Abstract
As humans age, the tonic level of activity in sympathetic vasoconstrictor nerves increases and may contribute to age-related increases in blood pressure. In previous studies in normotensive young men with varying levels of resting sympathetic nerve activity, we observed a balance among factors contributing to blood pressure regulation, such that higher sympathetic activity was associated with lower cardiac output and lesser vascular responsiveness to α-adrenergic agonists, which limited the impact of high sympathetic activity on blood pressure. In the present study, we tested the hypothesis that older normotensive men would exhibit a similar balance among these variables (sympathetic nerve activity, cardiac output, and α-adrenergic responsiveness) but that this balance would be shifted toward higher sympathetic nerve activity values. We measured muscle sympathetic nerve activity, cardiac output, arterial pressure, and forearm vasoconstrictor responses in 17 older men and compared these with previous data collected in 14 younger men. Muscle sympathetic activity (burst incidence) was positively related to diastolic blood pressure in the older men (r=0.49; P=0.05); this relationship was not observed in young men. In addition, there was no relationship between cardiac output and muscle sympathetic activity (r=0.29; P>0.05) or between muscle sympathetic activity and vasoconstrictor responses in the older men (eg, norepinephrine: r=−0.21; P>0.05). Although our older subjects were normotensive, the relationship between muscle sympathetic nerve activity and diastolic blood pressure and the lack of “balance” among the other variables suggest that these changes with aging may contribute to the risk of sympathetically mediated hypertension in older humans.
Keywords: age, cardiac output, sympathetic vasoconstriction, total peripheral resistance, hypertension
The risk for hypertension increases as humans age in most ethnic backgrounds 1,2; however, the mechanisms for this increased risk remain incompletely understood despite decades of investigation. In animal models, both central and peripheral sympathetic neural mechanisms have key roles in several models of hypertension.3 Studies in humans also suggest that changes in sympathetic neural control of the circulation contribute importantly to age-related hypertension.4–7 It is evident that the activity of sympathetic vasoconstrictor nerves increases with age in humans,4,8 and in older subjects there is a positive relationship between sympathetic nerve activity and blood pressure.6
We have recently become interested in interindividual differences in muscle sympathetic nerve activity (MSNA) and the regulation of arterial pressure in humans.9,10 In young, normotensive men at rest, MSNA is reproducible in a given person but exhibits a wide variability across a population. MSNA can vary as much as 7- to 10-fold.11 However, among young men there is no relationship between resting levels of MSNA and blood pressure,6,9,10,12 although at rest there is tight coupling between changes in MSNA and arterial pressure via the baroreflex.4 Thus, a healthy young person with a high level of MSNA can have a blood pressure that is similar to a person with very low MSNA. In recent studies we investigated this paradox and demonstrated that, despite a positive relationship between MSNA and total peripheral resistance (TPR) in young men, normal blood pressure is maintained by an inverse balance between MSNA and cardiac output.9 In addition, high levels of MSNA are counterbalanced by decreased α-adrenergic receptor sensitivity; therefore, there is no effect of high MSNA on arterial pressure in young men.13 In older subjects, however, there is a positive relationship between MSNA and blood pressure, and, therefore, we questioned whether these balances would be observed in older men. Our hypothesis was that older men would exhibit a similar balance among MSNA, cardiac output, and α-adrenergic responsiveness but that this balance would be shifted toward higher MSNA values.
Methods
Subjects
The protocol for this study was approved by the institutional review board of the Mayo Foundation. Seventeen healthy older men volunteered to participate and gave written informed consent (Table 1 for demographics). The subjects were nonsmokers with no history of cardiovascular or other chronic diseases. All of the older subjects participated in a full cardiovascular screen, including a treadmill test with 12-lead ECG to rule out occult cardiovascular disease. In addition, data from our previous study in 14 young men13 were used for comparison with our present group of older subjects. All of the subjects were moderately active as assessed by a physical activity questionnaire, but no subject was highly trained or participated in >1.5 hours of moderate-intensity exercise per week. Furthermore, participants were excluded if their body mass index was ≥28 kg/m2. Subjects were asked not to consume anything within 2 hours before the experiment and not to consume caffeine or alcohol for 24 hours before the experiment.
Table 1.
Demographic Variables in the Young (n=14) and Older Men (n=17)
| Demographics | Young Men | Older Men |
|---|---|---|
| Age, y | 26±1 | 60±2* |
| Body mass, kg | 78.3±3.0 | 81.2±2.6 |
| Height, m | 1.78±0.02 | 1.77±0.01 |
| BSA, m2 | 1.94±0.04 | 1.99±0.04 |
| BMI, kg m2 | 24.6±0.7 | 25.8±0.7 |
Data show the mean ±SEM. BMI indicates body mass index; BSA, body surface area.
P<0.05.
Measurements
All of the studies were performed in a Clinical Research Unit laboratory at the Mayo Clinic maintained at ≈22°C. On arrival to the laboratory, subjects rested in the supine position during instrumentation. After local anesthesia with 2% lidocaine, a 5-cm, 20-gauge arterial catheter was placed in the brachial artery, using an aseptic technique. This catheter was connected to a pressure transducer placed at heart level and used for measurement of arterial pressure and for simultaneous local infusions of vasoactive substances. A 3-lead ECG was used for continuous monitoring of the heart rate.
Cardiac output was measured using an open-circuit acetylene uptake technique, as described previously.14 The cardiac output was estimated immediately after each maneuver using the calculation method described by Gan et al15 and Stout et al.16 This technique has been validated against direct Fick measurements of cardiac outputs over a range of values and has a variability of ≈7% at rest.14 The instrumentation period included a practice cardiac output measurement to familiarize the subject with the procedure.
MSNA was recorded with a tungsten microelectrode in the peroneal nerve, posterior to the fibular head, as described by Sundlof and Wallin.11 The recorded signal was amplified 80 000-fold, bandpass filtered (700 to 2000 Hz), rectified, and integrated (resistance-capacitance integrator circuit; time constant: 0.1 seconds) by a nerve-traffic analyzer.
Forearm blood flow (FBF) was measured using mercury-in-silastic strain gauge plethysmography.17 Briefly, a pediatric blood pressure cuff was placed around the wrist and inflated to suprasystolic levels (220 mm Hg) to arrest the circulation of the hand, and a venous occlusion cuff was placed on the upper arm and rapidly inflated to 50 mm Hg every 7.5 seconds, yielding 1 blood flow every 15.0 seconds. FBF was expressed as milliliters per 100 mL of tissue per minute.
Brachial Artery Drug Administration
Study drugs were adjusted for forearm volume and administered via the brachial artery catheter at total rates of 2 to 4 mL/min−1. Norepinephrine (norepinephrine bitartrate, Bedford Laboratories) was administered at 2, 4, and 8 ng (100 mL)−1/min−1 and tyramine (tyramine hydrochloride >98%, Sigma-Aldrich) at 3, 6, and 12 μg (100 mL)−1/min−1. Before norepinephrine administration, propranolol was administered at 10 μg/100 mL−1 of forearm volume per minute for 5 minutes to inhibit β-receptor–stimulating effects of norepinephrine.18 This dose has been documented to block forearm vasodilatation to isoproterenol.19 A “maintenance” dose of propranolol (5 μg/min−1) was then infused throughout the norepinephrine and tyramine trials. These doses and infusion rates were identical to those published in our previous study in young men.13
Protocol
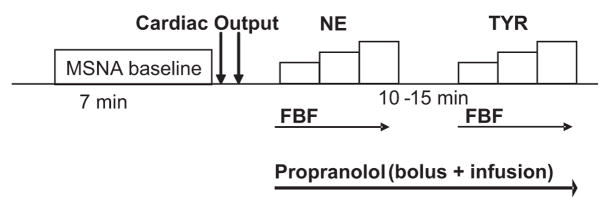
The time line for the experimental protocol is shown in Figure 1. After the placement of arterial catheters, ECG leads, and instrumentation for plethysmography, subjects rested supine during instrumentation for microneurography. Once a good electrode site for measurement of MSNA was found, 7 minutes of baseline data (cardiac output, arterial pressures, and MSNA) were recorded with the subject resting quietly. After this baseline period, forearm dose-response trials were performed for norepinephrine and tyramine, respectively, at the doses noted above. Each dose-response trial included 2 to 4 minutes of resting FBF measurement, followed by 2-minute infusions of each dose. We waited ≈15 minutes between trials to allow the FBF to return to baseline.
Figure 1.
Time line for experimental protocol. Resting baseline values for cardiac output, arterial pressures, and MSNA were measured before FBF dose-response relationships were evaluated for norepinephrine (NE) and tyramine (TYR; see text for details).
Data Analysis
Data were sampled at 240 Hz and stored on a personal computer for offline analysis. MSNA, heart rate, mean arterial pressure, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were assessed as 4-minute averages at the end of the initial baseline period. Heart rate variability was evaluated as the SD of resting heart rate in each group of subjects. Sympathetic bursts in the integrated neurogram were identified by a custom-manufactured semiautomated analysis program9,20; burst identification was controlled visually by a single investigator. The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
FBF was determined from the slope of the plethysmographic recording during venous occlusion.17 FBF data are presented as an average of 4 measurements during the last minute of each dose of drug infusion, when steady-state vasoconstriction was observed. Vasoconstrictor responses were analyzed both as absolute Δs and as the percentages of change in FBF from baseline.
Statistics
We evaluated the relationships of MSNA with vascular and hemodynamic variables in each group using a Pearson product moment correlation coefficient. On the basis of our previous data in younger men, we calculated that we had 83% power to detect a correlation of 0.65. We evaluated differences between groups in neural and cardiovascular variables using a Student t test. Differences in blood flow responses to increasing doses of norepinephrine and tyramine between the older and younger men were measured using a 2-way ANOVA. We accepted statistical significance for P<0.05.
Results
Group-Averaged Data for Cardiovascular and Neural Variables in Men and Women
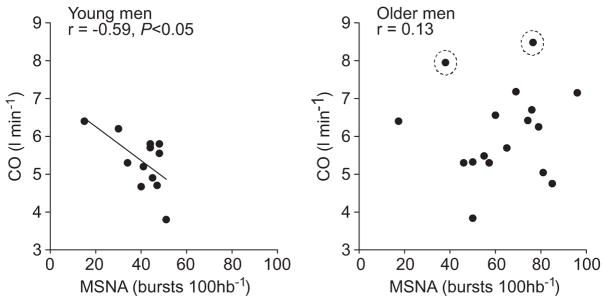
Table 2 shows average values for resting arterial pressures, heart rate, heart rate variability, cardiac output, and TPR in young and older men. Although resting heart rate was not different between groups, heart rate variability was lower in the older men compared with the younger men (P<0.05). There was a large variability in cardiac output, particularly among the older subjects. Two of the older subjects exhibited a very large cardiac output and stroke volume (see circled data points in Figure 3); when these were removed from the analysis, average cardiac output in the older men was 5.8±0.3 L/min−1. MSNA was greater in the older men compared with the younger men, both when expressed as burst frequency and as burst incidence. Furthermore, venous plasma norepinephrine was greater in the older men compared with the younger men.
Table 2.
Cardiovascular and Neural Variables in Young (n=14) and Older Men (n=17)
| Hemodynamics | Young Men | Older Men |
|---|---|---|
| Heart rate, beats/min−1 | 59±2 | 58±2 |
| Heart rate variability, s | 0.09±0.01 | 0.06±0.01* |
| Stroke volume, mL | 96±6 | 106±6 |
| Cardiac output, L/min−1 | 5.5±0.3 | 6.1±0.3 |
| SBP, mm Hg | 128±2 | 136±3* |
| DBP, mm Hg | 71±1 | 71±2 |
| MAP, mm Hg | 90±2 | 93±2 |
| TPR, mm Hg, L/min−1 | 16.7±0.8 | 15.8±1.0 |
| MSNA, bursts per min−1 | 24±2 | 37±2* |
| MSNA, bursts per 100 heart beats−1 | 41±3 | 64±4* |
| Norepinephrine, pg/mL−1 | 119±18 | 208±28* |
| Epinephrine, pg/mL−1 | 12±3 | 15±2 |
| FBF, mL (100 mL)−1/min−1 | 2.4±0.1 | 2.9±0.5 |
MAP indicates mean arterial pressure. Norepinephrine and epinephrine are venous samples. Data are mean±SEM. Data for catecholamines are from 10 young and 12 older men.
P<0.05.
Figure 3.
Regression analysis of MSNA with cardiac output in young (n=14) and older men (n=17). The inverse relationship between MSNA and cardiac output in young men was not observed in older men (r=0.13; P>0.05). This lack of relationship persisted when the older men (circled data points) with high cardiac outputs were removed from the analysis (burst incidence: r=0.29; burst frequency: r=0.35; P>0.05).
Interindividual Relationships Between Neural and Hemodynamic Variables in Young and Older Men
Relationships between MSNA and cardiovascular variables are demonstrated in Figure 2 through 5, with MSNA expressed as burst incidence (bursts per 100 heartbeats). The relationships between MSNA expressed as bursts frequency (bursts per minute) and hemodynamic variables showed similar trends and are reported in the text.
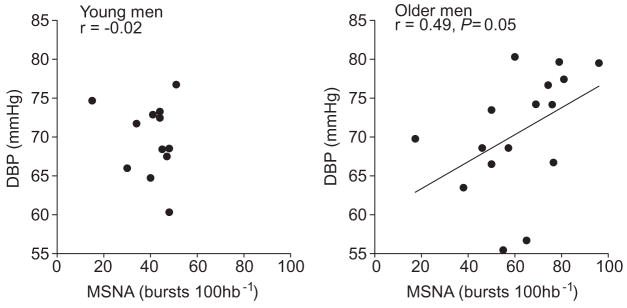
Figure 2.
Relationship of MSNA with DBP in young (n=14) and old men (n=17). There was no relationship of DBP with MSNA in the young men, but a positive relationship existed in the older men.
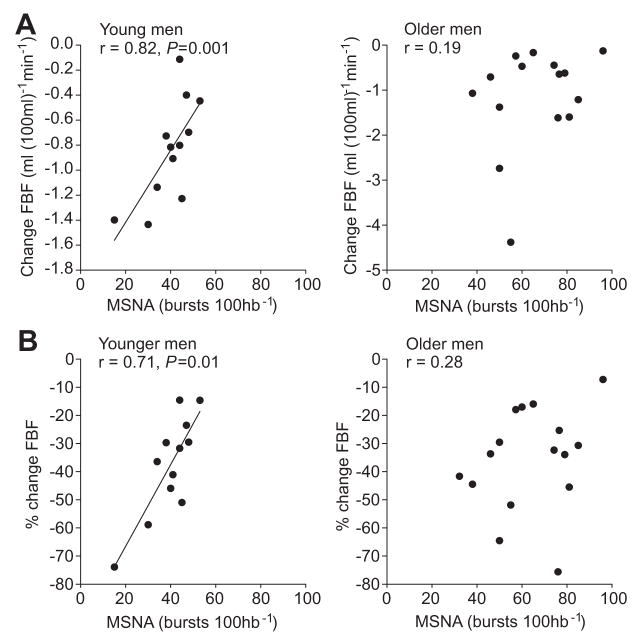
Figure 5.
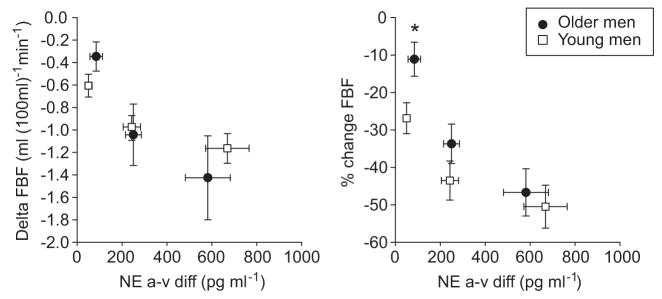
Regression analysis of MSNA with (A) change in FBF and (B) percentage change in FBF after intra-arterial infusion of norepinephrine (4 ng [100 mL−1]/min−1) in young (n=14; left) and older (n=16; right) men. The relationship between MSNA and change in FBF to norepinephrine that existed in the younger men did not exist in the older men.
In young men, there was no relationship between MSNA and DBP when MSNA was expressed as burst incidence (Figure 2) and burst frequency (r=−0.17), whereas in older men there was a positive correlation, which showed a strong trend for significance (burst incidence: P=0.05; Figure 2; burst frequency: r=0.47). As reported previously, there was a positive relationship between MSNA and TPR in the younger men (burst incidence: r=0.51; burst frequency: r=0.51; P<0.05). However, there was no relationship between MSNA and TPR in the older men (burst incidence: r=−0.06; burst frequency: r=−0.10; P>0.05). Figure 3 shows the relationship between MSNA and cardiac output in the young and older groups. As reported previously, MSNA was inversely correlated with cardiac output in the younger men (burst frequency: r=−0.64; P<0.05). Conversely, MSNA was unrelated to cardiac output in the older men (burst frequency: r=0.13; P<0.05). Because of the large variability seen in the cardiac output values in the older men, we also performed the analysis after removing the 2 subjects with the highest values (circled data in Figure 3) and found no significant relationship between cardiac output and MSNA (burst incidence: r=0.29, P=0.3; burst frequency: r=0.35, P=0.2). Furthermore, there was no correlation between heart rate and MSNA in the younger (burst incidence: r=−0.23; burst frequency: r=0.32; P>0.05) and older men (burst incidence: r=−0.24; burst frequency: r=0.20; P>0.05). Additionally, there was no correlation of MSNA with heart rate variability in the younger (burst incidence: r=0.30; burst frequency: r=−0.03; P>0.05) and = older men (burst incidence: r=−0.15; burst frequency: r=−0.25; P>0.05).
FBF Responses to α-Adrenergic Agonists
Because of problems with recording equipment, we excluded 1 older man from the FBF analyses. On average, resting FBF before norepinephrine and tyramine infusions was slightly higher in the older men (n=16) compared with the younger men (n=14), although the difference between groups was not significant (norepinephrine baseline, Table 2, P=0.3; tyramine baseline, 2.7±0.5 versus 2.4±0.1 mL (100 mL)−1/min−1, P=0.2). Therefore, we chose to express changes in FBF to drug infusions as a percentage change from baseline. Norepinephrine and tyramine caused dose-dependent reductions in FBF in all of the participants. Average percentage changes in FBF to norepinephrine were smaller in the older compared with the younger men (norepinephrine 2: −26±3% [older] versus −33±7% (younger); norepinephrine 4: −36±5% [older] versus −42±6% [older]; norepinephrine 8: −49±5% [older] versus −56±5% [younger]; PANOVA=0.04). Average percentage changes in FBF to tyramine were significantly smaller in the older men compared with the younger men (tyramine 3: −13±4% [older] versus 27±5% [younger]; tyramine 6: 36±5% [older] versus 43±5% [younger]; tyramine 12: 48±6% [older] versus 53±5% [younger]; PANOVA=0.04).
To address the potential for differences in norepinephrine release with tyramine among subjects, we measured the arteriovenous (a-v) difference in plasma norepinephrine during tyramine infusion in both groups. The a-v difference is reported as the absolute value of the difference, because our goal was to use this as an index of the amount of norepinephrine that the blood vessels were exposed to (ie, a positive value). Because of the vasoconstriction caused by tyramine infusion, we could not obtain venous blood from all of the participants; therefore, we were able to compare a-v differences of 12 older men with those of 10 younger men. Tyramine induced significant increases in the a-v difference of norepinephrine in both groups; however, the a-v difference was not different between the older men and younger men at all of the doses (tyramine 3: 92±21 pg/mL−1 [older] versus 50±12 pg/mL−1 [younger]; tyramine 6: 256±39 pg/mL−1 versus 243±38 pg/mL−1; tyramine 12: 550±110 pg/mL−1 versus 668±97 pg/mL−1).
Figure 4 shows changes in FBF plotted against the a-v difference of norepinephrine during tyramine infusion. Changes in FBF in response to tyramine infusion were not different between the 2 groups of men when expressed as absolute changes (PANOVA>0.5). However, when changes were expressed as a percentage of the baseline flow, the older men had a significantly smaller change in FBF at all of the levels of tyramine infusion (PANOVA<0.05).
Figure 4.
Vasoconstrictor responses to tyramine (TYR) as a function of the a-v difference in norepinephrine (NE) in young (n=10) and older (n=12) men. Changes in FBF expressed in absolute numbers (left) and percentage (right). The older men exhibited a blunted vasoconstriction vs younger men to TYR-induced release of NE when FBF was expressed as a percentage change (PANOVA<0.05). However, the difference was only significant at the lowest TYR dose. Data show the mean±SEM; *P<0.05.
MSNA and FBF Responses to α-Adrenergic Stimulation
In young men, as reported previously, the change in FBF to norepinephrine was positively related to MSNA. This is shown for burst incidence for the middle dose of norepinephrine (4 ng [100 mL−1]/min−1) in Figure 5 (for burst frequency; r=0.63; P<0.05), such that individuals with high MSNA showed less vasoconstriction to a given dose of norepinephrine and vice versa. This relationship was not observed in the older men in the present study (burst incidence; Figure 5; burst frequency: r=−0.19; P>0.05).
Similarly, in young men, the change in FBF to tyramine was positively related to MSNA. For example, for the middle dose of tyramine (6 μg [100 mL−1]/min−1), individuals with a high MNSA exhibited a lower vasoconstriction, and those with lower MSNA has a higher vasoconstrictor response (burst incidence: r=0.56; burst frequency: r=0.62; P< 0.05). In older men, there was no relationship between MSNA and the decrease in FBF during tyramine (burst incidence: r=−0.19; burst frequency: r=−0.04).
Discussion
The major new findings of the present study are 2-fold. First, the relationships among MSNA, TPR, and cardiac output observed in young men are absent in older men. Second, the inverse relationship between MSNA and changes in FBF to α-adrenergic receptor agonists seen in young men was not seen in older men.
MSNA, TPR, and Blood Pressure
In previous studies, we have found that, in young normotensive men, higher levels of MSNA appear to be offset by lower cardiac output and decreased vascular α-adrenergic responsiveness9,13 so that there is no net effect of the level of MSNA on resting blood pressure (ie, in young men there is no relationship between MSNA and DBP). Conversely, in normotensive older men, we found a positive correlation between DBP and MSNA (Figure 2), which is consistent with the findings of Narkiewicz et al.6 However, despite a similar mean arterial pressure between young and older men and a relationship between DBP and MSNA in the older subjects, we found no relationship of TPR to MSNA in the older men. This suggests that MSNA contributes less to the overall level of resistance in the peripheral vessels in older men compared with younger men.
There are several possible explanations for the lack of relationship between TPR and MSNA in our older subjects. First, other circulating vasoconstrictors, eg, endothelin 1,21 may have larger contributions to TPR in older subjects. This would agree with the findings of Jones et al,5 who reported that, although older men have a greater autonomic support of blood pressure, increased sympathetic nerve activity was not a key contributor to TPR. Although α-adrenergic receptor responsiveness was decreased in our older men, the existence of a normal TPR in the face of increased MSNA makes it less intuitive to speculate that endogenous vasoconstrictors are increased in this group. In this context, there is a potential interaction between NO bioavailability and vasoconstrictor tone, and in older subjects this interaction may become more variable and influence the net impact of MSNA and changes in α-adrenergic responsiveness on TPR.22 For example, even modest levels of physical activity may enhance NO bioavailability in older subjects,23 and, conversely, higher levels of MSNA could increase oxidative stress in blood vessel walls and limit NO bioavailability.24 These changes with aging, including less ability of the baroreflex to buffer changes in arterial pressure,7 suggest that determinants of TPR become more complex in older men. In addition, if different mechanisms are affected in different subjects, systematic relationships to single factors may become difficult to detect. It should be noted that, in young women, there is also no relationship between MSNA and TPR, likely as a result of enhanced NO bioavailability and perhaps enhanced β2-mediated vasodilation10,25 associated with higher levels of MSNA.
MSNA and Cardiac Output
In young men, high levels of MSNA and TPR are balanced by a lower cardiac output and vice versa for low levels of MSNA.9,10 We originally hypothesized that baroreflexes might have a role in the cardiac output-MSNA balance that we observed in young men.9 This could be via 1 of 2 mechanisms: first, it is possible that cardiopulmonary receptors cause reflex inhibition of MSNA with higher cardiac output (ie, higher cardiac volume). It is unlikely that heart rate regulation, per se, contributes to this relationship, because neither resting heart rate nor heart rate variability showed consistent relationships with MSNA in either younger or older men in this study. Second, blood flow through baroreceptive regions may have a pressure-independent effect on baroreflex-afferent input into the central nervous system.26 Higher flow (via higher cardiac output) would, therefore, cause greater afferent input and greater efferent sympathoinhibition. Because aging is known to blunt arterial cardiac-baroreflex sensitivity (but not sympathetic-baroreflex sensitivity),27 a potential mechanism for the uncoupling between cardiac output and MSNA in our older subjects may be attributable to a decrease in responsiveness of the cardiac baroreflex.27,28 In addition, it is unknown whether aging influences any potential blood flow–sensitive elements of baroreflex function. Along these lines, it is possible that baroreceptor control of heart rate and blood pressure becomes less coupled with aging in a way that disrupts the inverse relationships among MSNA, TPR, and cardiac output observed in young men.
MSNA and α-Adrenergic Receptor Responsiveness
The ultimate effect of sympathetic nerve activity depends on the strength of the activity and the vascular response to that activity. On average, MSNA is increased in older compared with younger men (although it can be seen in Figure 4 that there is some overlap between groups). However, the vascular response to α-adrenergic agonists is decreased in older compared with younger men in the forearm29 and leg.30 The identification of cause and effect for the increased sympathetic nerve activity and decreased α-adrenergic responsiveness in older men is complex. One logical possibility is that increases in sympathetic nerve activity lead to decreased α-adrenergic sensitivity via agonist-mediated receptor down-regulation. Alternatively, the increased sympathetic nerve activity in older individuals could be a response to the decrease in vascular adrenergic sensitivity (ie, more sympathetic activity is “required” for a given level of vascular tone).
Figure 5 shows the relationships in young and older men between resting MSNA and vascular responses to norepinephrine. As reported previously, vasoconstrictor responses in young men with higher MSNA were less than those with lower MSNA, suggesting that α-adrenergic responsiveness helps to balance the impact of varying levels of sympathetic nerve activity on blood pressure. Unexpectedly, there was no relationship between baseline MSNA in our present group of older men and the extent of vasoconstriction (expressed either as absolute change in FBF or as percentage of change). As noted above, it is possible that other endogenous vasoconstrictors that increase with age in men, eg, endothelin 1,21 offset the balance between α-receptor sensitivity and the level of MSNA. In addition, age-related changes in NO bioavailability might further limit this relationship.22,31
Limitations
Our study focuses on correlational analyses to investigate how older men maintain arterial pressure in the face of increased levels of MSNA. Therefore, the mechanisms that underlie the lack of balance between neural and hemodynamic variables in this group of older men are not established. Consequently, we can only postulate on the possible mechanisms that might be involved in this lack of relationship. Furthermore, the present data do not provide information about the roles of specific α-adrenergic receptor subtypes in the balance of arterial pressure and sympathetic activity. Another limitation to the current study would appear to be the high levels of resting cardiac output observed in some of our older subjects. First, resting cardiac output measured using any technique is more variable than measurements made during exercise.14 Second, the range of values that we report in both older and younger subjects is consistent with values obtained from classic invasive studies completed in similar age groups in the 1960s.32 Finally, if we assume that several of the high cardiac output values obtained represent outliers, excluding these subjects from the analysis does not change our fundamental conclusions.
Although our goal in the present study was not to determine the cause of the increase in resting MSNA with aging, previous studies have indicated that waist circumference or abdominal adiposity appear to have important causative roles in age-related chronic sympathoexcitation.33,34 We did not evaluate the relationship between waist circumference or abdominal fat and MSNA in the present study, although this could provide important additional information regarding the relationships that we have reported.
With regard to the clinical interpretation for hypertension, our data show changes in the relationships that could affect either SBP (ie, cardiac output versus MSNA) or DBP (TPR versus MSNA). In older individuals, systolic hypertension appears to be the primary hypertensive phenotype.35 Although our older men were not hypertensive, they did exhibit a slightly higher SBP compared with the young men. Systolic hypertension is most often related to vascular stiffness,35 and, in the present study, SBP was not related to MSNA. Therefore, our data are limited in their ability to draw conclusions regarding systolic hypertension specifically.
Perspectives
Our major findings in the present study were that MSNA was significantly related to DBP in older men but was not related to cardiac output or TPR. In our view, the clinical relevance of our findings lies in the lack of a balance between neural and hemodynamic contributors to blood pressure in older men. We know that aging is associated with increased risk for hypertension, and our present findings suggest that neither cardiac output nor vasoconstrictor responsiveness relate to sympathetic neural activity in a way that helps to minimize its effect on blood pressure. In addition, we were struck by the apparent greater variability of a number of responses in the older men. Thus, the lack of balance noted above, in combination with the influences of aging on vascular stiffening, loss of NO bioavailability, and increased levels of nonadrenergic vasoconstrictors, eg, endothelin, likely contribute to the increased risk of hypertension with advancing age. Our findings also help to explain the complex relationship between sympathetic outflow and blood pressure in humans.
Acknowledgments
We thank Shelly Roberts, Karen Krucker, Shirley Kingsley-Berg, Jessica Sawyer, and Nicholas Strom for their assistance in the conduct of the studies and analysis of the data. Finally, we thank the subjects for their participation.
Sources of Funding
This study was supported by National Institutes of Health grant HL083947 and Swedish Medical Council grant 12170, and CTSA-UL-1-RR-024150.
Footnotes
Disclosures
None.
References
- 1.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 3.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 4.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest: relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- 6.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 7.Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 8.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275:R1600–R1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- 9.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundlof G, Wallin BG. Muscle-nerve sympathetic activity in man: relationship to blood pressure in resting normo- and hypertensive subjects. Clin Sci Mol Med Suppl. 1978;4:387s–389s. doi: 10.1042/cs055387s. [DOI] [PubMed] [Google Scholar]
- 13.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 15.Gan K, Nishi I, Chin I, Slutsky AS. On-line determination of pulmonary blood flow using respiratory inert gas analysis. IEEE Trans Biomed Eng. 1993;40:1250–1259. doi: 10.1109/10.250579. [DOI] [PubMed] [Google Scholar]
- 16.Stout RL, Wessel HU, Paul MH. Pulmonary blood flow determined by continuous analysis of pulmonary N2O exchange. J Appl Physiol. 1975;38:913–918. doi: 10.1152/jappl.1975.38.5.913. [DOI] [PubMed] [Google Scholar]
- 17.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 18.Torp KD, Tschakovsky ME, Halliwill JR, Minson CT, Joyner MJ. β-Receptor agonist activity of phenylephrine in the human forearm. J Appl Physiol. 2001;90:1855–1859. doi: 10.1152/jappl.2001.90.5.1855. [DOI] [PubMed] [Google Scholar]
- 19.Johnsson G. The effects of intra-arterially administered propranolol and H 56–28 on blood flow in the forearm–a comparative study of two beta-adrenergic receptor antagonists. Acta Pharmacol Toxicol (Copenh) 1967;25:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 20.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 22.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 23.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res. 2004;94:37–45. doi: 10.1161/01.RES.0000109412.80157.7D. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- 26.Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circ Res. 1987;61:648–658. doi: 10.1161/01.res.61.5.648. [DOI] [PubMed] [Google Scholar]
- 27.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263:H798–H803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- 28.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- 29.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- 30.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 32.Julius S, Conway J. Hemodynamic studies in patients with borderline blood pressure elevation. Circulation. 1968;38:282–288. doi: 10.1161/01.cir.38.2.282. [DOI] [PubMed] [Google Scholar]
- 33.Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Physiol. 1997;272:E976–E980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- 34.Jones PP, Davy KP, Seals DR. Relations of total and abdominal adiposity to muscle sympathetic nerve activity in healthy older males. Int J Obes Relat Metab Disord. 1997;21:1053–1057. doi: 10.1038/sj.ijo.0800515. [DOI] [PubMed] [Google Scholar]
- 35.Duprez DA. Systolic hypertension in the elderly: addressing an unmet need. Am J Med. 2008;121:179–184. e3. doi: 10.1016/j.amjmed.2007.10.027. [DOI] [PubMed] [Google Scholar]