Full text
PDF










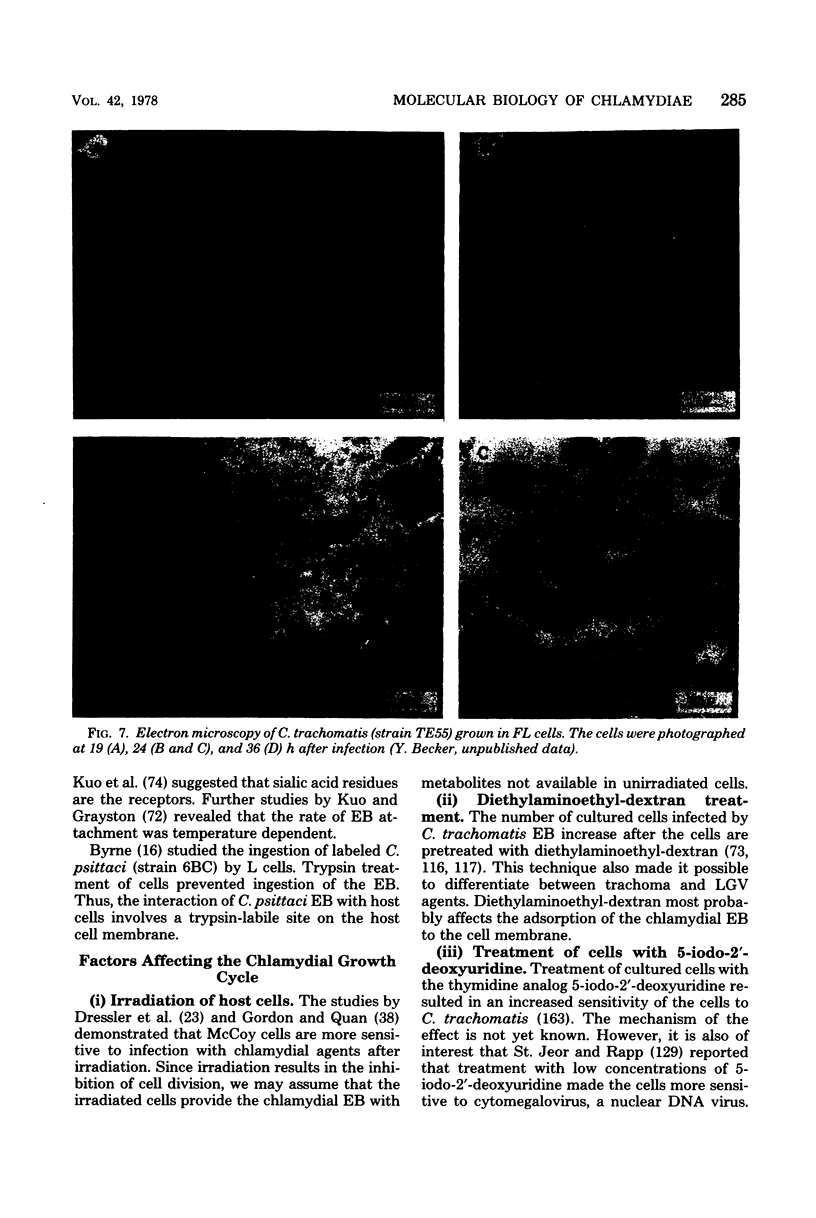





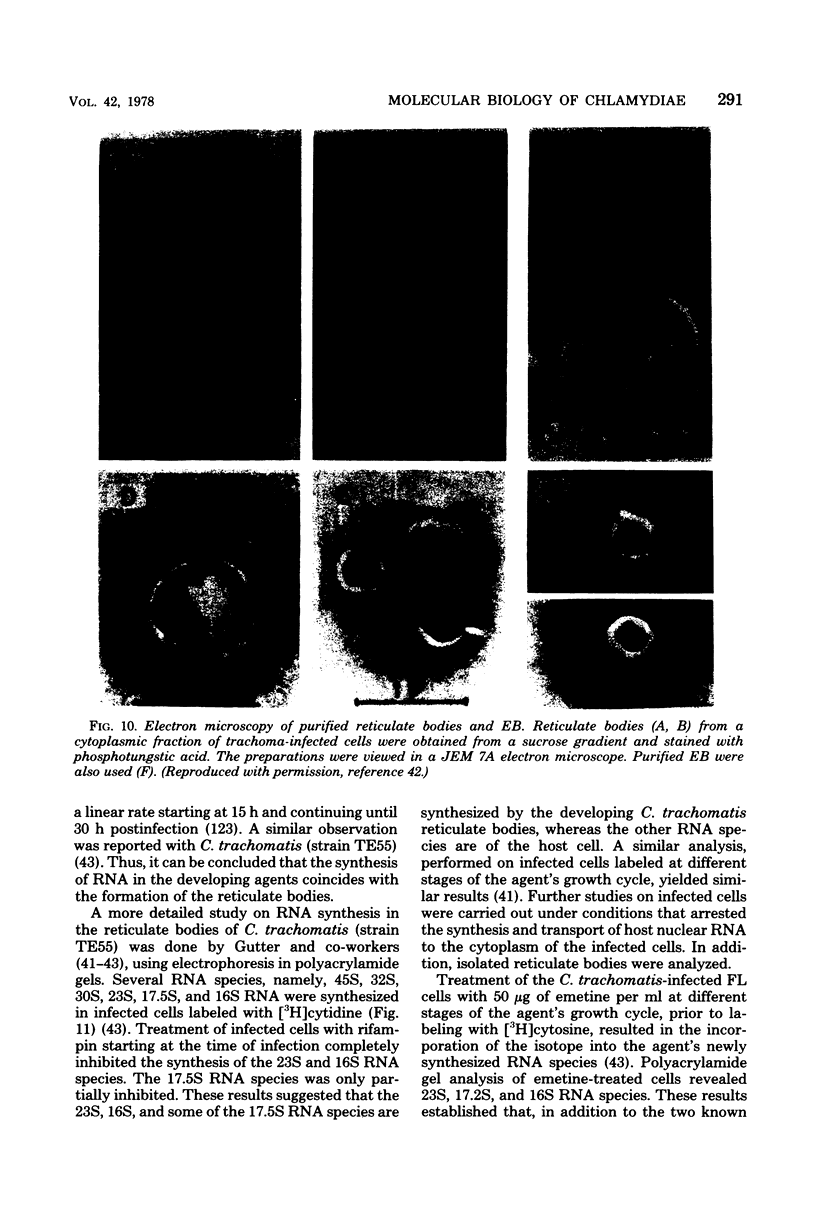
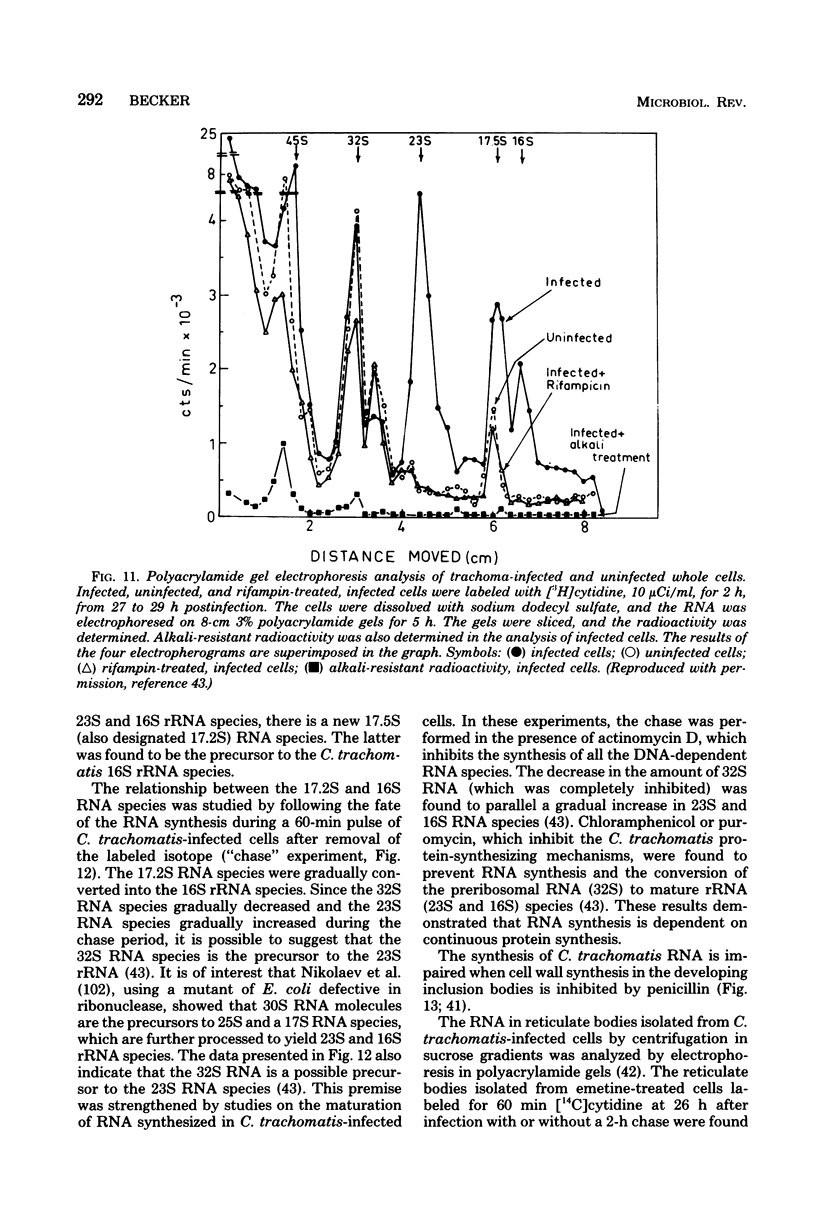

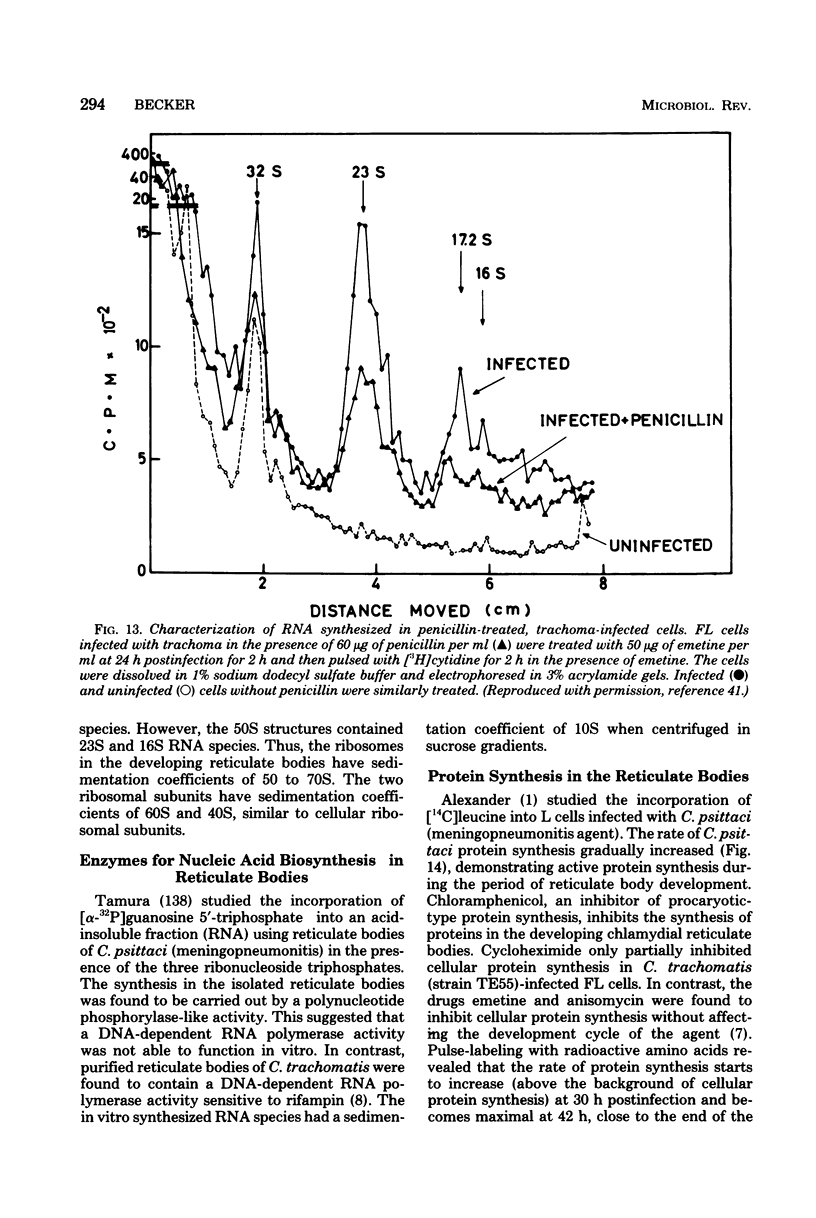












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. G., BOVARNICK M. R. Enzymatic activity associated with meningopneumonitis. Ann N Y Acad Sci. 1962 Mar 5;98:229–233. doi: 10.1111/j.1749-6632.1962.tb30547.x. [DOI] [PubMed] [Google Scholar]
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON A. L., ZAKAY-RONESS Z., BERNKOPF H. HEMAGGLUTINATION OF CHICKEN ERYTHROCYTES BY THE AGENT OF PSITTACOSIS. Proc Soc Exp Biol Med. 1965 Jun;119:377–381. doi: 10.3181/00379727-119-30186. [DOI] [PubMed] [Google Scholar]
- BECKER Y., MASHIAH P., BERNKOPF H. Biochemical changes in FL cell-cultures infected with a trachoma agent. Nature. 1962 Jan 20;193:271–272. doi: 10.1038/193271a0. [DOI] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P. The growth cycle of a trachoma agent in FL cell cultures. J Immunol. 1962 May;88:570–571. [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y., Asher Y. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in purified Chlamydia trachomatis initial bodies. Isr J Med Sci. 1974 Jul;10(7):777–781. [PubMed] [Google Scholar]
- Becker Y., Asher Y. Obligate parasitism of trachoma agent: lack of trachoma development in ethidium bromide-treated cells. Antimicrob Agents Chemother. 1972 Feb;1(2):171–173. doi: 10.1128/aac.1.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y., Asher Y. Synthesis of trachoma agent proteins in emetine-treated cells. J Bacteriol. 1972 Mar;109(3):966–970. doi: 10.1128/jb.109.3.966-970.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y., Hochberg E., Zakay-Rones Z. Interaction of trachoma elementary bodies with host cells. Isr J Med Sci. 1969 Jan-Feb;5(1):121–124. [PubMed] [Google Scholar]
- Becker Y., Zakay-Rones Z. Rifampicin--a new antitrachoma drug. Nature. 1969 May 31;222(5196):851–853. doi: 10.1038/222851a0. [DOI] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H. Growth characteristics of inclusion blennorrhea virus in cell cultures. Ann N Y Acad Sci. 1962 Mar 5;98:42–49. doi: 10.1111/j.1749-6632.1962.tb30530.x. [DOI] [PubMed] [Google Scholar]
- CROCKER T. T., EASTWOOD J. M. Subcellular cultivation of a virus: growth of ornithosis virus in nonnucleate cytoplasm. Virology. 1963 Jan;19:23–31. doi: 10.1016/0042-6822(63)90020-5. [DOI] [PubMed] [Google Scholar]
- CROCKER T. T., PELC S. R., NIELSEN B. I., EASTWOOD J. M., BANKS J. POPULATION DYNAMICS AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN HELA CELLS INFECTED WITH AN ORNITHOSIS AGENT. J Infect Dis. 1965 Apr;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Dhir S. P., Boatman E. S. Location of polysaccharide on Chlamydia psittaci by silver-methenamine staining and electron microscopy. J Bacteriol. 1972 Jul;111(1):267–271. doi: 10.1128/jb.111.1.267-271.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Elvin-Lewis P. F., Czekalowski J. W. Haemagglutination by the TRIC group of Chlamydia. J Med Microbiol. 1971 Feb;4(1):31–41. doi: 10.1099/00222615-4-1-31. [DOI] [PubMed] [Google Scholar]
- Evans A. The development of TRIC organisms in cell cultures during multiple infection. J Hyg (Lond) 1972 Mar;70(1):39–48. doi: 10.1017/s0022172400022075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan V. S., Jenkin H. M. Biosynthesis of Phospholipids and neutral lipids of monkey kidney cells (LLC-MK-2) infected with Chlamydia trachomatic strain lymphogranuloma venereum (38538). Proc Soc Exp Biol Med. 1975 Feb;148(2):351–357. doi: 10.3181/00379727-148-38538. [DOI] [PubMed] [Google Scholar]
- Fan V. S., Jenkin H. M. Glycogen metabolism in Chlamydia-infected HeLa-cells. J Bacteriol. 1970 Oct;104(1):608–609. doi: 10.1128/jb.104.1.608-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- GREENLAND R. M. A nonlethal mutant of penicillin-resistant feline pneumonitis virus. J Infect Dis. 1961 May-Jun;108:287–292. doi: 10.1093/infdis/108.3.287. [DOI] [PubMed] [Google Scholar]
- GREENLAND R. M., MOULDER J. W. A search for genetic interaction in the psittacosis group. J Infect Dis. 1961 May-Jun;108:293–303. doi: 10.1093/infdis/108.3.293. [DOI] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Garrett A. J. Some properties of the polysaccharide from cell cultures infected with TRIC agent (Chlamydia trachomatis). J Gen Microbiol. 1975 Sep;90(1):133–139. doi: 10.1099/00221287-90-1-133. [DOI] [PubMed] [Google Scholar]
- Gaugler R. W., Neptune E. M., Adams G. M., Sallee T. L., Weiss E., Wilson N. N. Lipid synthesis by isolated Chlamydia psittaci. J Bacteriol. 1969 Nov;100(2):823–826. doi: 10.1128/jb.100.2.823-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff R. K., Ritter D. B., Watson R. O. DNA homology between the meningopneumonitis agent and related microorganisms. J Infect Dis. 1966 Apr;116(2):197–202. doi: 10.1093/infdis/116.2.197. [DOI] [PubMed] [Google Scholar]
- Gerloff R. K., Ritter D. B., Watson R. O. Studies on thermal denaturation of DNA from various chlamydiae. J Infect Dis. 1970 Jan;121(1):65–69. doi: 10.1093/infdis/121.1.65. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Becker Y. Effect of emetine on ribonucleic acid biosynthesis in HeLa cells. Eur J Biochem. 1971 Nov 11;23(1):143–149. doi: 10.1111/j.1432-1033.1971.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Gutter B., Asher Y., Cohen Y., Becker Y. Studies on the developmental cycle of Chlamydia trachomatis: isolation and characterization of the initial bodies. J Bacteriol. 1973 Aug;115(2):691–702. doi: 10.1128/jb.115.2.691-702.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutter B., Becker Y. Synthesis and maturation of ribosomal RNA during the developmental cycle of trachoma agent, a prokaryotic obligate parasite of eukaryocytes. J Mol Biol. 1972 May 14;66(2):239–253. doi: 10.1016/0022-2836(72)90476-7. [DOI] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- HIGASHI N., TAMURA A., IWANAGA M. Developmental cycle and reproductive mechanism of the meningopneumonitis virus in strain L cells. Ann N Y Acad Sci. 1962 Mar 5;98:100–121. doi: 10.1111/j.1749-6632.1962.tb30536.x. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Effect of interferon on TRIC agents and induction of interferon by TRIC agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1115–1119. doi: 10.1016/0002-9394(67)94092-5. [DOI] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of exogenous thymidine by Chlamydia psittaci growing in the thymidine kinase-containing and thymidine kinase-deficient L cells. J Bacteriol. 1976 Feb;125(2):706–712. doi: 10.1128/jb.125.2.706-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi N. The mode of reproduction of psittacosis-lymphogranuloma-trachoma (PLT) group viruses. Int Rev Exp Pathol. 1964;3:35–64. [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M. Comparative lipid composition of psittacosis and trachoma agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1087–1098. doi: 10.1016/0002-9394(67)94087-1. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M., Lu Y. K. Induction of interferon by the Bour strain of trachoma in HeLa 229 cells. Am J Ophthalmol. 1967 May;63(5 Suppl):1110–1115. doi: 10.1016/0002-9394(67)94091-3. [DOI] [PubMed] [Google Scholar]
- Kajima M., Miyamoto H., Mitsui Y. [Fine structure of trachoma agent as observed by freeze-etching electron microscopy (author's transl)]. Nippon Ganka Gakkai Zasshi. 1973 Sep;77(9):1184–1193. [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo L. R., Hanna L., Keshishyan H. Reduction in chlamydial infectivity by lysozyme. Proc Soc Exp Biol Med. 1973 Jan;142(1):131–132. doi: 10.3181/00379727-142-36974. [DOI] [PubMed] [Google Scholar]
- Kordová N., Hoogstraten J., Wilt J. C. Lysosomes and the "toxicity" of Rickettsiales. IV. Ultrastructural studies of macrophages infected with a cytopathic L cell-grown C. psittaci 6BC strain. Can J Microbiol. 1973 Mar;19(3):315–320. doi: 10.1139/m73-052. [DOI] [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- LWOFF A. The concept of virus. J Gen Microbiol. 1957 Oct;17(2):239–253. doi: 10.1099/00221287-17-2-239. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepinay A., Robineaux R., Orfila J., Orme-Rosselli L., Boutry J. M. Ultrastructure et cytochimie ultrastructurale des membranes de Chalamydia psittaci. Arch Gesamte Virusforsch. 1971;33(3):271–280. [PubMed] [Google Scholar]
- Lin H. S. Stability of the nucleic acids of L cells after infection with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2049–2093. doi: 10.1128/jb.96.6.2049-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwoff A., Tournier P. The classification of viruses. Annu Rev Microbiol. 1966;20:45–74. doi: 10.1146/annurev.mi.20.100166.000401. [DOI] [PubMed] [Google Scholar]
- MATTHIESEN M., VOLKERT M. An ornithosis related antigen from a coccoid bacterium. Acta Pathol Microbiol Scand. 1956;39(2):117–126. doi: 10.1111/j.1699-0463.1956.tb03383.x. [DOI] [PubMed] [Google Scholar]
- MITSUI Y., KAJIMA M., NISHIMURA A., KONISHI K. Morphology and developmental cycle of the trachoma agent. Morphology of trachoma agent in conjunctiva and chick embryo. Ann N Y Acad Sci. 1962 Mar 5;98:131–144. doi: 10.1111/j.1749-6632.1962.tb30538.x. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., GRISSO D. L., BRUBAKER R. R. ENZYMES OF GLUCOSE CATABOLISM IN A MEMBER OF THE PSITTACOSIS GROUP. J Bacteriol. 1965 Mar;89:810–812. doi: 10.1128/jb.89.3.810-812.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., OFFICER J. E. INHIBITION OF THE GROWTH OF AGENTS OF THE PSITTACOSIS GROUP BY D-CYCLOSERINE AND ITS SPECIFIC REVERSAL BY D-ALANINE. J Bacteriol. 1963 Mar;85:707–711. doi: 10.1128/jb.85.3.707-711.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., TRIBBY I. C. DIAMINOPIMELIC ACID DECARBOXYLASE OF THE AGENT OF MENINGOPNEUMONITIS. J Bacteriol. 1963 Mar;85:701–706. doi: 10.1128/jb.85.3.701-706.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W. Some basic properties of the psittacosis-lymphogranuloma venereum group of agents. Structure and chemical composition of isolated particles. Ann N Y Acad Sci. 1962 Mar 5;98:92–99. doi: 10.1111/j.1749-6632.1962.tb30535.x. [DOI] [PubMed] [Google Scholar]
- Makino S., Jenkin H. M., Yu H. M., Townsend D. Lipid composition of Chlamydia psittaci grown in monkey kidney cells in defined medium. J Bacteriol. 1970 Jul;103(1):62–70. doi: 10.1128/jb.103.1.62-70.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. Fine structures of cell envelopes of Chlamydia organisms as revealed by freeze-etching and negative staining techniques. J Bacteriol. 1973 Dec;116(3):1355–1363. doi: 10.1128/jb.116.3.1355-1363.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Higashi N., Tamura A. Electron microscope observations on the effects of polymixin B sulfate on cell walls of Chlamydia psittaci. J Bacteriol. 1973 Jan;113(1):357–364. doi: 10.1128/jb.113.1.357-364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui Y. Electron microscopy of the trachoma agent. Rev Int Trach. 1971;48(2 Suppl):9–31. [PubMed] [Google Scholar]
- Moore D. E., Moulder J. W. Autoradiographic study of deoxyribonucleic acid synthesis in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 Oct;92(4):1128–1132. doi: 10.1128/jb.92.4.1128-1132.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Jenkin H. M. Growth of Chlamydia psittaci strain meningopneumonitis in mouse L cells cultivated in a defined medium in spinner cultures. In Vitro. 1972 Sep-Oct;8(2):94–100. doi: 10.1007/BF02615966. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Narita T., Manire G. P. Protein-carbohydrate-lipid complex isolated from the cell envelopes of Chlamydia psittaci in alkaline buffer and ethylenediaminetetraacetate. J Bacteriol. 1976 Jan;125(1):308–316. doi: 10.1128/jb.125.1.308-316.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., WEISS E. TRACHOMA AGENT: GLUCOSE UTILIZATION BY PURIFIED SUSPENSIONS. Science. 1963 Nov 22;142(3595):1077–1078. doi: 10.1126/science.142.3595.1077. [DOI] [PubMed] [Google Scholar]
- OSSOWSKI L., BECKER Y., BERNKOPF H. AMINO ACID REQUIREMENTS OF TRACHOMA STRAINS AND OTHER AGENTS OF THE PLT GROUP IN CELL CULTURE. Isr J Med Sci. 1965 Mar;1:186–193. [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R., ALLISON A. C. Cell-wall constituents of rickettsiae and psittacosis-lymphogranuloma organisms. J Gen Microbiol. 1963 Mar;30:469–480. doi: 10.1099/00221287-30-3-469. [DOI] [PubMed] [Google Scholar]
- POLLARD M., STARR T. J., MOORE R. W., TANAMI Y. Cytochemical changes in human amnion cell, infected with psittacosis virus. Nature. 1960 Nov 26;188:770–770. doi: 10.1038/188770a0. [DOI] [PubMed] [Google Scholar]
- POLLARD M., TANAMI Y. Cytochemistry of trachoma virus replication in tissue cultures. Ann N Y Acad Sci. 1962 Mar 5;98:50–61. doi: 10.1111/j.1749-6632.1962.tb30531.x. [DOI] [PubMed] [Google Scholar]
- ROSS M. R., JENKIN H. M. Cell wall antigens from members of the psittacosis group of organisms. Ann N Y Acad Sci. 1962 Mar 5;98:329–336. doi: 10.1111/j.1749-6632.1962.tb30555.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Gutter B., Becker Y. Studies on the developmental cycle of Chlamydia trachomatis: selective inhibition by hydroxyurea. J Bacteriol. 1973 Aug;115(2):682–690. doi: 10.1128/jb.115.2.682-690.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Chlamydin trachomatis in cell culture. I. Comparison of efficiencies of infection in several chemically defined media, at various pH and temperature values, and after exposure to diethylaminoethyl-dextran. Appl Microbiol. 1973 Oct;26(4):560–565. doi: 10.1128/am.26.4.560-565.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Infection of cell cultures by trachoma agent: enhancement by DEAE-dextran. J Infect Dis. 1971 Oct;124(4):419–421. doi: 10.1093/infdis/124.4.419. [DOI] [PubMed] [Google Scholar]
- SHIMIZU Y., BANKOWSKI R. A. THE NATURE OF THE CROSS REACTIONS BETWEEN A BACTERIUM OF THE GENUS HERELLEA AND THE ORNITHOSIS VIRUS IN COMPLEMENT FIXATION. Am J Vet Res. 1963 Nov;24:1283–1290. [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]
- STARR T. J., SHARON N. AUTORADIOGRAPHY WITH THE AGENTS OF PSITTACOSIS AND TRACHOMA. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:912–914. doi: 10.3181/00379727-113-28529. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in purified trachoma elementary bodies: effect of sodium chloride on ribonucleic acid transcription. J Bacteriol. 1971 Sep;107(3):593–598. doi: 10.1128/jb.107.3.593-598.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Becker Y. RNA in the elementary bodies of trachoma agent. Nature. 1968 Mar 2;217(5131):849–852. doi: 10.1038/217849b0. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Trachoma agent DNA. J Mol Biol. 1969 Jun 28;42(3):581–589. doi: 10.1016/0022-2836(69)90245-9. [DOI] [PubMed] [Google Scholar]
- Schechter E. M. Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 May;91(5):2069–2080. doi: 10.1128/jb.91.5.2069-2080.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Bankowski R. A. The characteristics of a bacterium used in complement fixation tests for ornithosis. Avian Dis. 1963 Aug;7(3):331–341. [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Cycloheximide-resistant glycosylation in L cells infected with Chlamydia psittaci. Infect Immun. 1974 Mar;9(3):497–499. doi: 10.1128/iai.9.3.497-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Proteinase produced by Chlamydia psittaci in L cells. J Bacteriol. 1974 May;118(2):616–620. doi: 10.1128/jb.118.2.616-620.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A. Isolation of ribosome particles from meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2009–2016. doi: 10.1128/jb.93.6.2009-2016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Cytochrome C reductase activity of meningopneumonitis organisms at different stages of development. Proc Soc Exp Biol Med. 1968 Nov;129(2):390–393. doi: 10.3181/00379727-129-33328. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Hemagglutinin in cell walls of Chlamydia psittaci. J Bacteriol. 1974 Apr;118(1):144–148. doi: 10.1128/jb.118.1.144-148.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Preparation and chemical composition of the cell membranes of developmental reticulate forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1184–1188. doi: 10.1128/jb.94.4.1184-1188.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Tanaka A., Manire G. P. Separation of the polypeptides of Chlamydia and its cell walls by polyacrylamide gel electrophoresis. J Bacteriol. 1974 Apr;118(1):139–143. doi: 10.1128/jb.118.1.139-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A. Detection of DNA polymerase activity in Chlamydia psittaci. Jpn J Exp Med. 1976 Jun;46(3):181–185. [PubMed] [Google Scholar]
- Tanami Y., Yamada Y. Miniature cell formation in Chlamydia psittaci. J Bacteriol. 1973 Apr;114(1):408–412. doi: 10.1128/jb.114.1.408-412.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuhaft M. W., Moulder J. W. Biosynthesis of arginine in L cells infected with chlamydiae. J Bacteriol. 1968 Dec;96(6):2004–2011. doi: 10.1128/jb.96.6.2004-2011.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Inhibition of Deoxyribonucleic Acid Synthesis in Synchronized Populations of L Cells Infected with Chlamydia psittaci. Infect Immun. 1971 Feb;3(2):363–364. doi: 10.1128/iai.3.2.363-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vender J., Moulder J. W. Initial step in catabolism of glucose by the meningopneumonitis agent. J Bacteriol. 1967 Oct;94(4):867–869. doi: 10.1128/jb.94.4.867-869.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilagines R., Atanasiu Action of ethidium bromide on growth of herpes virus in cell cultures. Nature. 1967 Jul 1;215(5096):87–88. doi: 10.1038/215087a0. [DOI] [PubMed] [Google Scholar]
- WEISS E., MYERS W. F., DRESSLER H. R., CHUN-HOON H. GLUCOSE METABOLISM BY AGENTS OF THE PSITTACOSIS-TRACHOMA GROUP. Virology. 1964 Apr;22:551–562. doi: 10.1016/0042-6822(64)90076-5. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. The rifamycins--relation of chemical structure and action on RNA polymerase. Biochim Biophys Acta. 1969 May 20;182(1):24–29. doi: 10.1016/0005-2787(69)90516-4. [DOI] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]







