Abstract
Using a novel analytical tool, this study investigates the relative roles of actin, microtubules, myosin, and Golgi bodies on form and movement of the endoplasmic reticulum (ER) in tobacco (Nicotiana tabacum) leaf epidermal cells. Expression of a subset of truncated class XI myosins, which interfere with the activity of native class XI myosins, and drug-induced actin depolymerization produce a more persistent network of ER tubules and larger persistent cisternae. The treatments differentially affect two persistent size classes of cortical ER cisternae, those >0.3 μm2 and those smaller, called punctae. The punctae are not Golgi, and ER remodeling occurs in the absence of Golgi bodies. The treatments diminish the mobile fraction of ER membrane proteins but not the diffusive flow of mobile membrane proteins. The results support a model whereby ER network remodeling is coupled to the directionality but not the magnitude of membrane surface flow, and the punctae are network nodes that act as foci of actin polymerization, regulating network remodeling through exploratory tubule growth and myosin-mediated shrinkage.
INTRODUCTION
The endoplasmic reticulum (ER) is the port of entry into the secretory pathway, yet very little is understood about the dynamic nature of this pleomorphic structure (Sparkes et al., 2009a). Actomyosin-based directional movement and cytoplasmic streaming drives the movement and dispersion of endomembrane compartments, such as the Golgi apparatus in plants (Avisar et al., 2008, 2009; Peremyslov et al., 2008; Prokhnevsky et al., 2008; Sparkes et al., 2008). Plant myosins, especially class XI myosins, including XIK, motorize Golgi bodies (and mitochondria and peroxisomes) just as myosin-V motorizes endomembrane organelles in yeast (Estrada et al., 2003) and nerve cells (Brown et al., 2004). While expression of the tail domain of certain Arabidopsis thaliana class XI myosins perturb organelle movement, these fluorescent fusions do not collocate to the organelles whose movement they effect, thus indicating titration of accessory factors or low levels of organelle binding. Here, analytical software has been developed to address questions of whether the increased movement and dispersion that characterize plant organelles, relative to organelles of animal cells, also pertain to the ER and how this movement and dispersion is driven by the actomyosin system.
As in cultured animal cells and yeast, the ER of higher plants is made of two primary domains, a polygonal tubule network, and cisternae (flattened membrane saccules) that are coextensive with the network. The movement of the network is not an entire shifting of a stationary set of polygons relative to, for instance, the plasma membrane. Indeed, the network appears more anchored to the plasma membrane than are other organelles (Quader et al., 1987). In the absence of global shifting of the network, movement within the network is either the movement of persistent network structures or the remodeling of the network through the formation or movement of network nodes and branches (Wolfram, 2002). Therefore, movement of the ER can be assessed by determining the persistency of network structures, such as nodes and branches. Here, this is done with persistency maps, images that show the level of persistency of different network structures in the ER, including tubules (branches and polygons), nodes (punctae or cisternae with an area between 0.1 and 0.3 μm2), and cisternae (membrane sheets with an area more than 0.3 μm2).
ER movement includes movement of proteins within the ER membrane and network remodeling. Directional, actin-dependent movement of membrane protein in the ER has recently been demonstrated in plants (Runions et al., 2006). The remodeling of the cisternal domain into tubules through the recruitment of reticulons, proteins that drive tubulation, may lead to the dynamic transition between tubular and cisternal domains (Tolley et al., 2008). In yeast (De Craene et al., 2006) and mammalian cells (Voeltz et al., 2006), the nuclear envelope is the most reliably visible cisternal domain of the ER. In plants, tubular-cisternal transitions are developmental (Ridge et al., 1999) and may generally represent regions of differing ER function (Staehelin, 1997).
The plant ER network overlies the actin cytoskeleton (Boevink et al., 1998), but does the actomyosin system regulate the forms of movement described above? Here, changes in the persistency of the cortical ER of tobacco (Nicotiana tabacum) leaf epidermal cells are determined after treatments that depolymerize actin filaments (latrunculin b) or microtubules (oryzalin), perturb organelle motility (coexpression of a truncated class XI myosin) or remove Golgi bodies (Brefeldin A). Although the results indicate that some form of linkage between the actin cytoskeleton and the ER membrane might drive ER movement and change the mobile fraction of ER surface proteins, they do not support the notion that the Golgi bodies, which are intimately associated with the ER (Boevink et al., 1998; Runions et al., 2006; Sparkes et al., 2009b) and which are also driven by the actomyosin system (Boevink et al., 1998; Nebenführ et al., 1999), mediate ER movement.
RESULTS
ER Network Persistence
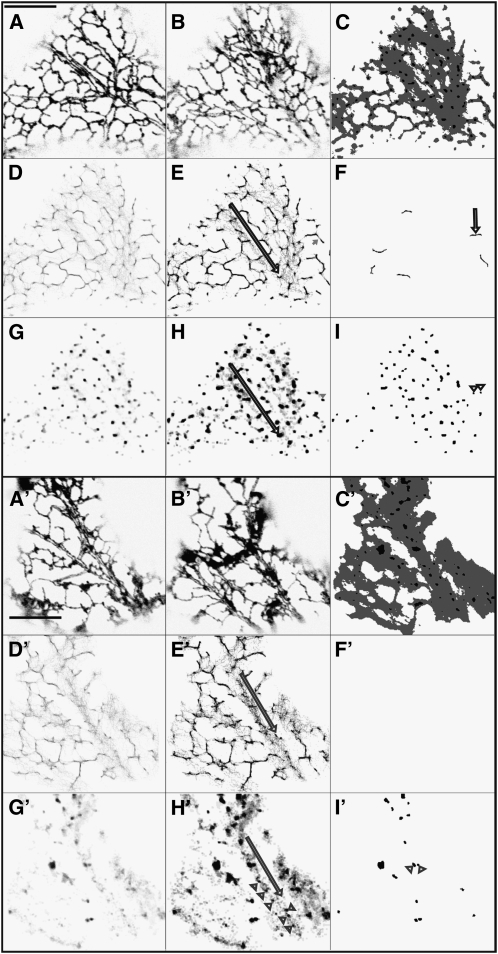
Image maps showing the persistence of tubules and cisternae are called persistency maps. Two different control tobacco epidermal cells, representative of 30 such cells, expressing the fluorescent marker green fluorescent protein (GFP)-HDEL (see Supplemental Movie 1 online) are shown in Figure 1, which includes the first and last frames of a 50-frame movie (Figures 1A, 1A′, 1B, and 1B′), the total imaged membrane over the time of imaging (image sum) used for normalization (Figures 1C and 1C′), and tubule (Figures 1D, 1D′, 1F, and 1F′) and cisternal persistency maps (Figures 1G, 1G′, 1I, and 1I′). Persistence is displayed as a gray level gradient (Figures 1D, 1D′, 1G, and 1G′) or discrete gray levels (Figures 1E, 1E′, 1H, and 1H′). The darkest, most persistent tubules above a certain size (Figures 1F and 1F′) are quantified (see below). Likewise, the most persistent cisternal structures, punctae that are smaller than 0.3 μm2 and cisternae that are larger than 0.3 μm2, are quantified (see below). Tubule and cisternal persistence are lower in some cells (Figures 1A′ to 1I′) than in others (Figures 1A to 1I). Both contain fast lanes of ER movement with low persistence gray regions adjacent to the arrow (Figures 1E and 1E′), where tubules change rapidly and active streaming occurs in the direction indicated. Neither example has persistent polygons within the network, nor do less persistent polygonal structures move (no gray tubules remain as polygons in Figures 1E and 1E′). There are few persistent cisternae, but less persistent ones (dark gray, particularly punctae, Figures 1H and 1H′) move. Therefore, as expected, and thus validating the modeling system, the movement of the network is not movement of polygonal subdomains of the network but of small nodal punctae and tubule branches that form, grow, and shrink or generate cisternae.
Figure 1.
Persistency of Tubules, Punctae, and Cisternae of Cortical ER in Two Different Control Tobacco Epidermal Cells Expressing GFP-HDEL.
The first ([A] and [A′]) and last frame ([B] and [B′]) of a 50-frame 80-s movie. Summed frames of the entire movie ([C] and [C′]), showing total imaged membrane (gray plus black) and the brighter punctae in the summed image (black). The tubule persistency map ([D] and [D′]) shows more persistent tubules as darker values. The posterized gray-level tubule persistency map ([E] and [E′]) shows high persistency (black), mid-range persistency (dark gray), and low persistency (light-gray gradient). The black arrow is positioned adjacent to an area of active streaming, showing the direction of streaming. High persistency tubules ([F] and [F′]) from (E) and (E′) that were >0.3 μm2 in area are the tubules counted in Figure 4A. In (F), the arrow indicates a persistent tubule that is subtended by persistent punctae ([I], below). The persistency map ([G] and [G′]) shows more persistent cisternae and punctae as darker values. The posterized gray-level cisternal persistency map ([H] and [H′]) shows high persistency (black), mid-range persistency (dark gray), and low persistency (light gray gradient) cisternae and punctae (arrowheads [H′] show midrange persistency punctae). The black arrow is positioned adjacent to an area of active streaming, showing the direction of streaming. The high persistency map ([I] and [I′]) from (G) and (G′) shows both the punctae (0.1 to 0.3 μm2, circularity > 0.5) and cisternae (0.3 μm2). Arrowheads show persistent punctae connected by a persistent tubule. Bar = 10 μm.
Actomyosin Requirement for ER Network Remodeling
To ascertain the contribution of the cytoskeleton to ER remodeling, tobacco epidermal cells were treated with drugs to depolymerize either actin filaments or microtubules or subjected to overexpression of class XI myosin tail domains, XIK or XIJ, fused to enhanced yellow fluorescent protein (eYFP). To confirm that latrunculin b and oryzalin depolymerize actin filaments and microtubules, respectively, pieces of tobacco leaf epidermal cells expressing fluorescent markers for both were treated and are shown in Supplemental Figure 1 online. Previous studies have shown that XIK tail fusions perturb Golgi, peroxisome, and mitochondria movement (Sparkes et al., 2008). Proposed explanations for these effects include competition for myosin effectors and cargo binding sites; however, since little is known about the latter, the formal proof for the mode of action of plant myosin tail domains remains elusive (Sparkes et al., 2008; Avisar et al., 2009). This fusion, along with other class XI tail domain fusions, does not collocate to these organelles, is present in motile puncta, and is diffuse throughout the cytosol. This diffuse cytoplasmic distribution could reflect a low level of binding to their target organelles. Similarly, the tail domain of XIJ fused to eYFP is diffuse throughout the cytosol but has a negligible affect on Golgi movement (Avisar et al., 2009). Therefore, as a control for XIK specificity on ER modeling, the effects of the tail domain of XIJ fused to eYFP were tested in parallel.
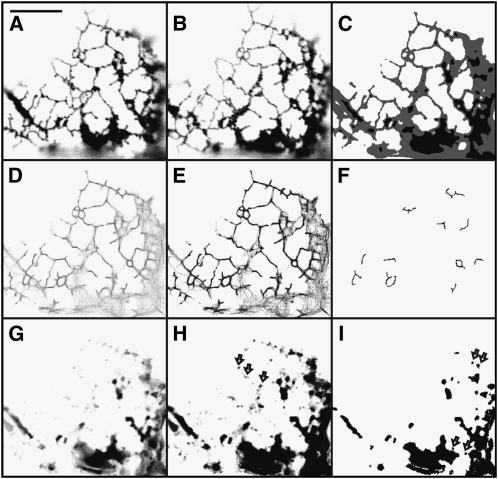
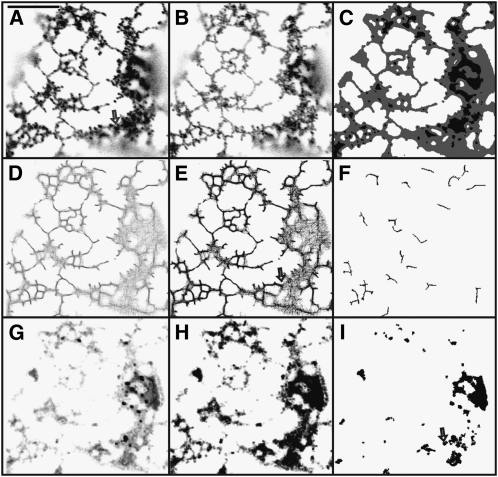
ER network movement is quite different when cells coexpress the myosin XIK tail domain (Figure 2; see Supplemental Movie 2 online, representative of 27 cells) or are treated with latrunculin b (Figure 3; see Supplemental Movie 3 online, representative of 14 cells). The pattern of tubules and cisternae changes less (Figures 2, 3A, and 3B; see Supplemental Movies 2 and 3 online) than in controls (Figures 1A, 1B, 1A′, and 1B′; see Supplemental Movie 1 online). The tubule persistency maps of the treatments (Figures 2D to 2Fand 3D to 3F) show a connected network of persistent tubules that include polygonal rings not found in control cells (Figures 1D to 1F and 1D′ to 1F′). The most persistent tubules (Figures 2E, 2F, 3E, and 3F) are longer and more abundant throughout the network than in control cells (Figures 1E, 1F, 1E′, and 1F′). The two treatments produce more and longer persistent tubules than control (Figure 4A). However, there is little difference in persistent tubules between the latrunculin b treatment and myosin XIK tail coexpression (Figure 4A). Both treatments produce more persistent cisternae (Figures 2G to 2I and 3G to 3I) than found in the controls (Figures 1G to 1I and 1G′ to 1I′). Although there is little difference between the number of persistent punctae per 100 μm2 of imaged membrane in the control and cells coexpressing truncated myosin XIK, there is a significant increase in punctae in the latrunculin b treatment compared with the control (Figure 4B).
Figure 2.
Persistency of Tubules, Punctae, and Cisternae of Cortical ER in a Tobacco Epidermal Cell Coexpressing GFP-HDEL and the eYFP-XIK Tail Domain.
(A) to (I) Same as in Figure 1. Because there are fewer or no fast lanes in cells coexpressing myosin-XIK tails and treated with latrunculin b, the gray regions in (E) indicate regions of remodeling cisternae that are differentially skeletonized. Arrows in (H) indicate less persistent punctae associated with tubules. Arrows in (I) indicate more persistent punctae associated with cisternae. Bar = 10 μm.
Figure 3.
Persistency of Tubules, Punctae, and Cisternae of Cortical ER in a Tobacco Epidermal Cell Expressing GFP-HDEL and Treated with Latrunculin b.
(A) to (I) Same as in Figure 1. Arrows in (A), (E), and (I) indicate similar location near the periphery of cisternae where a persistent puncta occurs. Bar = 10 μm.
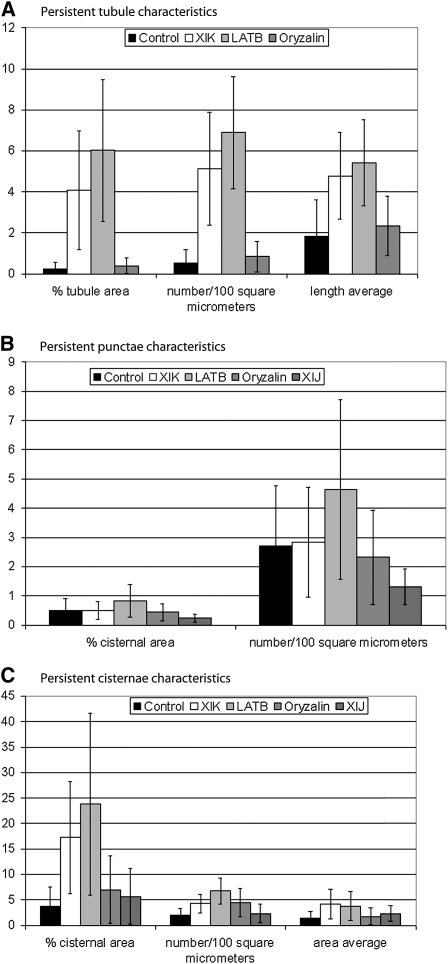
Figure 4.
Quantification of Persistent ER Tubules and Cisternae under Various Treatment Conditions.
(A) Persistent tubules in control GFP-HDEL–expressing cells (black, Control), GFP-HDEL, and eYFP-XIK tail coexpressing cells (white, XIK), latrunculin b–treated GFP-HDEL–expressing cells (light gray, LATB), and oryzalin-treated GFP-HDEL–expressing cells (gray). Columns show average value, and error bars show sd in total number of movies evaluated, n (Control, n = 30; XIK, n = 27; LATB, n = 14; oryzalin, n = 16). Percentage of tubule area is the percentage of the total imaged membrane (from summed images, refer to Figures 1 to 3C, black and gray regions) that is persistent tubules (high persistency tubules, refer to Figures 1 to 3F). Number/100 μm2 is the number of persistent tubules per 100 μm2 of total imaged membrane. Length average is the average length in micrometers of the persistent tubules. The differences between all except the control-oryzalin pair (all categories) and the XIK-LATB pair (tubule length category) are significant (P < 0.05, Tukey's HSD multiple comparisons of means, 95% family-wise confidence level).
(B) Persistent punctae in control GFP-HDEL–expressing cells (black, Control), GFP-HDEL, and eYFP-XIK tail coexpressing cells (white), latrunculin b (light gray), and oryzalin (medium gray) treated GFP-HDEL–expressing cells, and GFP-HDEL and eYFP-XIJ tail coexpressing cells (dark gray). Columns show average value, and error bars show sd in total number of movies evaluated, n (Control, n = 30; XIK, n = 27; LATB, n = 14; oryzalin, n = 16; XIJ, n = 4). Percentage of cisternal area is the percentage of the total imaged membrane (from summed images, refer to Figures 1 to 3C, black and gray regions) that is persistent punctae (high persistency cisternae with areas between 0.1 and 0.3 μm2 and circularity > 0.5; refer to Figures 1 to 3I; see Supplemental Figures 2 and 3F online). Number/100 μm2 is the number of persistent punctae per 100 μm2 of total imaged membrane. The LATB treatment is significantly different (P < 0.05) from all of the other treatments, if the XIJ data are not included in the set because of low sample size. The other treatments do not significantly differ from each other (Tukey's HSD multiple comparison of means, 95% family-wise confidence level).
(C) Persistent cisternae in control GFP-HDEL–expressing cells (black), GFP-HDEL and eYFP-XIK tail coexpressing cells (white), latrunculin b–treated (light gray) and oryzalin-treated (medium gray) GFP-HDEL–expressing cells, and GFP-HDEL and eYFP-XIJ tail coexpressing cells (dark gray). Columns show average value, and error bars show sd in total number of movies evaluated, n (same as [B]). Percentage of cisternal area is the percentage of the total imaged membrane (from summed images, refer to Figures 1 to 3C, black and gray regions) that is persistent cisternae (high persistency cisternae >0.3 μm2, refer to Figures 1 to 3I; see Supplemental Figures 2 and 3F online). The Control-LATB and -XIK pairs are significantly different, as are the Oryzalin-LATB and -XIK pairs and the XIJ-LATB pair (P < 0.05). Number/100 μm2 is the number of persistent cisternae per 100 μm2 of total imaged membrane. All pair comparisons except the Control-XIJ, Oryzalin-XIJ, XIK-XIJ, and Oryzalin-XIK pairs are significant (P < 0.05). Area average is the average area in μm2 of the persistent cisternae. The only pair comparisons that are significant (P < 0.05) are Control-LATB, Control-XIK, and Oryzalin-XIK. Tukey's HSD multiple comparisons of means were done at 95% family-wise confidence level.
After myosin XIK tail coexpression, high persistency punctae are near the periphery of the larger persistent and less persistent cisternae (arrows, Figure 2I), whereas the lower persistency punctae subtend tubular networks (arrows, Figure 2H). Many punctae seen in the latrunculin b treatment are not so much nodal at the branching or bending points of tubules, as they are on the periphery of the large cisternae (arrows, Figures 3A, 3E, and 3I). Less persistent punctae sometimes appear at the blind end of tubules (arrowheads, Figures 3E and 3H). Persistent tubules in control cells terminate into a network of lower persistency tubules (Figures 1E and 1E′), whereas short persistent tubules in myosin XIK tail coexpression and latrunculin b treatments do not (Figures 2E and 3E).
Cisternae increase in both area and number after truncated myosin XIK coexpression and latrunculin b treatment (Figure 4C; compare Figures 1G and 1G′ with 2G and 3G). Although both treatments show a similar increase in the average area of the cisternae over control, there is a larger increase in the number of persistent cisternae after latrunculin b treatment than after truncated myosin XIK coexpression (Figure 4C).
To ascertain whether microtubules and another class XI myosin (XIJ) affect ER remodeling, movies were captured of cells coexpressing eYFP-XIJ tail domain and GFP-HDEL and after oryzalin treatment of cells expressing GFP-HDEL (see Supplemental Figures 2 and 3 and Supplemental Movies 4 and 5 online). Oryzalin has no significant effect on tubule length and persistency (Figure 4A) but does slightly increase persistent cisternae size compared with the control (Figure 4C; see Supplemental Figure 2 online for persistency maps). Prior to quantification, it was apparent that coexpression of the XIJ tail domain did not perturb ER remodeling. A small sample size was analyzed and indicated that the XIJ tail domain did not affect ER cisternal size or number, but there were no persistent tubules above the threshold level of 0.3μm2 (see Supplemental Figure 3 online for persistency maps).
Actomyosin Requirement for ER Membrane Protein Mobility
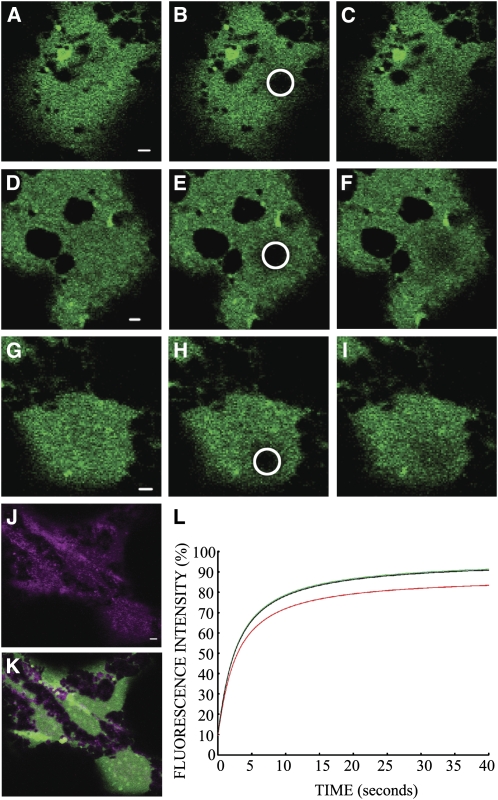
Fluorescence recovery after photobleaching (FRAP) and photoactivation studies reveal the dynamics of ER membrane proteins after disruption of the actomyosin system. Overexpression of GFP-CXN generates large ER cisternae over time (Figure 5A). Cisternalization provides large two-dimensional sheets desirable for FRAP photobleaching. FRAP analysis of cisternal GFP-CXN yielded rates (t1/2 values) of protein movement and a measure of the mobile fraction of membrane protein. Control cells (Figures 5A to 5C), latrunculin b–treated cells (Figures 5D to 5F), and cells coexpressing GFP-CXN and the eYFP-XIK tail domain (Figures 5G to 5I) had similar recovery rates (t1/2 = ∼2.4 s; Table 1, Figure 5L). However, coexpression of GFP-CXN and the eYFP-XIK tail domain resulted in a significantly lower mobile fraction of fluorescent protein, for example, exchange of bleached molecules with fluorescent molecules outside of the spot bleach region (79.09% ± 13.20%, Tukey's HSD P < 0.001) compared with control and latrunculin b treatments, which were the same (87.72% ± 10.18% and 89.14% ± 7.39%, respectively; Table 1, Figure 5L).
Figure 5.
The Effect of the eYFP-XIK Tail Domain and Latrunculin b on FRAP of Tobacco Leaf Epidermal ER Marker GFP-CXN.
FRAP of a bleached spot area in control cells ([A] to [C]), cells treated with latrunculin b ([D] to [F]), and those coexpressing eYFP-XIK tail domain ([G] to [I]) were quantified where prebleach ([A], [D], and [G]), bleach ([B], [E], and [H]) and 1.21 s postbleach ([C], [F], and [I]) are shown. Coexpression of GFP-CXN and the eYFP-XIK tail domain ([J]; merged GFP-CXN image in [K]) prior to the bleach experiment shown in (G) to (I) is indicated. The bleached area of GFP-CXN (circle) was monitored over time, and the fluorescence recovery was plotted ([L]; where the green line is control, black line is latrunculin b treatment, and red line is coexpression of GFP-CXN and the eYFP-XIK tail domain). Bars = 2 μm.
Table 1.
ER Membrane Dynamics Determined with FRAP of GFP-CXN under Various Treatment Conditions
| Treatment | t1/2 (s) | Mobile Fraction (Plateau) (%) |
|---|---|---|
| Control (n = 57) | 2.37 ± 0.49 | 87.72 ± 10.18 |
| Latrunculin B (n = 16) | 2.45 ± 0.44 | 89.14 ± 7.39 |
| eYFP-XIK tail (n = 48) | 2.33 ± 0.58 | 79.09 ± 13.20 |
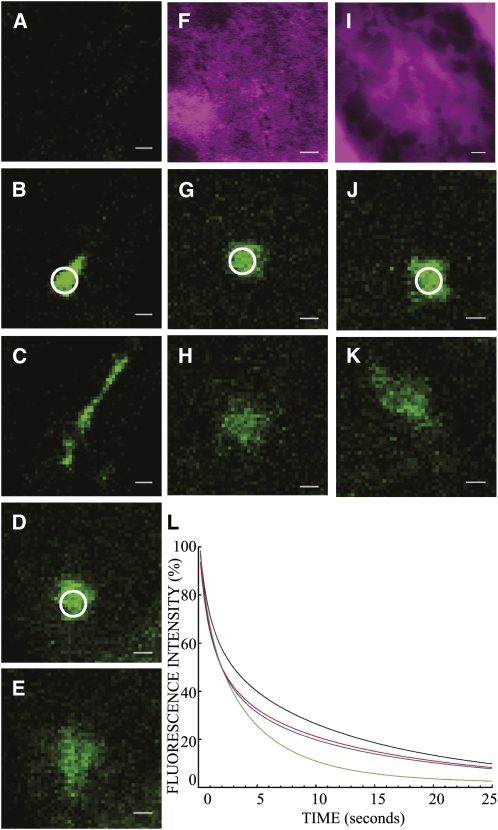
Photoactivation studies using CXN-paGFP (Runions et al., 2006) measured protein mobility and mobile fraction independently of FRAP. Protein mobility is measured as fluorescence intensity decline as photoactivated CXN-paGFP disperses from the activation spot (Figure 6). The t1/2 values of ∼2 s for movement away from the activation spot were the same in all cases (Figures 6A to 6K; see also Table 2). Mobile fractions, however, of cells coexpressing the mRFP-XIK tail domain (91.27% ± 6.12%) and those treated with latrunculin b (89.42% ± 7.04%) were significantly lower than in control cells (97.63% ± 4.26%, Tukey's HSD, P < 0.001; Figure 6L).
Figure 6.
The Effect of mRFP-XIK Tail Domain, Latrunculin b, and BFA on the Mobility of CXN-Photoactivated GFP in Tobacco Leaf Epidermal ER.
A spot activated area (circle) of CXN-photoactivatable GFP was generated, and its mobility out of the activation spot was monitored over time ([A] to [K]) and quantified ([L]; where the green line is control, black line is latrunculin b treatment, red line is coexpression of myosin XIK tail domain, and the blue line is BFA treatment). Mobility of CXN-paGFP in control cells ([A] to [C]), cells treated with latrunculin b ([D] and [E]), those coexpressing CXN-paGFP and the mRFP-XIK tail domain ([F] to [H]) or treated with BFA ([I] to [K]) were quantified where activation ([B], [D], [G], and [J]) and 1.93 s postactivation ([C], [E], [H], and [K]) are shown. An example of preactivation (A) highlights the lack of fluorescence prior to photoactivation in control cells. Expression of the mRFP-XIK tail domain (F) and the effect of relocation of ST-mRFP back to the ER (I) in the activation experiments shown ([F] to [K]) are indicated. Bars = 2 μm.
Table 2.
ER Membrane Dynamics Determined with Photoactivation of CXN-paGFP under Various Treatment Conditions
| Treatment | t1/2 (s) | Plateau (%) | Mobile Fraction (%) |
|---|---|---|---|
| Control (n = 45) | 2.01 ± 1.15 | 2.37 ± 4.26 | 97.63 ± 4.26 |
| Latrunculin B (n = 20) | 2.31 ± 1.22 | 10.58 ± 7.04 | 89.42 ± 7.04 |
| mRFP-XIK tail (n = 31) | 1.85 ± 1.11 | 8.73 ± 6.12 | 91.27 ± 6.12 |
| BFA (n = 28) | 1.79 ± 0.98 | 8.12 ± 8.08 | 91.88 ± 8.08 |
Are Golgi Bodies Involved in ER Remodeling and ER Membrane Mobility?
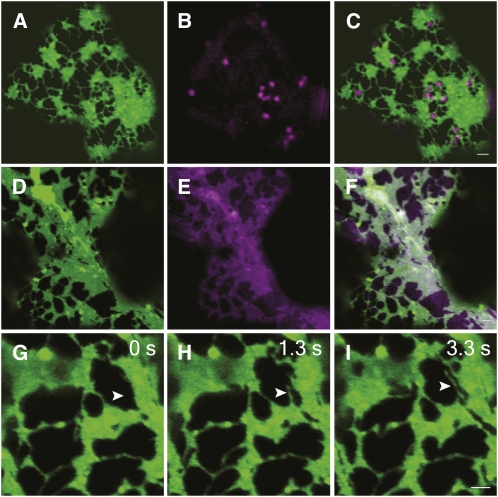
In various plant tissues, Golgi bodies appear to be associated with the ER and move either over, or with the ER membrane, but as seen here do not collocate with persistent cortical ER puncta (Figures 7A to 7C).
Figure 7.
Golgi Bodies Do Not Collocate with Persistent ER Puncta in Tobacco Epidermal Cells and Do Not Affect ER Membrane Remodeling.
Cells coexpressing GFP-HDEL ([A]; green) and ST-mRFP ([B]; magenta) treated with latrunculin b highlight the persistent puncta on the cisternal ER and show that the Golgi bodies do not collocate to these regions (C). Cells coexpressing GFP-HDEL and ST-mRFP were treated with BFA (3 h, 100 μg/mL) ([D] to [I]), and remodeling of the ER network was monitored over time. The Golgi marker ST-mRFP has relocated to the ER after BFA treatment, indicating Golgi body absence ([E] and merged image in [F]). Consecutive images ([G] to [I]) show that the ER network is able to remodel and tubule outgrowth still occurs (arrowhead) after BFA treatment. Bars = 2 μm.
To test the putative role of Golgi bodies in ER remodeling and membrane flow, cells were treated with Brefeldin A (BFA), which deconstructs Golgi and results in varying degrees of cisternalization of the ER (Boevink et al., 1999). This cisternalization precludes comparison of tubule and cisternal persistency with control cells because drug effects could be confused with the direct effect of Golgi body absence.
Cells coexpressing GFP-HDEL and the Golgi marker ST-mRFP were treated with BFA for 3 h, resulting in disappearance of Golgi bodies and redistribution of ST-mRFP to the ER (Figures 7D to 7F). Stills from a representative movie (Figures 7G to 7I; see Supplemental Movie 6 online) show that the ER remodels in the absence of Golgi bodies. Photoactivation analysis of cells coexpressing CXN-paGFP and ST-mRFP after treatment with BFA for 3 h reveals t1/2 values for movement away from the activation spot (1.79 ± 0.98 s) similar to control (2.01 ± 1.15 s). The mobile fraction (91.88% ± 8.08%) was significantly lower after BFA than in control samples (97.63% ± 4.26%, Tukey's HSD P = 0.001; Table 2, Figures 6I to 6K).
DISCUSSION
Visualization of Cortical ER Network Movement and Cytoplasmic Streaming
The nature of cortical ER network movement in plant cells is not widely understood. In higher plants, the ER cisternal/tubular network does not move as a whole or as cisternal/tubular network fragments, as is often illustrated for the involvement of ER in charophyte cytoplasmic streaming (Kachar and Reese, 1988; Lodish et al., 2008). Instead, about 5% of the total imaged ER is very persistent (Figure 4A) and forms the framework upon which movement occurs. This framework is revealed with persistency maps, visual maps of the ER that show its recent degree of persistency. The maps show ER features that remain relatively static for periods of longer than 8 s (five time-lapse frames) in an 80-s data set (Figures 1D, 1D′, 1I, and 1I′; see Supplemental Movie 1 online). These persistency maps do not have persistently connected networks of polygonal rings (Figures 1D, 1D′, 1E, and 1E′); polygonal subunits do not persist. Polygon ring formation and closure are two of the more transient events in the network. Until now, most analysis of the tubular network has focused on these transient events that generate and remove the tubule polygons that make up the network (Lee and Chen, 1988; Knebel et al., 1990; Lichtscheidl and Url, 1990), but some have noted that punctate structural features persist (Knebel et al., 1990; Lichtscheidl and Url, 1990). Observations using persistency maps confirm that there is a population of distributed, persistent punctae (cisternae between 0.1 and 0.3 μm2; Figures 1G to 1I and 1G′ to 1I′) that could function as the nodal framework upon which network movement takes place. Although these punctae may serve to anchor persistent features in the otherwise dynamic network, they are less persistent and may move (gray punctae near arrowheads, Figure 1H′) in regions of rapid streaming. Therefore, instead of referring to them as anchor sites, here we prefer to describe them as anchor/growth sites, and we predict that they are the same anchor sites around which the ER tubules can be remodeled manually using laser-trapped Golgi bodies (Sparkes et al., 2009b).
The Role of the ER-Golgi Interface in ER Remodeling
There is an intimate association of Golgi bodies with moving ER exit sites (daSilva et al., 2004), ER tubule growth appears to follow Golgi body movement (Brandizzi et al., 2002), and the flow of proteins within the ER membrane corresponds in direction and magnitude to that of streaming nearby Golgi (Runions et al., 2006). These observations prompted the notion that Golgi-associated motors might mediate the actomyosin dependence of ER movement (Brandizzi et al., 2002; Hawes et al., 2008). However, the results presented here indicate that Golgi bodies do not affect ER remodeling (Figure 7) because when the Golgi have been deconstructed with BFA, new ER tubules still actively form and retract (see Supplemental Movie 6 online). The rate of movement of ER membrane protein (t1/2 values) after BFA treatment, as estimated by photoactivation (Figures 6I to 6L), did not differ from other treatments, but the mobile fraction was lower, matching myosin XIK tail coexpression values most closely (Figure 6L). Although Golgi bodies do not appear to drive ER, an as yet unanswered question is, does ER drive Golgi bodies? However, the ER does appear to exert an effect on Golgi stack location in terms of constraining them to the tubular ER or to the rims of cisternal regions (Figure 7) (Boevink et al., 1998; Runions et al., 2006; Hawes et al., 2008). While a comprehensive study of all the Arabidopsis myosins, through transient expression studies in tobacco epidermal cells, has been implemented, it has been shown that the XIK tail domain, but not the XIJ tail, significantly affects movement of Golgi and mitochondria (Avisar et al., 2009). Similarly, we show a comparable differential effect on ER movement with the same myosin tail domains, perhaps indicating an interdependent motion of the two organelles driven by the same myosin.
The Effect of the Actomyosin System and Microtubules on ER Remodeling and Movement
Both eYFP-XIK myosin tail coexpression and latrunculin b treatment affect the appearance and dynamics of the ER (Figures 2 and 3; see Supplemental Movies 2 and 3 online). The eYFP-XIK myosin tail domain perturbs streaming of Golgi, mitochondria, and peroxisomes (Peremyslov et al., 2008; Sparkes et al., 2008), presumably by interfering with the activity of endogenous myosin XIK through titration of accessory factors required for binding cargo or through titration of active myosin dimers required to bind cargo. Latrunculin b treatment reduces the pool of polymerized F-actin by complexing G-actin monomers so that actin filaments disappear (Coue et al., 1987; see Supplemental Figures 1C and 1D online). Not only do both these treatments result in an increase in the presence of persistent ER tubules (Figure 4A; number/100 μm2 and length average), but they also produce a pattern of more persistent polygonal ring structures (cf. Figures 2, 3D, and 3E with Figures 1D, 1D′, 1E, and 1E′), resulting in a persistency map of polygonal ring structures connected by persistent tubules. Furthermore, although both treatments give rise to increased persistent cisternal area (Figure 4C, area average), latrunculin b treatment appears to generate more of an increase in the number of persistent punctae (Figure 4B, number/100 μm2) and persistent cisternae (Figure 4C; number/100 μm2) than truncated myosin XIK coexpression.
The increase in the size of persistent ER cisternae (Figure 4C, area average) may arise through a common mechanism, (i.e., inhibition of dynamic or exploratory tubular outgrowth of membranes from anchor sites on cisternae that would otherwise diminish cisternal area). The observation that persistent punctae are redistributed to cisternae and their periphery after XIK tail coexpression (arrows, Figure 2I) and latrunculin b treatment (arrowhead, Figure 3A, 3E, and 3I) indicates that the nodal punctae may not only be anchor sites but may also act as growth sites, stopped at some point in the tubule growth cycle.
We observed no significant effect of oryzalin on ER dynamics other than an increase in cisternae. Oryzalin at the concentration and time used is effective at depolymerizing microtubules (see Supplemental Figures 1A and 1B online). Recent studies have highlighted that oryzalin can have a secondary effect leading to ER nodulation (Langhans et al., 2009), which could explain the apparent increase in small persistent cisternae observed here. Unlike Knebel et al. (1990), who also noted some ER nodulation, Langhans et al. (2009) also reported an effect on ER dynamics but attributed this to the drug and not to the absence of microtubules. Our evidence supports that of Knebel et al. (1990); microtubule depolymerization has no effect on ER dynamics. In addition, the small persistent cisternae may actually be swollen anchor/growth sites, as suggested by Knebel et al. (1990). We should caution, however, that this by no means excludes a role for microtubules exerting a regulating influence on the dynamics of the ER at other stages of the cell cycle. Indeed, it is quite likely that during the transition to metaphase, while the nuclear envelope resorbs into the ER, which then forms aggregates at the spindle poles, there is a switch over from an actin-based movement to microtubule-based movement on spindle microtubules (Gupton et al., 2006). The association of ER membrane with spindle microtubules in plants is well established (Hepler and Wolniak, 1984).
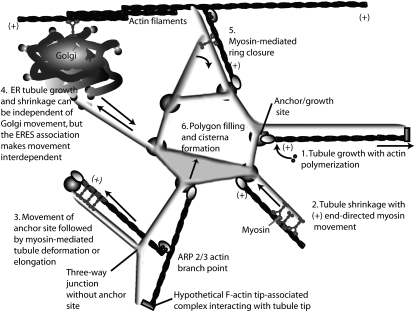
A Model for the Involvement of Actin and Myosin in ER Form and Remodeling
Observations of ER form reveal a dynamic structure that undergoes rapid remodeling in an actin-dependent manner. Here, we present the development of analytical software in order to quantify these observations, with some surprising revelations relating to persistent growth/anchor sites and the movement of the ER surface in relation to the active remodeling structure. Based on these results, we propose a model for how the actomyosin system regulates ER remodeling by interacting with key structural elements, including growing, shrinking, and stabilized tubules, three-way junctions, anchor/growth sites, and tubule/cisternal transitions (Figure 8). In plants, extremely little is understood regarding any of these key elements, and only recently has it emerged that reticulons are involved in constricting the ER, and we have proposed a role for them in modulating cisternalization versus tubulation (reviewed in Tolley et al., 2008; Sparkes et al., 2009a).
Figure 8.
Model of the Different Activities of F-Actin and Myosin at Anchor/Growth Sites in Tubule Dynamics at Nonanchored Sites and in Determining the Mobile Fraction of ER Membrane Proteins.
Process 1: Tubules grow outwards, driven by actin polymerization at an anchor/growth site. The anchor site is proposed to be a region where the plasma membrane and ER are closely associated. These sites may contain actin nucleation and growth proteins. The mechanism of actin attachment to the growing tubule is represented diagrammatically as a hypothetical protein associated with the growing tubule tip at the pointed (−) end of the actin filament. Process 2: Tubules shrink or retract in the (+) direction in a myosin-dependent fashion. This is supported by Figure 4A, where both latrunculin b and myosin XI-K expression increase the length and number of persistent tubules. We interpret this as arising from an absence of shrinkage and stabilization of untethered tubules. Process 3: Less persistent anchor/growth sites can move, perhaps recruited at branch points in the actin network, indicated here by the presence of an ARP2/3 complex. There are fewer driven junctions after latrunculin b and myosin XI-K expression (Figures 2 and 3), as indicated by persistent kinks. Blind-end tubules in Figures 2 and 3 may also be anchor sites that cannot move far from the point of origin but have tubules attached. Note that three-way junctions do not require tethering to an anchor site. Process 4: Golgi do not regulate all of the growth or shrinkage of ER tubules. After BFA treatment, which deconstructs the Golgi, new ER tubules still grow and shrink (Figure 7). However, since the moving ER and moving Golgi remain closely associated, an interdependence of ER membrane movement and Golgi movement seems likely. Process 5: Myosin mediates ring closure of polygons. The association of myosin with an unanchored three-way junction is postulated, and the special nature of the junction is inferred. The observation that intact rings of the polygonal network are persistent after myosin XIK tail expression (Figures 2D to 2F) and latrunculin b treatment (Figures 3D to 3F) is evidence to support this part of the model. Process 6: Actomyosin dependence of polygon filling. Removal of myosin may result in the collapse of the corner with high angle of curvature and polygon filling, leading to cisternae and giving rise to the larger cisternal area and larger number of cisternae after myosin XIK tail expression (Figure 4C).
ER Tubule Growth/Shrinkage
Since it is not clear how ER tubules and microfilaments mutually orient themselves during growing/shrinking cycles, the relative roles of myosin and actin polymerization in ER tubule remodeling remain speculative. However, generating a model that helps explain the results in the context of what is currently known about cytoskeletal dynamics is desirable. The central features of the model (Figure 8) are that the ER is centered on anchor/growth sites and moves along microfilaments, mainly through its attachment to a myosin such as XIK, but also via actin polymerization. This generates tubule nonpersistency through cycles of tubule elongation and shrinkage (Figure 8, processes 1 to 3) as well as mutual sliding of tubules generating polygonal ring closure (Figure 8, process 5). Both myosin XIK tail coexpression with GFP-HDEL and latrunculin b treatments diminish dynamic, exploratory tubules by blocking either tubule shrinkage or growth (Figure 8, processes 1 to 3). This is supported by Figure 4A, where both latrunculin b and myosin XI-K expression increase the length and number of persistent tubules. Inhibition of growing/shrinking cycles of tubules after F-actin has depolymerized is supported by the large number of blind ended tubules in Figures 2 and 3, which may have started a cycle but not completed it. We further postulate that there is a polarity for ER tubule remodeling and the actomyosin system (Figure 8, processes 1 to 3 and 5); growth is mainly driven by actin polymerization, as supported by the observation that the rate of in vivo actin polymerization (1.7 μm/s; Staiger et al., 2009) is very close to the rate of tubule growth previously reported (1.34 ± 0.54 μm/s; Sparkes et al., 2009b), while shrinkage is mediated by (+) end-directed myosin processivity. Myosins are classically defined as (+) end-directed motors. Although this is true for a tobacco myosin (Tominaga et al., 2003), the processivity and directionality of Arabidopsis myosins is not known at this time.
Based on the BFA studies presented here, ER tubule remodeling still occurs in the absence of Golgi bodies. Therefore, the potential role of ER tubule growth in Golgi body movement is also depicted whereby a physical link, be it direct membrane continuity or interacting protein tethers, between the two organelles results in interdependent movement (Figure 8, process 4).
Three-Way Junction and Anchor Sites Are Stabilization Factors to Maintain Tubules
Three-way junctions and anchor/growth sites are evident under all treatment conditions. Through micromanipulating Golgi bodies, we showed that the underlying ER did indeed remodel and resulted in the identification of ER anchor sites (Sparkes et al., 2009b). However, it is unclear at this time whether all anchor sites are indeed growth sites and vice versa. The data presented here indicate two populations of anchor sites/growth sites; a stable persistent group and a transitory nonpersistent group. Upon latrunculin b treatment, the latter become persistent, resulting in a significant increase in number over the other treatment conditions (Figure 4B). Therefore, perhaps an actin-dependent process is required to determine the extent of movement of anchor sites (Figure 8, process 3). Myosins may also be involved in the movement of tubules toward moving anchor points but do not affect the movement of anchor sites as much as latrunculin b because the number of persistent punctae do not increase after truncated myosin XIK expression (Figure 4B).
Proteinaceous factors regulating tubule stability can also be tested using this system. For example, in mammals, CLIMP63 (cytoskeletal linking membrane protein p63) is required to interact with microtubules to stabilize the cortical ER network (see review in Vedrenne and Hauri [2006] and references therein; Klopfenstein et al., 1998). Growth of tubules in animal systems is also dependent on integral ER membrane proteins (e.g., stromal interaction molecule 1) that bind the plus end of growing microtubules (Grigoriev et al., 2008). By isolating analogous actin-dependent factors in plants, such as the hypothetical minus-end tip associated complex in Figure 8, persistency maps can determine which proteins are required for tubule, anchor point, and three-way junction stability.
The model presented for cortical ER dynamics in higher plants relies heavily on myosin and actin dynamics. Therefore, one assumption would be that upon actin depolymerization, the ER network would disintegrate; however, it remained in place over the time frame of the experiment albeit with altered tubule length/dynamics/cisternalization (see Boevink et al., 1998). We hypothesize that the anchor points, which might be connected to the plasma membrane, are required to hold the ER network in place and that the tubules are stabilized by accessory scaffolding or coat proteins in the absence of actin. This is shown in the model in Figure 8, where the tubules forming the central polygon are tethered between anchor/growth sites in the absence of directly underlying microfilaments.
Tubule/Cisternal Transitions
The actomyosin system also appears to be involved to some extent in tubulation versus cisternalization as latunculin b treatment and overexpression of the myosin XIK tail domain resulted in increased persistent cisternae (Figure 4C) compared with control and other treatment conditions. Based on the large number of observed persistent polygonal structures (Figures 2 and 3) compared with the control (Figure 1), we propose that the actomyosin system is also required for polygon remodeling. This is achieved by the relative lateral sliding of newly formed interconnected tubules (Figure 8, process 5). Actomyosin involvement in polygon ring closure (Figure 8, process 5) may have a flip side. In the absence of functional actomyosin, branch sites making up the polygon ring may become unstable and the inner side of the tubule corner, where there are high degrees of curvature, would fill with membrane, causing more cisternalization (Figure 4C) through polygon filling (Figure 8, process 6).
The Effect of the Actomyosin System on ER Membrane Protein Mobility
The data presented here, based on the mobility of the transmembrane domain of calnexin (CXN) fused to GFP, indicate that ER remodeling and the direction of movement of the surface membrane could be linked but are not linked to the magnitude of the diffusive movement. Initial membrane diffusion rates (t1/2) are similar in control to a latrunculin b–treated immotile ER network system. Latrunculin treatment and overexpression of myosin XIK tail domain remove the directional restriction on diffusion, while restricting the available amount of molecules available for diffusion (mobile fraction, Tables 1 and 2).
We hypothesize that native myosin XIK might naturally maintain a high mobile fraction of the CXN-GFP construct in the ER membrane by destabilizing actin-membrane linkages during shrinkage (Figure 8, process 2) or outgrowth of the membrane (Figure 8, process 3). The eYFP-XIK myosin tail domain is present throughout the cytosol and could therefore directly interact with the cortical ER, producing a lower mobile fraction of ER membrane proteins by interfering with the activity of native myosin XIK. Alternatively, instead of interfering directly with native myosin, the tail domain could remove an active component required for membrane flow (through titration of accessory factors) or introduce a rigid locked cytoskeletal scaffold on the ER surface causing a barrier to free diffusive movement of proteins.
Latrunculin b treatment decreased the mobile fraction of ER membrane CXN fused to paGFP/GFP in the photoactivation experiments but not in the FRAP experiments. Photoactivation may target either tubules or cisternae (both are invisible prior to photoactivation), while FRAP targets preselected cisternae. Latrunculin b treatment produces more persistent tubules (Figures 2 to 4), stabilized perhaps in a way that would diminish the mobile fraction of protein in the membrane. Latrunculin b also produces cisternae with many peripheral punctae (Figures 3H and 3I, arrows) that may be stabilized by myosins, while the center of the cisternae remains relatively unaffected, with no apparent decrease in mobile fraction, as detected by FRAP.
Comparison of the results of this study with an earlier one using a similar technique to evaluate ER membrane dynamics (Runions et al., 2006) shows an apparent disparity in the diffusion rate of CXN-paGFP. Half-times of recovery in the previous study were reported to be 5 to 10 times longer than in this study, while mobile fraction percentages are comparable. Reexamination of the older data shows that in the previous study, entire small cisternal regions of ER were photoactivated so that diffusion of activated paGFP was only possible through connecting tubules of small radius. This resulted in a bottleneck effect and slower movement of GFP away from the activation region than measured in this study, where FRAP and photoactivation experiments were performed on the tubular network of ER or larger cisternal sheets that allowed a steeper diffusion gradient of GFP.
This study builds on the previous work by establishing the diffusive, nonmotorized nature of movement of ER membrane protein even during rapid ER remodeling. Previous work (Runions et al., 2006) demonstrated a directionality to this movement that can be seen in Figures 6B and 6C (control) and Figures 6J and 6K (BFA treatment). The directionality of this diffusion is changed when the actomyosin system is perturbed (Runions et al., 2006; Figures 6D, 6E, 6G, and 6H). Here, we show that the average rate of movement itself is not affected by the actomyosin network, indicating nonmotorized and diffusive movement. However, occasional fast, apparently nondiffusive movements were monitored but were infrequent in comparison to diffusional movements. A better quantification is required to determine vectorial, directional diffusive versus apparent active movements. The fact that directional diffusion occurs after BFA treatment helps establish that directional diffusion is not a property of tubules alone. This process of directional diffusion within tubules (near one-dimensional) and cisternae (near two-dimensional) may provide a mechanism whereby proteins and metabolites can be more efficiently transferred through the cell with minimal expenditure of energy at rates that far exceed those of nondirectional diffusion in three dimensions (Adam and Delbruck, 1968; Loverdo et al., 2008; Mirny, 2008; Pickard, 2003).
In conclusion, we have found that the cortical ER network in tobacco epidermal cells undergoes rapid remodeling that is coupled to the directionality but not the magnitude of ER surface flow. ER remodeling is an actomyosin-dependent process and is independent of microtubules. Quantification of ER tubule growth, cisternalization, and recognition of anchor/growth sites has led to a model defining the basic principles behind remodeling of the plant cortical ER in leaf epidermal cells. Additionally, ER network remodeling occurs in the absence of Golgi bodies, thereby addressing the long-held postulate that Golgi body movement might drive ER tubule growth. Future work using this model and persistency mapping will help identify factors required for macro- and microscopic changes in ER network remodeling.
METHODS
Plant Material and Constructs
Nicotiana tabacum plants were grown according to Sparkes et al. (2005). Fluorescent protein fusion constructs including the ER marker GFP-HDEL (Batoko et al., 2000), GFP-FABD2 (Sheahan, et al., 2004), GFP-TUA6 (Ueda et al., 1999), eYFP- and mRFP-myosin XIK tail domain (eYFP-XIK and mRFP-XIK; Sparkes et al., 2008), eYFP-XIJ tail domain (eYFP-XIJ; Avisar et al., 2009), transmembrane domain of calnexin (CXN-paGFP) (Runions et al., 2006), GFP transmembrane domain of CXN (GFP-CXN; Irons et al., 2003), and sialyl transferase signal anchor sequence-mRFP (ST-mRFP; Saint-Jore et al., 2002) were all infiltrated according to Sparkes et al. (2006) with an optical density of 0.1 except for GFP-FABD2 at 0.02 and GFP-HDEL, CXN fusions, and ST-mRFP, which required 0.04 optical density. The mRFP-XIK tail domain-pB7WGR2 construct was generated by Gateway cloning using the entry vector XIK tail-pDonor 207 (Sparkes et al., 2008).
Sample Preparation and Image Acquisition
The ER in the outermost cortical region of adaxial leaf epidermal pavement cells was imaged. Where stated, leaf segments of ∼25 mm2 were incubated either in BFA (100 μg/mL) for 3 h or in latrunculin b (25 μm) or oryzalin (14 μm) for 30 min prior to imaging.
Dual imaging of YFP and GFP was done using multitracking in line switching mode on a Zeiss LSM510 Meta confocal microscope. GFP was excited with the 458-nm line, and eYFP with the 514-nm line of an argon ion laser using a 458/514 dichroic mirror, and the subsequent emission was detected using 470- to 500-nm and 560- to 615-nm band-pass filters. Similarly, GFP and mRFP emission was captured using multitracking in line switching mode. GFP was excited with a 488-nm argon laser and mRFP with a 543-nm laser and their emissions detected using a 488/543 dichroic mirror and 505- to 530-nm and 560- to 615-nm band-pass filters. All imaging was performed using a ×63 1.4–numerical aperture oil immersion objective.
For persistency mapping, time-lapse images of GFP-HDEL were captured using a 2- to 3-μm pinhole, 512 × 512 pixel resolution, and ×2.3 digital zoom. To reduce noise, 4× line averaging was used. The scan rate was increased by imaging a 955- to 960-μm2 region of interest so that 50 frames per 80 s were captured (0.63 frames/s). For samples where cells were coexpressing a fluorescent myosin tail domain and GFP-HDEL, coexpression was verified before time-lapse imaging of the GFP-HDEL alone was performed.
FRAP and Photoactivation
For FRAP experiments, five prebleach scans were captured using settings as for GFP-HDEL (above) with the 488-nm laser transmission set to 1% transmission prior to bleaching of a 10- to 11-μm2 spot using 50 iterations of a 405-nm diode laser set to 100% transmission. Fast intensity recovery required a high scan rate (4.57 frames/s); therefore, time-lapse imaging was performed using 256 × 256 pixel resolution and 2.8× digital zoom.
For photoactivation of CXN-paGFP, 30 prephotoactivation scans were captured using settings as for GFP-HDEL (above) with the 488-nm laser transmission set to 1% transmission prior to activation of the 8- to 10-μm2 spot using five iterations of the 405-nm diode laser set at 100% transmission. Inherent high mobility of the ER required a high scan rate. Postactivation time-lapse imaging was therefore performed using 128 × 128 resolution (6.8 frames/s) and 2.8× digital zoom with the 488-nm laser set at 1% transmission.
Mean fluorescence intensity was calculated (Zeiss LSM software) within the bleach (FRAP) or activation (photoactivation) region during a 30- to 40-s postbleach or postactivation period. For FRAP, recovery of fluorescence occurred on a scale normalized from 0% for the first postbleach scan to 100% for the prebleach mean fluorescence level within the region of interest. For photoactivation, normalized intensity occurred on a scale between 0%, which represented mean preactivation intensity, and 100%, the immediate postactivation intensity (Graumann et al., 2007).
Normalized data from the time of bleaching/photoactivation were plotted against time using Prism 5 software (GraphPad Software). Nonlinear regression was used to fit curves.
The exponentially increasing curve used to fit the FRAP data took the form:
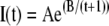 |
(1) |
The exponentially decreasing curve used to fit photoactivation data took the form:
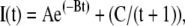 |
(2) |
where A, B, and C are parameters of the curve and e is the base of the natural logarithms.
Individual data sets within each experiment were fit independently to assess variation within groups, and data points from all sets within a group were fitted by sharing curve parameters in order to derive a master fit for graphical presentation. Comparison across experimental groups was done by deriving the mobile fraction of bleached/activated molecules (plateau) and the half-time of fluorescence recovery (t1/2). The plateau value was the highest-intensity value postbleach for FRAP and the lowest intensity value for photoactivation in each data set. To determine the plateau, the calculated parameters A, B, and C, which gave the best fit in Equations 1 and 2 were used to fit a theoretical curve to the duration of each data set. Half time of fluorescence recovery/decay is the time at which half of the fluorescence has recovered/decayed after bleaching/photoactivation. Half-fluorescence intensity (I1/2) was half the difference between minimum and plateau fluorescence values. Half-time of fluorescence recovery was interpolated at I1/2 using Prism 5 software (GraphPad Software).
Multivariate analysis of variance (MANOVA) was used to compare the families of curves derived in each of the experiments based on their plateau and t1/2 values using SPSS software. Wilks′ lambda was used as a test statistic for differences between experiments, and post-hoc tests to compare differences between individual pairs of experiments were done by computing Tukey's HSD statistic.
Modeling the Cortical ER in Tobacco Epidermal Cells
Persistency maps were generated in Image J (version 1.37v; Wayne Rasband, National Institutes of Health, Bethesda, MD). Beginning at frame number 5 of 50, every fifth frame was compared with frames 8 s (five frames) earlier with a Boolean “AND” operation and by subtraction. The subtraction result was subsequently subtracted from the AND result (with minor improvement), resulting in a set of 45 frames with objects that persisted, unmoving for at least 8 s. Binary conversion of objects in the 13 to 255 (the brightest pixels) gray level range was followed either by a closing operation (default in ImageJ, one iteration, one pixel count) that connected tubules that may have become discontinuous during the second subtraction operation or by an opening operation that removed tubules between cisternae. The closed binary images were skeletonized and summed, producing the tubule persistency map. The opened binary images were summed, producing the cisternal persistency map. To count and measure persistent tubules and cisternae, eight-bit persistency maps were segmented between 0 and 180 gray levels of 256 (the darkest tubules and cisternae). For tubule maps, the number and length (half the perimeter) of resultant persistent tubules were measured. For cisternae maps, the number and area of the resultant cisternae were measured.
To generate percentage area values and number per 100 μm2, the total imaged membrane for each movie was determined by summing the frames and segmenting between 15 and 255 gray levels (gray and black areas in Figures 1 to 3C). The brightest regions, between 50 and 255 gray levels (black areas in Figures 1 to 3C) look somewhat like persistent cisternae and punctae (Figures 1 to 3H) but do not show good spatial correlation. These regions may either be persistent or have high local fluorophore concentration.
Quantitation of the most persistent tubules, punctae, and cisternae was done, normalizing each movie to the total membrane area imaged (panel C in Figures 1 to 3) in the percentage area and number/100 μm2 values. Statistical comparison of the data was done using R version 2.7.1 (http://www.r-project.org/) using analysis of variance and Tukey's HSD two-way comparison at 95% confidence interval.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Oryzalin and Latrunculin b Depolymerize Microtubules and Actin, Respectively.
Supplemental Figure 2. Persistency of Tubules, Punctae, and Cisternae of Cortical ER in a Tobacco Epidermal Cell Expressing GFP-HDEL Treated with Oryzalin.
Supplemental Figure 3. Persistency of Tubules, Punctae, and Cisternae of Cortical ER in a Tobacco Epidermal Cell Coexpressing GFP-HDEL and eYFP-XIJ Myosin Tail Construct.
Supplemental Movie 1. Cortical ER Network Remodeling in Two Control Tobacco Epidermal Cells.
Supplemental Movie 2. Cortical ER Network Remodeling in Tobacco Epidermal Cells Coexpressing GFP-HDEL and the eYFP-XIK Tail Domain.
Supplemental Movie 3. Cortical ER Network Remodeling in Tobacco Epidermal Cells Treated with Latrunculin b.
Supplemental Movie 4. Cortical ER Network Remodeling in Tobacco Epidermal Cells Expressing GFP-HDEL Treated with Oryzalin.
Supplemental Movie 5. Cortical ER Network Remodeling in Tobacco Epidermal Cells Coexpressing GFP-HDEL and the eYFP-XIJ Tail Domain.
Supplemental Movie 6. Cortical ER Network Remodeling in Tobacco Epidermal Cells Treated with BFA.
Supplementary Material
Acknowledgments
We thank Janet Evins for technical support during the work, D.W. McCurdy for the GFP-FABD2 constructs, and T. Hashimoto for GFP-TUA6 constructs. I.S. was supported by a Leverhulme Trust grant (F/00382/G) to C.H. J.R. was funded by a Biotechnology and Biological Science Research Council grant (BB/F014074/1), and L.G. was funded by the International Research Travel/Award Grant and Faculty Development Leave programs at Texas A&M University.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: L. Griffing (griffing@tamu.edu).
Online version contains Web-only data.
References
- Adam, G., and Delbruck, M. (1968). Reduction of dimensionality in biological diffusion processes. In Structural Chemistry in Molecular Biology, A. Rich and N. Davidson, eds (San Francisco, CA: Freeman), pp. 198–215.
- Avisar, D., Abu-Abied, M., Belausov, E., Sadot, E., Hawes, C., and Sparkes, I.A. (2009). A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol. 150 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar, D., Prokhnevsky, A.I., Makarova, K.S., Koonin, E.V., and Dolja, V.V. (2008). Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 146 1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink, P., Martin, B., Oparka, K., Santa Cruz, S., and Hawes, C. (1999). Transport of virally expressed green fluorescent protein through the secretory pathway in tobacco leaves is inhibited by cold shock and brefeldin A. Planta 208 392–400. [Google Scholar]
- Boevink, P., Oparka, K., Santa Cruz, S., Martin, B., Betteridge, A., and Hawes, C. (1998). Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J. 15 441–447. [DOI] [PubMed] [Google Scholar]
- Brandizzi, F., Snapp, E.L., Roberts, A.G., Lippincott-Schwartz, J., and Hawes, C. (2002). Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: Evidence from selective photobleaching. Plant Cell 14 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.R., Stafford, P., and Langford, G.M. (2004). Short-range axonal/dendritic transport by myosin-V: A model for vesicle delivery to the synapse. J. Neurobiol. 58 175–188. [DOI] [PubMed] [Google Scholar]
- Coue, M., Brenner, S.L., Spector, I., and Korn, E.D. (1987). Inhibition of actin polymerization by latrunculin A. FEBS Lett. 213 316–318. [DOI] [PubMed] [Google Scholar]
- daSilva, L.L.P., Snapp, E.L., Denecke, J., Lippincott-Schwartz, J., Hawes, C., and Brandizzi, F. (2004). Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16 1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene, J.O., Coleman, J., Estrada de Martin, P., Pypaert, M., Anderson, S., Yates III, J.R., Ferro-Novick, S., and Novick, P. (2006). Rtn1p is involved in structuring the cortical endoplasmic reticulum. Mol. Biol. Cell 17 3009–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, P., Kim, J.W., Coleman, J., Walker, L., Dunn, B., Takizawa, P., Novick, P., and Ferro-Novick, S. (2003). Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J. Cell Biol. 163 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann, K., Irons, S.L., Runions, J., and Hawes, C. (2007). Retention and mobility of the mammalian lamin B receptor in the plant nuclear envelope. Biol. Cell 99 553–562. [DOI] [PubMed] [Google Scholar]
- Grigoriev, I., Gouveia, S.M., van der Vaart, B., Demmers, J., Smyth, J.T., Honnappa, S., Splinter, D., Steinmetz, M.O., Putney, J.W., Jr., Hoogenraad, C.C., and Akhmanova, A. (2008). STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol. 18 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton, S.L., Collings, D.A., and Allen, N.S. (2006). Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiol. Biochem. 44 95–105. [DOI] [PubMed] [Google Scholar]
- Hawes, C., Osterrieder, A., Hummel, E., and Sparkes, I. (2008). The Plant ER-Golgi interface. Traffic 9 1571–1580. [DOI] [PubMed] [Google Scholar]
- Hepler, P.K., and Wolniak, S.M. (1984). Membranes in the mitotic apparatus: Their structure and function. Int. Rev. Cytol. 90 169–238. [DOI] [PubMed] [Google Scholar]
- Irons, S.L., Evans, D.E., and Brandizzi, F. (2003). The first 238 amino acids of the human lamin B receptor are targeted to the nuclear envelope in plants. J. Exp. Bot. 384 943–950. [DOI] [PubMed] [Google Scholar]
- Kachar, B., and Reese, T.S. (1988). The mechanism of cytoplasmic streaming in characean algal cells: Sliding of endoplasmic reticulum along actin filaments. J. Cell Biol. 106 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein, D.R., Kappeler, F., and Hauri, H.P. (1998). A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 17 6168–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel, W.H., Quader, H., and Schnepf, E. (1990). Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: Short- and long-term observations with a confocal laser scanning microscope. Eur. J. Cell Biol. 52 328–340. [PubMed] [Google Scholar]
- Langhans, M., Niemes, S., Pimpl, P., and Robinson, D. (2009). Oryzalin bodies: In addition to its anti-microtubules properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum. Protoplasma 236 73–84. [DOI] [PubMed] [Google Scholar]
- Lee, C., and Chen, L.B. (1988). Dynamic behavior of endoplasmic reticulum in living cells. Cell 54 37–46. [DOI] [PubMed] [Google Scholar]
- Lichtscheidl, I.K., and Url, W.G. (1990). Organisation and dynamics of cortical endoplasmic reticulum in inner epidermal cells of onion bulb scales. Protoplasma 157 203–215. [Google Scholar]
- Lodish, H., Berk, A., Kaiser, C.A., Krieger, M., Scott, M.P., Bretscher, A., Ploegh, H., and Matsudaira, P. (2008). Molecular Cell Biology. (New York: W.H. Freeman and Co.).
- Loverdo, C., Benichou, O., Moreau, M., and Voituriez, R. (2008). Enhanced reaction kinetics in biological cells. Nat. Phys. 4 134–137. [Google Scholar]
- Mirny, L. (2008). Cell commuters avoid delays. Nat. Phys. 4 93–95. [Google Scholar]
- Nebenführ, A., Gallagher, L.A., Dunahay, T.G., Frohlick, J.A., Mazurkiewicz, A.M., Meehl, J.B., and Staehelin, L.A. (1999). Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 121 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov, V.V., Prokhnevsky, A.I., Avisar, D., and Dolja, V.V. (2008). Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis thaliana. Plant Physiol. 146 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard, W.F. (2003). The role of cytoplasmic streaming in symplastic transport. Plant Cell Environ. 26 1–15. [Google Scholar]
- Prokhnevsky, A.I., Peremyslov, V.V., and Dolja, V.V. (2008). Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation and organelle motility. Proc. Natl. Acad. Sci. USA 105 19744–19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader, H., Hofmann, A., and Schepf, E. (1987). Shape and movement of the endoplamic reticulum in onion bulb epidermis cells:possible involvement of actin. Eur. J. Cell Biol. 44 17–26. [Google Scholar]
- Ridge, R.W., Uozumi, Y., Plazinski, J., Hurley, U.A., and Williamson, R.E. (1999). Developmental transitions and dynamics of the cortical ER of Arabidopsis cells seen with green fluorescent protein. Plant Cell Physiol. 40 1253–1261. [DOI] [PubMed] [Google Scholar]
- Runions, J., Brach, T., Kuhner, S., and Hawes, C. (2006). Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J. Exp. Bot. 57 43–50. [DOI] [PubMed] [Google Scholar]
- Saint-Jore, C.M., Evins, J., Batoko, H., Brandizzi, F., Moore, I., and Hawes, C. (2002). Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J. 29 661–678. [DOI] [PubMed] [Google Scholar]
- Sheahan, M.B., Staiger, C.J., Rose, R.J., and McCurdy, D.W. (2004). A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiol. 136 3968–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes, I.A., Frigerio, L., Tolley, N., and Hawes, C. (2009. a). The plant endoplasmic reticulum: A cell-wide web. Biochem. J. 423 145–155. [DOI] [PubMed] [Google Scholar]
- Sparkes, I.A., Hawes, C., and Baker, A. (2005). AtPEX2 and AtPEX10 are targeted to peroxisomes independently of known endoplasmic reticulum trafficking routes. Plant Physiol. 139 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes, I.A., Ketelaar, T., Ruijter, N.C.A., and Hawes, C. (2009. b). Grab a Golgi: Laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10 567–571. [DOI] [PubMed] [Google Scholar]
- Sparkes, I.A., Runions, J., Kearns, A., and Hawes, C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sparkes, I.A., Teanby, N.A., and Hawes, C. (2008). Truncated myosin XI tail fusions inhibit peroxisome, Golgi and mitochondrial movement in tobacco leaf epidermal cells: A genetic tool for the next generation. J. Exp. Bot. 59 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin, A. (1997). The plant ER: A dynamic organelle composed of a large number of discrete functional domains. Plant J. 11 1151–1185. [DOI] [PubMed] [Google Scholar]
- Staiger, C.J., Sheahan, M.B., Khurana, P., Wang, X., McCurdy, D.W., and Blanchoin, L. (2009). Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol. 184 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley, N., Sparkes, I.A., Hunter, P.R., Craddock, C.P., Nuttall, J., Roberts, L.M., Hawes, C., Pedrazzini, E., and Frigerio, L. (2008). Overexpression of a plant reticulon remodels the lumen of the cortical endoplasmic reticulum but does not perturb protein transport. Traffic 9 94–102. [DOI] [PubMed] [Google Scholar]
- Tominaga, M., Kojima, H., Yokota, E., Orii, H., Nakamori, R., Katayama, E., Anson, M., Shimmen, T., and Oiwa, K. (2003). Higher plant myosin XI moves processively on actin with 35nm steps at high velocity. EMBO J. 6 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, K., Matsuyama, T., and Hashimoto, T. (1999). Visualisation of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma 206 201–206. [Google Scholar]
- Voeltz, G.K., Prinz, W.A., Shibata, Y., Rist, J.M., and Rapoport, T.A. (2006). A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124 573–586. [DOI] [PubMed] [Google Scholar]
- Vedrenne, C., and Hauri, H.P. (2006). Morphogenesis of the endoplasmic reticulum: Beyond active membrane expansion. Traffic 7 639–646. [DOI] [PubMed] [Google Scholar]
- Wolfram, S. (2002). A New Kind of Science. (Champaign, IL: Wolfram Media).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.