Abstract
Although CD8+ Treg-mediated suppression has been described, CD8+ Treg remain poorly characterized. Here we identify a novel subset of CD8+ Treg that express latency-associated peptide (LAP) on their cell surface (CD8+LAP+ cells) and exhibit regulatory activity in vitro and in vivo. Only a small fraction of CD8+LAP+ cells express Foxp3 or CD25, although the expression levels of Foxp3 for these cells are higher than their LAP− counterparts. In addition to TGF-β, CD8+LAP+ cells produce IFN-γ, and these cells suppress EAE that is dependent on both TGF-β and IFN-γ. In an adoptive co-transfer model, CD8+LAP+ cells suppress myelin oligodendrocyte glycoprotein (MOG)-specific immune responses by inducing or expanding Foxp3+ cells and by inhibiting proliferation and IFN-γ production in vivo. Furthermore, in vivo neutralization of IFN-γ and studies with IFN-γ-deficient mice demonstrate an important role for IFN-γ production in the function of CD8+LAP+ cells. Our findings identify the underlying mechanisms that account for the immunoregulatory activity of CD8+ T cells and suggest that induction or amplification of CD8+LAP+ cells may be a therapeutic strategy to help control autoimmune processes.
Keywords: Autoimmunity, Treg, Tolerance
Introduction
The immune system has evolved several mechanisms, including deletion, anergy, and regulation, to control the expansion and differentiation of activated T cells. Treg are specialized subpopulations of T cells that modulate the immune system to avert unwanted immune responses and thus contribute to maintenance of immunological self-tolerance and immune homeostasis [1–3]. Whereas CD4+ Treg subsets have been extensively studied, less is known about CD8+ Treg, their subsets, and modes of action. Several lines of evidence suggest that CD8+ T cells are important in regulation of EAE [4–6] even though there are reports that EAE can be induced by CD8+ T cells [7, 8]. Depletion of CD8+ T cells from mice that have recovered from EAE renders them susceptible to reinduction of disease [5] and CD8−/− mice develop more chronic EAE manifested by a higher incidence of relapse than WT animals [6]. Distinct subpopulations of CD8+ Treg, including CD8+CD28− and CD8+ CD122+ Treg, which can regulate EAE, have been identified [9, 10]. Moreover, experiments in β2 microglobulin−/− mice, in which CD8+ T cells do not develop due to MHC I deficiency, suggest a regulatory role for CD8+ T cells in EAE [11]. CD8+ T cells isolated from EAE-recovered mice specifically inhibit MBP-activated CD4+ T-cell clones in vitro and their depletion was followed by recurrence of EAE. The suppressive function of these CD8+ T cells is restricted by MHC I-like Qa-1 molecule (murine homologue of the human HLA-E) and adoptive transfer of these cells prevented disease in MBP-immunized mice [12–14]. The failure of resistance to EAE of Qa-1-deficient mice is associated with the escape of Qa-1-deficient CD4+ cells from CD8+ T-cell suppression [15]. Taken together, these results provide evidence that CD8+ T cells are important in both inducing resistance to EAE and abrogating recurrent relapsing episodes of pathogenic autoimmunity in vivo.
Various modes of action have been reported for CD8+ Treg, including the production of soluble factors, such as immuno-suppressive cytokines IL-10 or TGF-β [16–19], direct killing of target cells [12, 19–21], targeting APC and rendering them tolerogenic [19, 22], or by noncytolytic pathways that have not been clearly defined.
We and others have previously shown that CD4+ T cells expressing latency-associated peptide (LAP) actively suppress autoimmune diseases in experimental models of colitis and EAE and function in a TGF-β-dependent manner [23–25]. LAP is the N-terminal propeptide of TGF-β precursor peptide that remains noncovalently associated with TGF-β after cleavage from TGF-β precursor and forms the inactive latent TGF-β complex; it therefore contributes to the prevention of uncontrolled activation of the cognate TGF-β receptors [26, 27]. It is not known whether CD8+LAP+ cells exist in vivo in animal models and whether they function as regulatory cells in autoimmune diseases such as EAE.
In the present study, we identify a novel subset of CD8+ Treg that express LAP on their surface and suppress EAE in a TGF-β and IFN-γ-dependent fashion.
Results
CD8+LAP+ cells are regulatory in vitro
To investigate the role of CD8+LAP+ T cells, we first determined their presence and frequency in naïve mice. In naïve mice CD8+ cells constitute 10–12% of splenocytes, of which ~3.3% express LAP (Fig. 1A). CD8+LAP+ cells were also present in thymus and lymph nodes but at lower frequency (not shown). To further characterize the function of CD8+LAP+ cells, we examined whether CD8+LAP+ cells exhibit regulatory function in vitro. As shown in Fig. 1B, CD8+LAP+ cells significantly suppressed the proliferation of responder cells (CD4+CD25−LAP− cells), and this was evident for CD8+LAP+ cells sorted from both naïve SJL and B6 mice, although the cells from B6 mice appeared to have greater suppressive activity (Fig. 1B). No suppressive effect was observed for CD8+LAP− cells (Fig. 1B). The suppression mediated by CD8+LAP+ cells did not depend on cell-to-cell contact because CD8+LAP+ cells suppressed the proliferation of responder cells equally as well across a transwell membrane (not shown), and there was no evidence of cell death in the responder cell population as measured by CFSE labeling (not shown). We then measured the effect of CD8+LAP+ T cells on IFN-γ production by responder cells. As shown in Fig. 1C, CD8+LAP+ cells inhibited IFN-γ production by responder cells. These data indicate that CD8+LAP+ cells possess regulatory activity in vitro.
Figure 1.
Regulatory capacity of CD8+LAP+ cells in vitro. (A) Frequencies of CD8+LAP+ cells in splenocytes. Spleen cells obtained from naïve SJL mice were stained with CD8 and LAP-specific Ab or corresponding isotype control and analyzed by FACS. Percentage of LAP+ cells in CD8+ T cells is shown. (B) Suppressive function of CD8+LAP+ cells in vitro. 1 × 105 sorted CD8+LAP+ cells purified from SJL (left panel) or B6 (right panel) mice were cultured at a 1:1 ratio with syngeneic responder CD4+CD25−LAP− cells. Cells were stimulated with anti-CD3 Ab (1 μg/mL) in the presence of irradiated (3000 rad) syngeneic splenic APC and assayed as described in the Materials and methods section. Data are presented as mean + SD. Percent suppression of proliferation was also shown. (C) IFN-γ production of responder CD4+CD25−LAP− cells in vitro. CD4+CD25−LAP− responder cells were cultured alone or together with CD8+LAP+ cells (ratio 1:1) in the presence of anti-CD3 Ab (1 μg/mL) and irradiated (3000 rad) syngeneic splenic APC for 60 h; IFN-γ productions by responder cells were then determined by intracellular cytokine staining. Percentage of IFN-γ+ cells among responder cells is shown. All data are representative of at least two independent experiments.
Adoptive transfer of CD8+LAP+ T cells ameliorates EAE
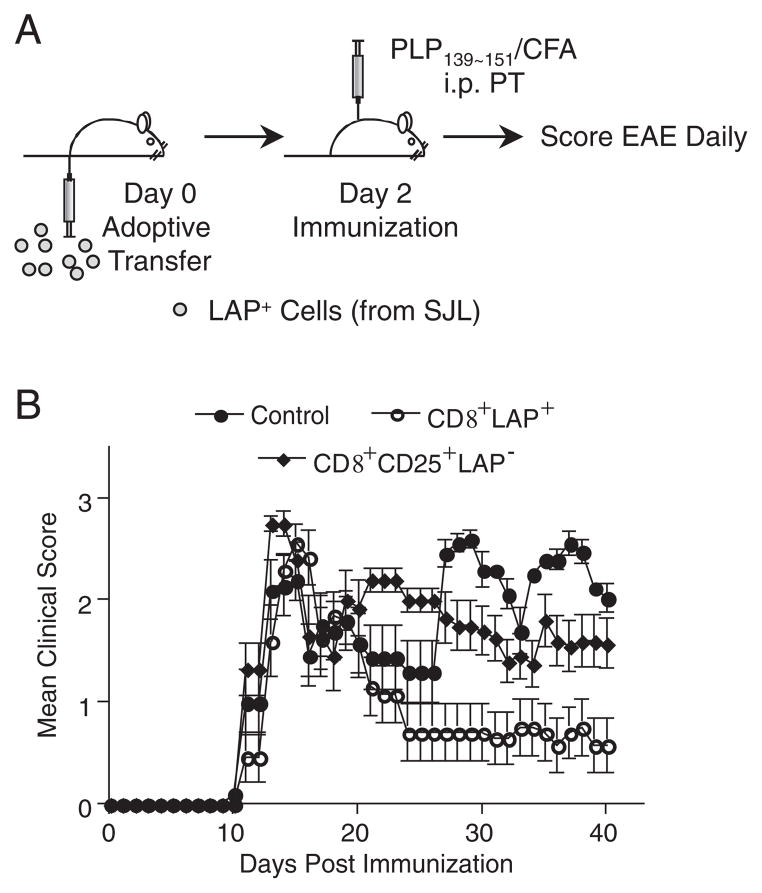
To analyze the function of CD8+LAP+ cells in vivo, we examined the ability of CD8+LAP+ cells to suppress EAE using the PLP139-151-induced EAE model. In addition to testing CD8+LAP+ cells, we tested CD8+CD25+LAP− cells. CD8+CD25+ T cells from MHC class II-deficient mice have been reported to exhibit regulatory activity in vitro [28]. Because both CD8+LAP+ and CD8+CD25+ LAP− cells show suppressive activity in vitro, we compared EAE suppression by CD8+LAP+ cells with CD8+CD25+LAP− cells. CD8+LAP+ or CD8+CD25+LAP− cells were adoptively transferred to SJL mice that were then immunized with PLP139-151 2 days after adoptive transfer to induce EAE (Fig. 2A). Control mice that did not receive cells showed the typical clinical course of EAE with disease onset on days 10–11 after PLP139–151 immunization, disease peak at day 15, and a relapsing phase beginning at day 20 (Fig. 2B). The mean day of onset and initial relapse was similar between control mice and those that received CD8+LAP+ cells. However, adoptive transfer of as few as 0.5 × 105 of CD8+LAP+ cells significantly reduced the disease severity at day 20 to day 40 compared with control mice (Fig. 2B, p<0.001) and with mice injected with CD8+CD25+LAP− cells (p<0.001). CD8+CD25+LAP− cells did not have a significant effect in vivo compared with control (Fig. 2B). The mean cumulative score of mice receiving CD8+LAP+ cells at day 20 to day 40 was significantly lower than control mice and mice transferred with CD8+CD25+LAP− cells (Table 1, p<0.01). Thus, CD8+LAP+ cells possess regulatory properties in vivo and their suppressive effect is statistically significant in the EAE model.
Figure 2.
Effect of adoptive transfer of CD8+LAP+ cells on EAE. (A) Schematic representation of experimental design. 0.5 × 105 sorted CD8+LAP+ or CD8+CD25+LAP− cells or PBS were injected intravenously into naïve SJL mice (five mice per group). Mice were then immunized with PLP139-151 in CFA to induce EAE 2 days after adoptive transfer. (B) Mean daily score±SEM for each group (five mice per group). Data are representative of at least two independent experiments. Group that received CD8+LAP+ cells had a significant reduction in disease severity compared with control mice (p<0.001, one-way ANOVA, followed by Turkey multiple comparisons).
Table 1.
Clinical parameters of EAE induced in SJL recipients of CD8+LAP+ and CD8+CD25+LAP− cells
| Treatmenta | nb | Incidence | Mean day of onsetc | Day 20 to 40 mean maximal scorec | Mean Cumulative scorecd | Mean maximal scorec |
|---|---|---|---|---|---|---|
| Control | 5 | 5/5 | 12.4±1.8 | 2.6±0.2 | 36.0±4.5e | 2.6±0.2 |
| CD8+LAP+ | 5 | 5/5 | 13.0±1.2 | 1.6±0.6 | 14.8±4.3ef | 2.5±0.3 |
| CD8+CD25+LAP− | 5 | 5/5 | 11.8±1.1 | 2.2±0.3 | 35.2±3.7f | 2.8±0.3 |
5 × 104 sorted CD8+LAP+ or CD8+CD25+LAP− cells were injected intravenously into naïve SJL mice. Mice were then immunized with PLP139-151 in CFA to induce EAE two days after adoptive transfer and signs of EAE were monitored.
n =mouse/group.
Data are presented as mean±SEM.
Mean cumulative score is defined as the mean of the sum of daily clinical scores observed between days 20 and 40.
p<0.01 When comparing control vs CD8+ LAP+ groups.
p<0.01 When comparing CD8+ LAP+ vs CD8+ CD25+ LAP− groups.
Analysis of cytokine profile, Foxp3 expression, and phenotype of CD8+LAP+ cells
To further characterize CD8+LAP+ cells, cytokine production of CD8+LAP+ and CD8+LAP− cells was compared. CD8+LAP+ cells produced IL-2, IFN-γ, and TGF-β in higher amounts than CD8+ LAP− cells (Fig. 3A). IL-6 and IL-12 were undetectable in both LAP+ and LAP− cells (not shown).
Figure 3.
Cytokine profile and phenotypic characterization of CD8+LAP+ and CD8+LAP− cells. (A) Cytokine production of CD8+LAP+ and CD8+LAP− cells. CD8+LAP+ and CD8+LAP− cells were sorted from pooled spleens and lymph nodes of naïve SJL mice as described in the Materials and methods section. After sorting, 1 × 105 cells from each sorted population were stimulated with plate-bound CD3-specific Ab (10 μg/mL). Cytokines were measured by ELISA. Data show mean+SD of triplicate wells. ***p<0.0001; Student’s t-test. (B–D), Phenotypic characterization of CD8+ populations sorted by LAP expression. Flow cytometry of expression of Foxp3 (assessed by intracellular staining) (B), CD25 (C), CD45RB, and intracellular CTLA4 (D) by CD8+LAP+ and CD8+LAP− cells from pooled spleens and lymph nodes of naïve SJL mice. Numbers next to outlined areas in (B) and (C) indicate percent cells positive for marker among each population. Red line in (D): marker specific staining for CD8+LAP− cells; blue line: marker specific staining for CD8+LAP+ cells. All data are representative of three independent experiments.
Since CD8+LAP+ cells exhibited regulatory activity, we determined whether they express signature molecules associated with CD4+CD25+ Treg and exhibit the characteristic phenotype of Treg. Although only a small fraction of CD8+LAP+ cells expressed Foxp3, a key molecular marker of CD4+CD25+ Treg [29], the Foxp3 expression (median fluorescence intensity, MFI) of CD8+LAP+ cells was higher than their LAP− counterparts (MFI 258 versus 166) (Fig. 3B). Unlike most CD4+CD25+ Treg that constitutively express the interleukin-2 (IL-2) receptor α chain (CD25), only a small percentage of CD8+LAP+ cells expressed CD25, and the expression level was lower than that of LAP− cells (Fig. 3C). Cytotoxic T-lymphocyte antigen-4 (CTLA4) has been shown to play an important role in the regulatory function of CD4+CD25+ cells both in vitro and in vivo [30]. Analysis of CTLA4 expression by CD8+ LAP+ cells revealed higher levels of expression as compared with LAP− cells (Fig. 3D). CD45RBlow is a phenotypic marker of Treg, and CD4+CD25−CD45RBlow cells suppress colitis in animal models [25, 31]. The expression of CD45RB on CD8+LAP+ cells was markedly downregulated compared with LAP− cells (Fig. 3D). We did not observe difference in the expression of glucocorticoid-induced TNFR-related gene (TNFRSF18) between CD8+LAP+ and LAP− populations (not shown).
CD8+LAP+ cells suppress MOG-specific immune responses and induce or expand Foxp3+ T cells in vivo
To further explore the mechanism of suppression mediated by CD8+ LAP+ cells in vivo, MOG TCR Tg Thy1.1+ T cells were depleted of CD25+ cells, CFSE-labeled, and adoptively co-transferred with CD8+LAP+ cells sorted from naïve B6 mice or transferred alone into WT B6 (Thy1.2+) mice, and the effect of CD8+LAP+ cells on the response of MOG TCR Tg T cells (CD4+Thy1.1+) after immunization with MOG35~55 in CFA was monitored (Fig. 4A). In the draining lymph nodes of mice receiving MOG TCR Tg T cells alone, 9% of MOG TCR Tg T cells (CD4+Thy1.1+) remained CFSE+, whereas 21% were CFSE+ when CD8+LAP+ cells were co-transferred, indicating that the proliferation of MOG TCR Tg T cells in draining lymph nodes was suppressed when mice were co-transferred with CD8+LAP+ cells (Fig. 4B).
Figure 4.
Effect of adoptive co-transfer of CD8+LAP+ cells on the effector function of MOG TCR Tg T cells in vivo. (A) Schematic representation of experimental design. MOG TCR Tg Thy1.1+ T cells depleted of CD25+ cells, CFSE-labeled (3 × 105) were transferred alone or together with CD8+ LAP+ cells into B6 (Thy1.2+) mice (three mice per group). Two days later, recipients were immunized with 50 μg of MOG35–55 peptide in CFA. Mice were killed 5 days after immunization, and cells from draining lymph nodes were harvested, stained, and analyzed by flow cytometry. (B) Proliferation of adoptively transferred MOG TCR Tg T cells (CD4+Thy1.1+) in the draining lymph nodes after immunization. The plots show the expression of CD4 versus CFSE fluorescence intensity on gated donor-derived cells (CD4+Thy1.1+). Numbers above bracketed areas indicate the frequency of CFSE+ cells among CD4+Thy1.1+ cells. p =0.015 (Student’s t-test). (C) IFN-γ production of transferred MOG TCR Tg T cells. Draining lymph node cells from mice adoptively transferred and immunized as described in (A) were restimulated ex vivo with PMA/ionomycin and stained for intracellular IFN-γ. Numbers next to bracketed areas indicate the frequency of IFN-γ+ cells among CD4+Thy1.1+ cells. p =0.015 (Student’s t-test). (D) Expression of Foxp3 of transferred MOG TCR Tg T cells. Draining lymph node cells from mice adoptively transferred and immunized as described in (A) were stained for intracellular Foxp3. Numbers next to bracketed areas indicate the frequency of Foxp3+ cells among CD4+Thy1.1+ cells. p =0.04 (Student’s t-test). (B–D) Data show mean±SD, n =3, and are representative of three independent experiments.
We next measured cytokine production of MOG TCR Tg T cells in draining lymph nodes after restimulation in vitro 5 days after immunization. In recipients of MOG TCR Tg T cells alone, 16.6% of MOG TCR Tg T cells (CD4+Thy1.1+) in the draining lymph nodes produced IFN-γ. In contrast, the fraction of IFN-γ-producing MOG TCR Tg T cells was reduced (7.9%) in mice that had received CD8+ LAP+ cells (Fig. 4C, p =0.015). IL-2 production of MOG TCR Tg T cells was similar between two groups of mice (not shown).
It has been reported that TGF-β can convert Foxp3− T cells into Foxp3+ Treg [32, 33]; in addition, the LAP molecule is closely associated with TGF-β and CD8+LAP+ cells produced high amounts of TGF-β (Fig. 3). We therefore determined whether co-transfer of CD8+LAP+ cells could convert MOG TCR Tg T cells to Foxp3+ cells. Although CD4+CD25− cells might contain some Foxp3+ cells, virtually no Foxp3+ cells were detected among CD4+CD25− fraction of MOG TCR Tg T cells from naïve MOG TCR Tg mice (not shown). Moreover, to insure that no Foxp3+ cells were being transferred, we depleted CD25+ cells from purified MOG TCR Tg T cells before adoptive co-transfer. As shown in Fig. 4D, there was an increase in the percentage of cells expressing Foxp3 among MOG TCR Tg T cells in draining lymph nodes of animals that had received both MOG TCR Tg T cells and CD8+LAP+ cells compared with mice receiving MOG TCR Tg T cells alone (5.4% versus 1.6%, p =0.04). Because the absolute number of cells recovered from draining lymph nodes and analyzed is comparable between two experimental groups (MOG TCR Tg T cells versus MOG TCR Tg T cells plus CD8+LAP+ cells: 1.7 × 107±1.4 × 106 versus 1.5 × 107±3.5 × 106) and the frequency of MOG TCR Tg T cells in draining lymph nodes is similar between the two groups (MOG TCR Tg T cells versus MOG TCR Tg T cells plus CD8+LAP+ cells: 1.93±0.52% versus 1.83±0.50%), these results suggest that CD8+ LAP+ cells induced or expanded Foxp3+ cells in vivo.
Regulatory function of CD8+LAP+ cells is TGF-β dependent in vivo
Because CD8+LAP+ cells produced a high amount of TGF-β and we and others have previously shown that LAP-expressing CD4+ T-cell subsets function in a TGF-β-dependent fashion [23–25], we investigated whether the in vitro suppression by CD8+LAP+ cells was dependent on TGF-β. We observed no reversal of in vitro suppression using anti-TGF-β Ab (not shown) and only 15% reversal of suppression using a specific inhibitor (ALK5 inhibitor II) of TGF-β signaling (Fig. 5A).
Figure 5.
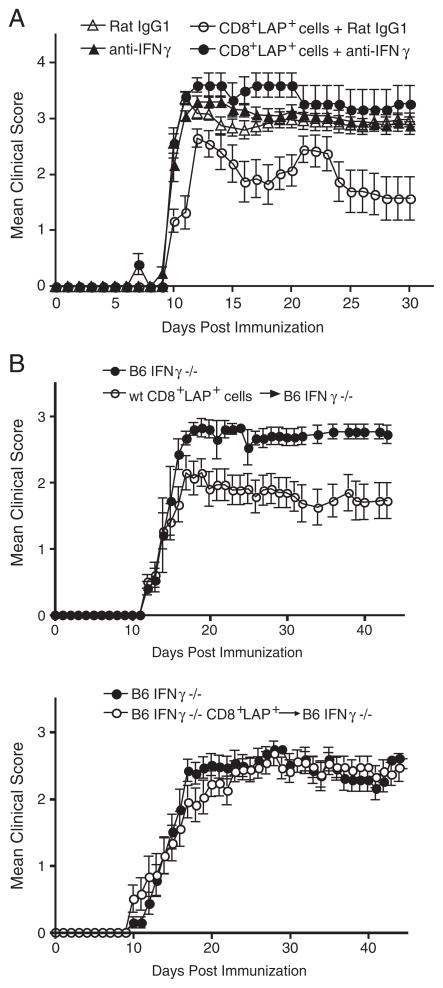
TGF-β-mediated suppressive function of CD8+LAP+cells in vitro and in vivo. (A) Effect of inhibitor of TGF-β signaling (ALK5 inhibitor II) on the in vitro suppressive function of CD8+LAP+ cells. CD4+CD25−LAP− responder cells were cultured alone or together with CD8+LAP+ at a 1:1 ratio and stimulated with anti-CD3 Ab (1 μg/mL) and irradiated (3000 rad) syngeneic splenic APC in the presence of indicated concentrations of ALK5 inhibitor and assayed as described in the Materials and methods section. Data are representative of at least two independent experiments and are presented as means±SD. Percent suppression of proliferation was shown. (B) Effect of neutralization of TGF-β on the regulatory function of CD8+LAP+ cells in vivo. SJL mice were adoptively transferred with 0.5 × 105 sorted CD8+LAP+ cells 2 days before EAE induction. Mice then received five intraperitoneal injections of 50 μg of neutralizing TGF-β specific or control Ab every other day starting 1 day before EAE induction. The mean daily score±SEM for each group (five mice per group) is shown. Data are representative of at least two independent experiments. In vivo administration of anti-TGF-β significantly reversed the suppression of EAE mediated by CD8+ LAP+ cells; p<0.001 (one-way ANOVA, followed by Turkey multiple comparisons) as compared between CD8+LAP+ cells+anti-TGF-β and CD8+LAP+ cells+Rat IgG1 groups.
Although the in vitro suppressive capacity of CD4+CD25+ Treg does not require TGF-β [1, 34], in vivo function of CD4+CD25+ Treg is dependent on TGF-β [35–37]. It is thus possible that the role of TGF-β in CD8+LAP+ cell-mediated suppression could be different in vitro and in vivo. To address the role of TGF-β in regulatory function of CD8+LAP+ cells in vivo, SJL mice receiving CD8+LAP+ cells were treated with five doses of neutralizing anti-TGF-β mAb (1D11) beginning 1 day before EAE induction. As shown in Fig. 5B, in vivo neutralization of TGF-β significantly reversed the suppression of EAE mediated by CD8+LAP+ cells (p<0.001). Thus, the function of CD8+LAP+ cells in vivo is TGF-β dependent.
Regulatory function of CD8+LAP+ cells is IFN-γ dependent both in vitro and in vivo
Given that IFN-γ is one of the predominant cytokines produced by CD8+LAP+ cells (Fig. 3) and has been shown to be important to the function of CD4+CD25+ Treg in vivo in an allogenic system [38] as well as to have a protective effect in EAE models [39–44], we examined whether IFN-γ contributed to the regulatory function of CD8+LAP+ cells both in vitro and in the EAE model. To delineate the role of IFN-γ in the suppressive function of CD8+LAP+ cells in vitro, CD8+LAP+ cells and responder (CD4+CD25−LAP−) cells sorted from WT B6, B6 IFN-γ−/−, or B6 IFN-γ receptor−/− (B6 IFN-γR−/−) mice were used for in vitro suppression assays. As shown in Fig. 6A, CD8+LAP+ cells from WT B6 mice (wt LAP+) significantly suppressed the proliferation of responder cells from both WT B6 and IFN-γR−/− mice. The suppressive effects were similar whether WT or IFN-γR−/− responder cells were used, indicating that the IFN-γ produced by CD8+LAP+ cells did not have direct suppressive effect on responder cells. We then asked whether CD8+LAP+ cells required IFN-γ signaling to exhibit suppressive activity by using CD8+LAP+ cells from IFN-γR−/− mice. We found that the suppressive capacity of CD8+LAP+ cells from IFN-γR−/− mice was only slightly decreased compared with WT CD8+LAP+ cells (Fig. 6A and B). In contrast, the suppressive function of CD8+LAP+ cells was markedly diminished when CD8+LAP+ cells were purified from IFN-γ−/− mice (Fig. 6C). These data demonstrate that although CD8+LAP+ cells did not require IFN-γ signaling to exhibit in vitro suppression and IFN-γ produced by these cells did not act directly on responder cells, the mechanism of CD8+LAP+ cell-mediated suppression requires IFN-γ.
Figure 6.
IFN-γ-mediated suppressive function of CD8+LAP+cells in vitro. Effect of IFN-γ and IFN-γ receptor deficiency on the in vitro suppressive function of CD8+LAP+ cells. Responder CD4+CD25−LAP− cells from WT or IFN-γR−/− mice were cultured alone or at 1:1 ratio with CD8+LAP+ cells from WT B6 mice (A), IFN-γR−/− mice (B); (C) responder from WT mice were cultured alone or at 1:1 ratio with CD8+LAP+ cells from WT B6 mice or IFN-γ−/− mice. In vitro proliferation assays were performed as described in the Materials and methods section. Data show mean+SD of triplicate wells and are representative of two independent experiments. Percent suppression of proliferation is also shown.
To address the role of IFN-γ in regulatory function of CD8+LAP+ cells in the EAE model, SJL mice adoptively transferred with CD8+LAP+ cells were injected intraperitoneally with five doses of neutralizing anti-IFN-γ mAb (R4-6A2) beginning 1 day before EAE induction. As shown in Fig. 7A, in vivo neutralization of IFN-γ significantly reversed the suppression of EAE mediated by CD8+LAP+ cells (p<0.001).
Figure 7.
IFN-γ-mediated suppressive function of CD8+LAP+ cells in vivo. (A) Effect of neutralization of IFN-γ on the regulatory function of CD8+LAP+ cells in vivo. SJL mice were adoptively transferred with 0.5 × 105 sorted CD8+LAP+ cells 2 days before EAE induction. Mice then received five intraperitoneal injections of 50 μg of neutralizing IFN-γ specific or control Ab every 3 days starting 1 day before EAE induction. Mean daily score±SEM for each group (five mice per group). Data are representative of at least two independent experiments. p<0.001 (one-way ANOVA, followed by Turkey multiple comparisons) as compared between CD8+LAP+ cells+Rat IgG1 and CD8+LAP+ cells+anti-IFN-γ groups. (B) Effect of IFN-γ deficiency on the regulatory function of CD8+LAP+ cells in vivo. 1.7 × 105 sorted CD8+LAP+ cells from WT or IFN-γ−/− mice were injected intravenously into naïve IFN-γ−/− mice. Mice were then immunized with MOG35–55 in CFA to induce EAE 2 days after adoptive transfer. Mean daily score±SEM for each group (five to six mice per group). Data are representative of three independent experiments. Group that received CD8+LAP+ cells purified from WT mice had a significant reduction in disease severity compared with control mice (p<0.0001, Mann–Whitney U test).
We next performed in vivo EAE experiments in which IFN-γ−/− mice were used as recipients for adoptive transfer to further confirm the importance of IFN-γ in CD8+LAP+ cell-mediated suppression in vivo. This experimental design allowed us to avoid the interference of endogenous IFN-γ produced in recipient mice. As shown in Fig. 7B, adoptive transfer of CD8+LAP+ cells purified from WT mice significantly suppressed EAE induced in IFN-γ−/− mice (p<0.0001); in contrast, CD8+LAP+ cells from IFN-γ−/− mice (B6 IFN-γ−/− CD8+LAP+) were unable to suppress EAE. The percentage of CD8+LAP+ cells in lymphoid organs is similar between WT and IFN-γ−/− mice (not shown), indicating that the absence of IFN-γ does not affect the generation and survival of CD8+LAP+ cells. Therefore, our observation that CD8+ LAP+ cells from IFN-γ−/− mice were unable to suppress EAE did not result from the disappearance of transferred CD8+LAP+ cells in IFN-γ−/− mice. Taken together, these results demonstrate that IFN-γ production is important for the in vivo function of CD8+ LAP+ cells. Since IL-17 producing-Th17 cells are increased in IFN-γ−/− mice and are the major pathogenic T cells in EAE induced in IFN-γ−/− mice [45], our results also implicate that CD8+LAP+ cells efficiently downregulate pathogenic Th17 responses in vivo.
Discussion
We have identified a novel subset of CD8+ Treg that express LAP on their cell surface (CD8+LAP+ cells). CD8+LAP+ cells possess regulatory activity in vitro and are regulatory in vivo, since transfer of as few as 5 × 104 CD8+LAP+ cells reduced disease severity in the EAE model. Also, in an adoptive co-transfer model, CD8+LAP+ cells suppressed proliferation and IFN-γ production while inducing Foxp3 expression of co-transferred MOG specific TCR Tg T cells or expanding Foxp3+ MOG specific TCR Tg T cells, although the majority (~97%) of CD8+LAP+ cells are Foxp3 negative. The regulatory function of CD8+LAP+ cells is both TGF-β and IFN-γ dependent in vivo.
CD8+ Treg expressing distinct cell surface markers have been identified, most of which were induced by Ag stimulation or by in vivo administration of immunomodulating agents [18, 46, 47]. The CD8+LAP+ Treg identified in our study are present in naïve animals without prior Ag encounter. Unlike other CD8+ Treg, CD8+LAP+ Treg are unique, as they are both TGF-β and IFN-γ dependent. Only a small fraction of freshly isolated CD8+LAP+ cells express Foxp3 and CD25, which suggests that the regulatory function of these cells is independent of Foxp3 expression. However, since CD8+LAP+ cells produced high amounts of TGF-β, the production of TGF-β by CD8+LAP+ cells could stabilize and enhance Foxp3 expression in CD8+LAP+ cells in vivo, thus amplifying their suppressive function.
Although anti-TGF-β has been reported to exacerbate auto-immune diseases including EAE, most studies used a relatively high dose (400 μg to 2 mg) of anti-TGF-β for in vivo neutralization [48, 49]. We have shown in our previous work that 50 μg of anti-TGF-β, the dose we also used in this study, did not affect EAE in WT mice [23, 24]. Our data suggest that the dose (50 μg) we used in this study might be only sufficient for neutralizing the TGF-β produced locally by the transferred CD8+LAP+ cells but is too low to neutralize most endogenous TGF-β and enhance EAE.
In addition to TGF-β, IFN-γ is also required for CD8+LAP+ cell-mediated suppression. That is distinct from CD4+LAP+ cell-mediated suppression, which is in a TGF-β-dependent fashion [23–25]. Although IFN-γ has been conventionally considered as a proinflammatory cytokine that plays a key role in inflammation and autoimmune disease, accumulating evidence indicates that IFN-γ also possesses regulatory and anti-inflammatory activities [38, 50, 51]. IFN-γ has been shown to function as a protective factor preventing the onset of autoimmune diseases including EAE, experimental autoimmune uveitis, and collagen-induced arthritis [41, 44, 52, 53]. The current view regarding the role of IFN-γ in EAE is that IFN-γ modulates or alleviates EAE, based on the observation that administration of IFN-γ ameliorated EAE, anti-IFN-γ Ab exacerbated EAE, as well as the observation that EAE was worsened in mice in which the gene for IFN-γ or its receptor was deleted [39, 41–44]. The fact that in vivo neutralization of IFN-γ abrogated the suppression of EAE mediated by CD8+LAP+ cells and that CD8+LAP+ cells lost the capacity to suppress if they were defective in IFN-γ production indicates that IFN-γ production is important for the regulatory capacity of CD8+ LAP+ cells in vivo. It is known that IFN-γ-deficient animals have a more severe form of EAE. Given our findings, this may in part be related to the loss of suppressive capacity of the IFN-γ-dependent CD8+LAP+ Treg that we have identified. These findings further support the notion that IFN-γ acts as a protective factor in autoimmune diseases such as EAE. Previous studies showed that administration of anti-IFN-γ mAb enhanced EAE in low susceptibility mice (B6), whereas it had no effect on EAE of high susceptibility mice (SJL) [39, 41–44]. Others reported that 1 mg of anti-IFN-γ mAb had a disease-enhancing effect in the development of EAE in SJL mice [54]. In our studies, anti-IFN-γ mAb administration did not affect EAE in SJL mice (Fig. 7A). The differences can be explained by the fact that we used a relatively low dose (50 μg) of anti-IFN-γ mAb for in vivo neutralization and the dose might be only sufficient for neutralizing the IFN-γ produced locally by the transferred CD8+LAP+ cells but is too low to block most endogenous IFN-γ and enhance EAE.
Several studies have demonstrated a role of IFN-γ in the suppression mediated by CD8+ T cells [47, 55] and CD4+CD25+ Treg [38]; however, the molecular mechanisms by which IFN-γ contribute to immunoregulation mediated by Treg are far from clear. Apart from induction of apoptosis and inhibition of proliferation of T cells [56–58], IFN-γ has been shown to induce Foxp3 expression and convert CD4+CD25− T cells to Treg [59]. Moreover, IFN-γ produced by Treg may also regulate the function of APC [60]. It has been shown that IFN-γ produced by activated CD4+CD25+ Treg led to greater IDO production and tolerogenic potential of the cocultured DC [60]. More recent studies showing the role of IFN-γ in antagonizing the function of IL-17, a key pathogenic cytokine in autoimmune settings [45, 61], further support the regulatory role of IFN-γ in inflammation. Although IFN-γ is required for CD8+LAP+ cell-mediated suppression, the results from our in vitro studies suggest that IFN-γ produced by CD8+LAP+ cells downregulated immune responses through its effect on APC, as neither was IFN-γ signaling crucial for CD8+LAP+ cells to exert suppressive activity nor did IFN-γ produced by these cells act directly on responder cells. Moreover, CD8+LAP+ cells significantly suppressed EAE in IFN-γ−/− mice in which Th17 cells play a central role in the disease process. Thus, the protective role of IFN-γ produced by CD8+LAP+ cells in the EAE model may result from a direct effect of IFN-γ on counteracting IL-17 or indirectly as a result of regulatory activity of tolerogenic APC.
Bystander suppression and infectious tolerance are mechanisms by which Treg may establish long-lasting and stable tolerance [62, 63]. Our observation that in vivo neutralization of either TGF-β or IFN-γ reversed the suppression of EAE mediated by CD8+LAP+ cells suggest that CD8+LAP+ cell-mediated control of EAE utilizes multiple mechanisms working in concert and thus that the presence of TGF-β and IFN-γ are necessary for complete protection. TGF-β together with APC induce naïve T cells to differentiate into Foxp3+ Treg [62, 64] and IFN-γ produced by activated Treg promotes greater IDO production and tolerogenic potential of the cocultured APC [60]. It is likely that by producing TGF-β and IFN-γ, CD8+LAP+ cells establish a regulatory milieu to promote the production of new Treg cell populations and enhance the tolerogenic potential of APC. These processes enable bystander suppression and infectious tolerance by which CD8+LAP+ cells can amplify their immunomodulatory effects and exert long-term and efficient control over EAE.
In the experiment presented in Fig. 4, we showed that CD8+ LAP+ cells suppressed proliferation and IFN-γ production of autoreactive MOG TCR Tg T cells and induced or expanded Foxp3+ MOG TCR Tg T cells (IL-2 production of MOG TCR Tg T cells was not affected) in the draining lymph nodes at an early time point after priming (5 days after priming). This result together with the EAE data presented in Fig. 2B suggest that although the transferred CD8+LAP+ cells had a suppressive effect on the pathogenic autoreactive CD4 T cells at an early time point after priming, the suppression is not sufficient to cause difference in the EAE onset. However, as presented in Fig. 2B, our results that adoptive transfer of CD8+LAP+ cells significantly reduced the disease severity at day 20 to day 40 compared with control mice suggests that the suppressive effect of CD8+LAP+ cells causes a significant difference in disease at later time points of EAE.
In addition to their role in disease protection and recovery from EAE, CD8+ Tregs have been implicated in regulating MS [65, 66]. Defects in “suppressive” CD8+ T cells have been reported [55, 67] and glatiramer acetate (Copaxone), an FDA approved treatment for relapsing forms of MS [68], restores CD8+ T-cell responses in MS patients [69]. Whether a counterpart of CD8+LAP+ cells exists in human and whether they play a role in modulating MS and/or are functionally defective in MS patients remain to be determined.
In summary, we have identified and characterized a novel subset of CD8+ Treg that express LAP on their surface and act in an IFN-γ- and TGF-β-dependent mechanism. Induction or amplification of CD8+LAP+ cells may be a therapeutic strategy to help control autoimmune processes.
Materials and methods
Mice
Female SJL, C57BL/6 (B6), B6 IFN-γ−/−, and B6 IFN-γ receptor−/− (B6 IFN-γR−/−) mice at 6–8 wk of age were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). MOG TCR Tg (2D2) mice were kindly provided by Dr. Vijay K. Kuchroo (Center for Neurologic Diseases, Brigham and Women’s Hospital, Boston, MA, USA) and have been described [70]. Mice were kept in a conventional, specific pathogen-free facility at the Harvard Institutes of Medicine according to the animal protocol with the full knowledge and permission of the Standing Committee on Animals at Harvard Medical School (Protocols 02683).
Induction and assessment of EAE
Female SJL mice were immunized subcutaneously in the flanks with 50 μg PLP139-151 (HSLGKWLGHPDKF) in CFA containing 250 μg of Mycobacterium tuberculosis H37RA (Difco, Detroit, MI, USA), followed by intraperitoneal injection of 100 ng of pertussis toxin (List Biological Laboratories, Campbell, CA, USA). To induce EAE in C57BL/6 IFN-γ−/− mice, female mice were immunized subcutaneously in the flanks with 50 μg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) in CFA containing 200 μg of M. tuberculosis H37RA (Difco), followed by intraperitoneal injection of 100 ng of pertussis toxin (List Biological Laboratories). Clinical assessment of EAE was performed according to the following criteria: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, front and hind limb paralysis; 5, moribund state. In some experiments, indicated number and sorted populations of cells were adoptively transferred i.v. 2 days before immunization. All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School.
Immunization
Mice were immunized s.c. with 50 μg of peptide MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA (Difco Bacto) containing 200 μg of M. tuberculosis H37RA (Difco). At the indicated time points the animals were sacrificed, and draining lymph nodes were harvested for analysis.
Ab, reagents, and FACS analysis
CD16/CD32-specific Ab (FcBlock), fluorescein-conjugated mAbs to CD8 (53–6.7), PE-conjugated mAbs to CD8 (53–6.7), CD25 (PC61), IL-2 (JES6–5H4), IFN-γ (XMG.1.2), CTLA4 (UC10-4F10-11), CD45RB (16A), CD90.1 (OX-7), rat IgG1 isotype control (R3-34), and rat IgG2b isotype control (A95-1), streptavidin-PE (SAv-PE), PerCp conjugated mAbs to CD8 (53–6.7), CD90.1 (OX-7), PerCP-Cy5.5-conjugated mAbs to CD25 (PC61), allophycocyanin-conjugated mAbs to CD8 (53–6.7), CD25 (PC61), SAv-allophycocyanin, normal mouse IgG and Ab for cytokine assays were purchased from Becton Dickinson. PE-conjugated anti-mouse glucocorticoid-induced TNFR-related gene/TNFRSF18, affinity-purified biotinylated goat anti-LAP polyclonal Ab, biotinylated normal goat IgG, and normal goat IgG were purchased from R&D Systems (Minneapolis, MN, USA). PE-conjugated anti-mouse Foxp3 (clone FJK-16s) and Rat IgG2a isotype control were purchased from eBioscience. Surface stainings were performed according to standard procedures at a density of 1–2 × 106 cells per 50 μL, and volumes were scaled up accordingly. Flow-cytometric analysis was performed on a FACSCalibur (Becton Dickinson) by using CELLQUEST software (Becton Dickinson). FoxP3 staining and analysis was performed by flow cytometry using the FoxP3 staining set (clone FJK-16s; eBioscience) according to the manufacturer’s instruction. ALK5 (TGF-Beta RI Kinase Inhibitor II) inhibitor II (EMD/Calbiochem 616452) was kindly provided by Dr. Taka Oida.
Cytokine assay
All cytokines except TGF-β1 were measured by ELISA using Ab sets from BD PharMingen as described [71]. TGF-β1 was measured by the Quantikine® ELISA Kit (R&D Systems) with acidification according to the manufacturer’s instruction.
Intracellular cytokine staining
Cultured cells or cells from the draining lymph nodes of immunized animals were stimulated in culture medium containing PMA (20 ng/mL, Sigma), ionomycin (250 ng/mL, Sigma), and monensin (GolgiStop 1 μL/mL, BD Biosciences, Mountain View, CA, USA), and cultures were incubated at 37°C in a humidified 5% CO2 atmosphere for 4–6 h. Cells were harvested and incubated with 2.4G2 Fc-receptor-blocking Ab and then stained for surface markers. After staining of surface markers, cells were fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer (Becton Dickinson) according to the manufacturer’s instructions. Cells were then incubated at 4°C for 30 min with indicated Ab to cytokines and corresponding isotype controls and washed twice in Perm/Wash buffer before analysis.
Purification and adoptive transfer of cells
Pooled cells from spleens and peripheral lymph nodes (mesenteric, axillary, popliteal, inguinal, and cervical) of female SJL, B6, B6 IFN-γ−/−, or B6 IFN-γ receptor−/− (B6 IFN-γR−/−) mice (6–10 wk) were subjected to erythrocyte lysis. After incubation with Fc-receptor-blocking Ab 2.4G2, cells were incubated with anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA, USA) and CD8+ cells were positively selected on LS MACS columns (Miltenyi Biotec), routinely achieving purities >95%. Purified CD8+ cells then were stained with biotinylated LAP-specific Ab followed by SAv-PE, anti-CD25 allophycocyanin, and fluorescein-labeled anti-CD8. CD8+LAP+ and CD8+LAP− cells were further sorted by using a FACSAria cell sorter (BD Biosciences); in some experiments, CD8+CD25+LAP− cells were also sorted. The purity of each population was >98% by FACS analysis. To purify CD25- depleted MOG TCR Tg T cells, pooled cells from spleens and lymph nodes of MOG TCR Tg mice were stained with biotinylated anti-CD25 followed by SAv microbeads (Miltenyi Biotec); after depletion of CD25+ cells by LD MACS column (Miltenyi Biotec), CD4+ cells in the CD25− flow-through fraction were further purified by staining with CD4 microbeads and separated by MS or LS MACS columns (Miltenyi Biotec). Cells were injected into the lateral tail vein in a volume of 200 μL of PBS. Where indicated, cells were labeled with CFSE (Molecular Probes) by incubation for 10 min at 37°C in 10 μM CFSE in PBS/0.1% BSA at a density of 1 × 107 cells per mL.
Proliferation assays
For suppression assays, 1 × 105 sorted CD8+LAP+ or CD8+LAP− cells were cultured at a 1:1 ratio with syngeneic CD4+ CD25−LAP− cells. Cells were stimulated with anti-CD3 Ab (1 μg/mL) in the presence of irradiated (3000 rad) syngeneic splenic APC in 200 μL of RPMI 1640 medium supplemented with 10% FBS in 96-well round-bottom plates. Proliferation was measured by scintillation counting after pulsing with 1 μCi per well [3H]thymidine (1 Ci =37 Gbq) for the last 16 h of a 72-h incubation period.
Neutralization of TGF-β
For in vivo neutralization of TGF-β, SJL mice were immunized with 50 μg of PLP139-151 2 days after adoptive transfer. Mice received five intraperitoneal injections of anti-mouse TGF-β (clone 1D11, BioExpress) or isotype control on alternating days beginning 1 day post adoptive transfer.
Neutralization of IFN-γ
For in vivo neutralization of IFN-γ, SJL mice were immunized with 50 μg of PLP139-151 2 days after adoptive transfer. Mice received five intraperitoneal injections of anti-mouse IFN-γ (clone R4-6A2, BioExpress) or isotype control every 3 days beginning 1 day post adoptive transfer.
Statistical analysis
Statistical significance was assessed by the two-tailed Student’s t-test. For in vivo EAE experiments, differences in clinical scores were analyzed using one-way ANOVA, followed by Turkey multiple comparisons or two-tailed Mann–Whitney U-test. p values <0.05 were regarded as significant.
Acknowledgments
We thank Dr. V. K. Kuchroo and Dr. B. Waksman for discussions and H. Ishikawa for technical assistance. MOG35–55 peptide was provided by Dr. Teplow (David Geffen School of Medicine, University of California, Los Angeles). This work was supported by National Institutes of Health Grants AI435801 and NS38037 (to H. L. W.).
Abbreviations
- LAP
latency-associated peptide
- MFI
median fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- SAv
streptavidin
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 3.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 6.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 7.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 9.Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, Clarkson MR, et al. Regulatory functions of CD8+ CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 11.Linker RA, Rott E, Hofstetter HH, Hanke T, Toyka KV, Gold R. EAE in beta-2 microglobulin-deficient mice: axonal damage is not dependent on MHC-I restricted immune responses. Neurobiol Disord. 2005;19:218–228. doi: 10.1016/j.nbd.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, Ware R, Stall A, Flaherty L, Chess L, Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 1995;2:185–194. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Curran S, Ruiz-Vazquez E, Liang B, Winchester R, Chess L. Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor V beta repertoire during experimental auto-immune encephalomyelitis. Proc Natl Acad Sci USA. 2003;100:8378–8383. doi: 10.1073/pnas.1432871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 16.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endharti AT, Rifa IMS, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 18.Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 T regulatory cells use IFN-gamma to elaborate TGF-beta-based suppression. J Immunol. 2005;174:7625–7632. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 19.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1988;334:530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- 21.Simpson E. Suppression of the immune response by cytotoxic T cells. Nature. 1988;336:426. doi: 10.1038/336426a0. [DOI] [PubMed] [Google Scholar]
- 22.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–5914. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 23.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 25.Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 26.Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14:657–659. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Ichijo H, Heldin CH. Transforming growth factor-beta: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 28.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175:246–253. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 29.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 30.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 31.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 34.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 36.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorthuis JA, Uitdehaag BM, De Groot CJ, Goede PH, van der Meide PH, Dijkstra CD. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990;81:183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, et al. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- 41.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental auto-immune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 42.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 43.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 44.Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 45.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 46.Izawa A, Yamaura K, Albin MJ, Jurewicz M, Tanaka K, Clarkson MR, Ueno T, et al. A novel alloantigen-specific CD8+PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J Immunol. 2007;179:786–796. doi: 10.4049/jimmunol.179.2.786. [DOI] [PubMed] [Google Scholar]
- 47.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, Heslan M, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1096–1106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cautain B, Damoiseaux J, Bernard I, van Straaten H, van Breda Vriesman P, Boneu B, Druet P, Saoudi A. Essential role of TGF-beta in the natural resistance to experimental allergic encephalomyelitis in rats. Eur J Immunol. 2001;31:1132–1140. [PubMed] [Google Scholar]
- 49.Gonzalez-Garcia I, Zhao Y, Ju S, Gu Q, Liu L, Kolls JK, Lu B. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bocek P, Jr, Foucras G, Paul WE. Interferon gamma enhances both in vitro and in vivo priming of CD4+ T cells for IL-4 production. J Exp Med. 2004;199:1619–1630. doi: 10.1084/jem.20032014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 53.Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158:5997–6005. [PubMed] [Google Scholar]
- 54.Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–115. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- 55.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shtrichman R, Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001;4:251–259. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 57.Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, Rothstein DM, et al. Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest. 2002;109:1471–1479. doi: 10.1172/JCI14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 61.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 62.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 67.Antel JP, Bania MB, Reder A, Cashman N. Activated suppressor cell dysfunction in progressive multiple sclerosis. J Immunol. 1986;137:137–141. [PubMed] [Google Scholar]
- 68.Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci USA. 2004;101:14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, Lovett-Racke AE, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cautain B, Damoiseaux J, Bernard I, Xystrakis E, Fournie E, van Breda Vriesman P, Druet P, Saoudi A. The CD8 T cell compartment plays a dominant role in the deficiency of Brown-Norway rats to mount a proper type 1 immune response. J Immunol. 2002;168:162–170. doi: 10.4049/jimmunol.168.1.162. [DOI] [PubMed] [Google Scholar]