (p = 0.004 vs. control for version A, p = 0.006 for version B of the model). KW3902 caused a lesser trend in the same direction (p = 0.014 vs. control by version A, p = 0.016 by version B of the model). In both versions of the model there was a tendency for addition of KW3902 to reverse the impact of BNZ (p = 0.058 for version A, p = 0.20 for version B).
Discussion
These studies examine individual and combined effects of two putative proximal diuretics. One of these (BNZ) is a CAI and the other (KW3902) is an A1R antagonist. The studies were motivated by interest in two areas. First, we were interested in learning whether one can prevent a CAI from lowering GFR by blocking the A1R-mediated TGF response. Second, we were interested to learn whether the diuretic effects of CAI and A1R blockade are additive or redundant. The novel findings are that: (1) systemic A1R blockade acutely increases GFR; (2) A1R blockade and CAI exert opposing effects on renal hemodynamics that are essentially additive; (3) a TGF stimulus is not necessary for A1R blockade to increase SNGFR; (4) A1R blockade and CAI are additively diuretic/natriuretic, whereas their effects on CLi are less than additive, to wit, they are redundant; (5) given alone, A1R blocker or CAI both increase the relative contribution of chloride transport to overall proximal reabsorption, whereas in the presence of CAI, A1R blockade reduces the relative contribution of chloride to overall proximal reabsorption. The implications of these findings for kidney physiology are discussed in the following paragraphs.
Effects on Glomerular Filtration
CAIs activate TGF [15, 28] and have been recurrently exploited for the purpose of studying TGF [29,30,31,32]. It was recently shown, however, that absence of a functional TGF mechanism does not prevent the reduction in GFR or RBF caused by BNZ in the A1R knockout mouse in which BNZ acutely stimulates renin release [33]. In the present study, KW3902 increased GFR, BNZ decreased GFR and combining them left GFR unchanged. Quantitatively, the result of combining KW3902 and BNZ appeared quite close to the vector sum of the two individual effects, which is the result one would expect if the two agents had affected GFR independent of one another. But this result might also arise by coincidence if KW3902 were only partially effective at blocking TGF and managed to block just enough of it to obtain this outcome. It is not possible to attach a p value to the likelihood of such a coincidence and, overall, the clearance data support a hypothesis of independent effects, which is quite opposite to our original working model.
As illustrated in figure 1, there appears to be a dissociation between GFR and RBF as GFR increased with KW3902 alone and with KW3902 + BNZ, while RBF did not. This implies increased ultrafiltration pressure during KW3902 since GFR cannot be increased by raising the glomerular ultrafiltration coefficient in hydropenic rats [34]. To raise ultrafiltration pressure at constant RBF requires simultaneous afferent arteriole dilation matched with efferent arteriole constriction. Blocking A1R should dilate the afferent arteriole without doing anything directly to raise efferent resistance. But A1R, on JGA cells, tonically inhibit renin secretion when the macula densa flow rate is not low and A1R blockade in this setting will de-repress renin leading to angiotensin II-mediated efferent constriction [35]. The time for a renin-angiotensin response to develop is on the order of 2–4 min [36], which is longer than the time required for KW3902 to directly reverse adenosine-mediated afferent tone. The disparate kinetics of these two events likely explains the transient increase in RBF with the KW3902 bolus that disappeared after 3–5 min. Less angiotensin II is required to affect the glomerular hemodynamics than to raise the systemic blood pressure [37] which was unaffected by KW3902. This model would allow KW3902 to dissociate increases in GFR from RBF under some circumstances, but not others, depending on what the relative activity of the various renin controllers in a given circumstance. For example, KW3902 clearly increases both GFR and RBF in humans with congestive heart failure [38]. While the hypothesis herein outlined is the most plausible explanation for dissociating GFR from RBF during A1R blockade, actual proof of this is beyond the scope of the current project.
There is additional suggestion from the present micropuncture data that our current understanding of A1R in the control of glomerular hemodynamics is incomplete and that A1R in the glomerular microvessels may be activated independent of TGF. In these experiments, TGF was rendered inactive by the method of collecting from the proximal tubule to measure SNGFR. As a result, BNZ, which reduced whole-kidney GFR, had no effect on SNGFR. Meanwhile, KW3902, which was expected to have no effect on SNGFR in the absence of a TGF signal, actually brought about a major increase in SNGFR. Therefore, while A1R are essential to the TGF response [10, 13], TGF appears to not account for all A1R-mediated vasoconstriction in the glomerulus. This is a novel conclusion but should not be surprising since most renal epithelia appear to secrete ATP [39], and since ecto-5′-nucleotidase is active in and around the glomerulus [40]. It is possible that the macula densa continues to secrete ATP at a low level even when there is no flow in Henle's loop.
Effects on the Tubule
Both BNZ and KW3902 increased urinary excretion of sodium and water and their effects were additive. Furthermore, both agents increased CLi, which is a rough measure of delivery up to the macula densa. But unlike the additive effect on sodium and water excretion, the effects on CLi were redundant. This combination of effects on sodium excretion and CLi implies that one or the other agent(s) must inhibit reabsorption in the distal tubule or collecting duct. In all likelihood, this falls to KW3902 in the collecting duct, since A1R blockers are reported to have potassium-sparing effects [41, 42]. Questions also arise from the clearance data as to whether KW3902 is, in fact, a proximal diuretic. There is no doubt that BNZ inhibited proximal reabsorption, since there is no other way to reconcile the simultaneous decrease in GFR and increase in CLi that occurred with BNZ. The direct impact of KW3902 on proximal reabsorption is less obvious, however, since KW3902 increased GFR and, all else remaining equal, increasing GFR will increase CLi.
The proximal tubule is a nearly isosmotic transporting epithelium across which glucose and bicarbonate ions manifest a higher reflection coefficient than chloride ions [25]. Hence, when glucose and bicarbonate are reabsorbed, the TF/P chloride rises. Given a 5-fold molar excess of bicarbonate relative to glucose, elimination of the transtubular chloride gradient by BNZ is not unexpected.
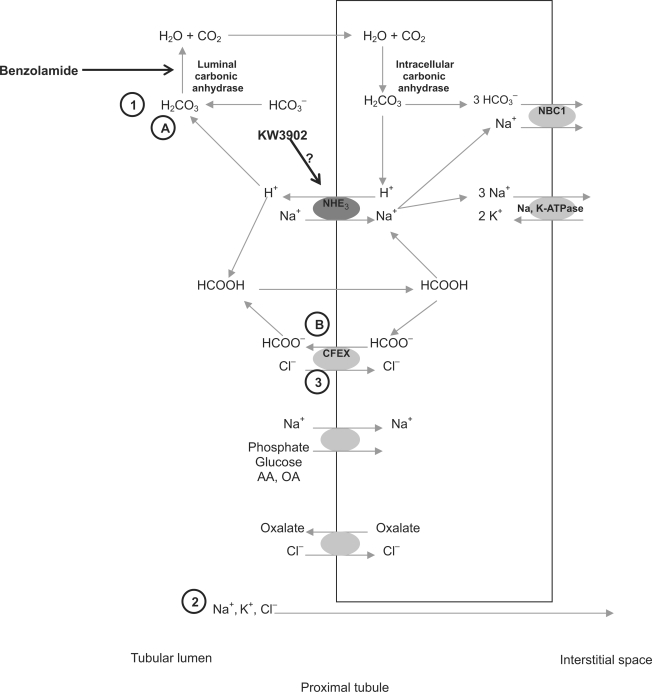
These micropuncture experiments were not designed to confirm that KW3902 and BNZ are proximal diuretics. Instead, they were designed to shed light on their mechanisms and interactions in the proximal tubule. Given the tools available to us, we chose to describe changes in the amount of chloride that must be reabsorbed to accomplish any particular amount of overall fluid reabsorption. Changes in the dependence of overall reabsorption on chloride will mirror changes in the ratio of chloride reabsorbed to bicarbonate + glucose that is reabsorbed. Given alone, either BNZ or KW3902 increase the chloride dependence of proximal reabsorption, possibly for different reasons (fig. 7). By blocking the luminal carbonic anhydrase only [43], BNZ suppresses active reabsorption of sodium bicarbonate, and at the same time, it acidifies the tubular lumen, thereby providing a direct stimulus for chloride reabsorption via the chloride-formate exchange mechanism (CFEX) [44, 45]. BNZ is a potent proximal diuretic because it is a strong inhibitor of bicarbonate reabsorption, which is normally responsible, directly or indirectly, for most of proximal reabsorption. Whatever proximal reabsorption does occur in the presence of BNZ should be more dependent on CFEX, which it activates. KW3902, on the other hand, not being a CAI, is not expected to acidify the tubule or to activate CFEX while it inhibits reabsorption. Data on the mechanism whereby A1R activation stimulates proximal reabsorption are not wholly convincing, with arguments having been made for activation of the apical sodium-hydrogen exchanger isoform-3 (NHE3) [46, 47] or the basolateral sodium-bicarbonate co-transport (NBC1) [48]. The former seems likely based on the fact that NHE3 is the control point for the major hormone (angiotensin II [49]) and neurotransmitter (norepinephrine [50]) that stimulate proximal reabsorption. Inhibiting NHE3 will suppress bicarbonate transport and CFEX to varying degrees, but will leave intact reabsorption mediated by chloride-oxalate exchange and sodium-glucose co-transport (SGLT).NHE3 blockade inhibits proximal reabsorption of sodium more so than of chloride [23]. Based on the overall response, KW3902 cannot be a strong inhibitor of NHE3, but owing to the primacy of bicarbonate reabsorption, inhibiting this, even slightly, will increase the overall chloride-dependence of residual reabsorption, which likely explains the intermediate increase in chloride dependence with KW3902. But when bicarbonate transport is previously eliminated and CFEX activity previously increased by BNZ, the only thing left to be accomplished by KW3902 is to offset the increase in CFEX, which will, in turn, accentuate the relative impact of whatever mechanisms for reabsorption are left over. This would likely be SGLT and chloride-oxalate exchange. Whether or not this turns out to be an accurate accounting of the mechanisms whereby KW3902 exerts contrary effects in the absence or presence of BNZ, it is the simplest explanation of the micropuncture results that takes into account what is known about the physiology of proximal reabsorption.
Fig. 7.
BNZ blocks selectively the luminal carbonic anhydrase, which increases dependency of water reabsorption on chloride reabsorption due to (1) loss of bicarbonate reabsorption with subsequent (2) decreased passive (paracellular) chloride reabsorption but (3) stimulation of active chloride reabsorption through chloride/ formate exchange (CFEX) following luminal acidification. Our hypothesis for the effects of KW3902 is that it may increase dependency of water reabsorption on chloride reabsorption, but to lesser extent than BNZ due to weak inhibition of NHE3, which lowers H+ secretion, leading to (A) a decrease in bicarbonate reabsorption but also to (B) a weak decrease in chloride/formate exchange. When the drugs are administered together, there would be greater inhibition of NaHCO3 reabsorption and decreased passive chloride reabsorption (due to BNZ) but the chloride formate exchanger would be partially inhibited (due to KW3902). This would lead to a decreased dependency of water reabsorption on HCO3− but only a limited ability to shift dependency of water reabsorption to chloride through CFEX or chloride/oxalate exchange. Water reabsorption would be more dependent on nonbicarbonate and non-chloride reabsorption than when either drug is given alone.
A problem remains to explain how, if KW3902 is a proximal diuretic, adding KW3902 to BNZ increased the GFR versus BNZ alone, without increasing the CLi. The answer presumably lies in the thick ascending loop of Henle (TALH) where locally-derived adenosine serves as a negative feedback inhibitor of NaCl reabsorption [reviewed in [1]]. Since NaCl reabsorption in TALH increases markedly during CAI [51], BNZ will heighten the activity of this braking system. To the extent that lithium reabsorption spills over to the TALH, this will temper the effect of the higher GFR on CLi.
Perspectives
A1R are being developed as diuretic therapy for edematous conditions. CAI are potent diuretics, but never caught on as therapy for edematous conditions. There are any number of ways to inhibit TGF by paralyzing the afferent arteriole with agents such as calcium channel blockers or K-channel activators. However, as predicted by the Guyton model [36], there will be no lasting benefit, vis-à-vis negative salt balance, from any vasodilator except to the extent that such vasodilator is selective for the kidney. Since the kidney is the only peripheral organ expected to vasodilate in response to A1R antagonists, these A1R antagonists have obvious therapeutic potential. The present data aid understanding of the role of A1R in proximal and distal reabsorption but also as a mediator of renal hemodynamics. In addition, our data highlight a role for A1R antagonists as diuretics, not only alone, but as agents to increase the diuretic efficacy of other proximal diuretics.
Acknowledgements
This work was supported by the VA San Diego Healthcare System Research Service, a grant from NovaCardia, Inc. (San Diego, Calif., USA) and by NIH RO1 grant DK-28602. Dr. Miracle is supported by a NIH training grant (NIH NIDDK T32 DK0076711-11). Dr. Rieg is supported by the Deutsche Forschungsgemeinschaft (TR 1535/3-1).
References
- 1.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 2.Collis MG, Baxter GS, Keddie JR. The adenosine receptor antagonist, 8-phenyltheophylline, causes diuresis and saliuresis in the rat. J Pharm Pharmacol. 1986;38:850–852. doi: 10.1111/j.2042-7158.1986.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 3.Yao K, Kusaka H, Sano J, Sato K, Karasawa A. Diuretic effects of KW3902, a novel adenosine A1-receptor antagonist, in various models of acute renal failure in rats. Jpn J Pharmacol. 1994;64:281–288. doi: 10.1254/jjp.64.281. [DOI] [PubMed] [Google Scholar]
- 4.Yamagata T, Kobayashi T, Kusaka H, Karasawa A. Diuretic effects of KW3902, a novel adenosine A1-receptor antagonist, in anesthetized dogs. Biol Pharm Bull. 1994;17:1599–1603. doi: 10.1248/bpb.17.1599. [DOI] [PubMed] [Google Scholar]
- 5.Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett D, Liittschwager EB. Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol. 2000;35:56–59. doi: 10.1016/s0735-1097(99)00532-x. [DOI] [PubMed] [Google Scholar]
- 7.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004;65:1180–1190. doi: 10.1111/j.1523-1755.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol. 1999;10:714–720. doi: 10.1681/ASN.V104714. [DOI] [PubMed] [Google Scholar]
- 9.Knight RJ, Bowmer CJ, Yates MS. The diuretic action of 8-cyclopentyl-1,3-dipropylxanthine, a selective A1 adenosine receptor antagonist. Br J Pharmacol. 1993;109:271–277. doi: 10.1111/j.1476-5381.1993.tb13564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine-1 receptors. Proc Natl Acad Sci USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflügers Arch. 2004;448:214–221. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- 12.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 13.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest. 2000;106:289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker BJ, Blantz RC. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978;235:F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- 15.Tucker BJ, Steiner RW, Gushwa LC, Blantz RC. Studies on the tubulo-glomerular feedback system in the rat. The mechanism of reduction in filtration rate with benzolamide. J Clin Invest. 1978;62:993–1004. doi: 10.1172/JCI109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg CK, Taymor R, Minor JB, Halpern M. The use of Diamox, a carbonic anhydrase inhibitor, as an oral diuretic in patients with congestive heart failure. N Engl J Med. 1953;248:883–889. doi: 10.1056/NEJM195305212482102. [DOI] [PubMed] [Google Scholar]
- 17.Hosokawa T, Yamauchi M, Yamamoto Y, Iwata K, Nakamura A, Kato Y. Evaluation of the carrier potential for the lipid dispersion system with lipophilic compound. Biol Pharm Bull. 2003;26:994–999. doi: 10.1248/bpb.26.994. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen K. Lithium clearance: a new method for determining proximal and distal tubular reabsorption of sodium and water. Nephron. 1984;37:217–223. doi: 10.1159/000183252. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen K, Shirley DG. Analysis of the relation between lithium clearance and sodium clearance in rats. Scand J Clin Lab Invest. 1990;50:209–215. doi: 10.1080/00365519009089156. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77:125–138. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey J, Brown R, Crogham P. Electrometric titration of chloride in small volumes. J Exp Biol. 1955;32:822–829. [Google Scholar]
- 22.Windhager EE, Giebisch G. Micropuncture study of renal tubular transfer of sodium chloride in the rat. Am J Physiol. 1961;200:581–590. doi: 10.1152/ajplegacy.1961.200.3.581. [DOI] [PubMed] [Google Scholar]
- 23.Vallon V, Schwark J-R, Richter K, Hropot M. Role of Na+/H+ exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol. 2000;278:F375–F379. doi: 10.1152/ajprenal.2000.278.3.F375. [DOI] [PubMed] [Google Scholar]
- 24.Haberle DA, Shiigai TT, Maier G, Schiffl H, Davis JM. Dependency of proximal tubular fluid transport on the load of glomerular filtrate. Kidney Int. 1981;20:18–28. doi: 10.1038/ki.1981.99. [DOI] [PubMed] [Google Scholar]
- 25.Neumann KH, Rector FC., Jr Mechanism of NaCl and water reabsorption in the proximal convoluted tubule of rat kidney. J Clin Invest. 1976;58:1110–1118. doi: 10.1172/JCI108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronson PS. Ion exchangers mediating NaCl transport in the renal proximal tubule. Cell Biochem Biophys. 2002;36:147–153. doi: 10.1385/CBB:36:2-3:147. [DOI] [PubMed] [Google Scholar]
- 27.Rose BD. Clinical Physiology of Acid Base and Electrolyte Disorders. New York: McGraw-Hill; 1989. [Google Scholar]
- 28.Tucker BJ, Blantz RC. Studies on the mechanism of reduction in glomerular filtration rate after benzolamide. Pflügers Arch. 1980;388:211–216. doi: 10.1007/BF00658483. [DOI] [PubMed] [Google Scholar]
- 29.Thomson SC, Bachmann S, Bostanjoglo M, Ecelbarger CA, Peterson OW, Schwartz D, Bao D, Blantz RC. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J Clin Invest. 1999;104:1149–1158. doi: 10.1172/JCI5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson SC, Vallon V, Blantz RC. Reduced proximal reabsorption resets tubuloglomerular feedback in euvolemic rats. Am J Physiol. 1997;273:R1414–R1420. doi: 10.1152/ajpregu.1997.273.4.R1414. [DOI] [PubMed] [Google Scholar]
- 31.Deng A, Hammes JS, Thomson SC. Hemodynamics of early tubuloglomerular feedback resetting during reduced proximal reabsorption. Kidney Int. 2002;62:2136–2143. doi: 10.1046/j.1523-1755.2002.00682.x. [DOI] [PubMed] [Google Scholar]
- 32.Ichihara A, Imig JD, Inscho EW, Navar LG. Cyclooxygenase-2 participates in tubular flow-dependent afferent arteriolar tone: interaction with neuronal NOS. Am J Physiol. 1998;275:F605–F612. doi: 10.1152/ajprenal.1998.275.4.F605. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto S, Huang YG, Castrop H, Hansen PB, Mizel D, Briggs J, Schnermann J. Effect of carbonic anhydrase inhibition on GFR and renal hemodynamics in adenosine-1 receptor-deficient mice. Pflügers Arch. 2004;448:621–628. doi: 10.1007/s00424-004-1330-1. [DOI] [PubMed] [Google Scholar]
- 34.Arendshorst WJ, Gottschalk CW. Glomerular ultrafiltration dynamics: historical perspective. Am J Physiol. 1985;248:F163–F174. doi: 10.1152/ajprenal.1985.248.2.F163. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- 36.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 37.Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976;57:419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S: The effect of KW3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. Eur Heart J 2007 (in press). [DOI] [PubMed]
- 39.Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 40.Satriano J, Wead L, Cardus A, Deng A, Boss GR, Thomson SC, Blantz RC. Regulation of ecto-5′-nucleotidase by NaCl and nitric oxide: potential roles in tubuloglomerular feedback and adaptation. Am J Physiol Renal Physiol. 2006;291:F1078–F1082. doi: 10.1152/ajprenal.00043.2006. [DOI] [PubMed] [Google Scholar]
- 41.Gellai M, Schreiner GF, Ruffolo RR, Jr, Fletcher T, DeWolf R, Brooks DP. CVT-124, a novel adenosine A1 receptor antagonist with unique diuretic activity. J Pharmacol Exp Ther. 1998;286:1191–1196. [PubMed] [Google Scholar]
- 42.Zhou X, Kost CK., Jr Adenosine A1 receptor antagonist blunts urinary potassium excretion, but not renal hemodynamic effects, induced by carbonic anhydrase inhibitor in rats. J Pharmacol Exp Ther. 2006;316:530–538. doi: 10.1124/jpet.105.091462. [DOI] [PubMed] [Google Scholar]
- 43.Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967;47:595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 44.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Planelles G. Chloride transport in the renal proximal tubule. Pflügers Arch. 2004;448:561–570. doi: 10.1007/s00424-004-1309-y. [DOI] [PubMed] [Google Scholar]
- 46.Di Sole F, Casavola V, Mastroberardino L, Verrey F, Moe OW, Burckhardt G, Murer H, Helmle-Kolb C. Adenosine inhibits the transfected Na+-H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1) J Physiol. 1999;515:829–842. doi: 10.1111/j.1469-7793.1999.829ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Sole F, Cerull R, Petzke S, Casavola V, Burckhardt G, Helmle-Kolb C. Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger-3 in opossum kidney cells. J Am Soc Nephrol. 2003;14:1720–1730. doi: 10.1097/01.asn.0000072743.97583.db. [DOI] [PubMed] [Google Scholar]
- 48.Takeda M, Yoshitomi K, Imai M. Regulation of Na+-3HCO3– cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol. 1993;265:F511–F519. doi: 10.1152/ajprenal.1993.265.4.F511. [DOI] [PubMed] [Google Scholar]
- 49.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285:F152–F165. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 50.Yang LE, Leong PK, Ye S, Campese VM, McDonough AA. Responses of proximal tubule sodium transporters to acute injury-induced hypertension. Am J Physiol Renal Physiol. 2003;284:F313–F322. doi: 10.1152/ajprenal.00134.2002. [DOI] [PubMed] [Google Scholar]
- 51.Malnic G, Mello Aires M, Lacaz Vieira F. Chloride excretion in nephrons of rat kidney during alterations of acid-base equilibrium. Am J Physiol. 1970;218:20–26. doi: 10.1152/ajplegacy.1970.218.1.20. [DOI] [PubMed] [Google Scholar]