Abstract
Background
It is becoming increasingly clear that stress and metabolic signalling networks interact and that this interaction is important in plant responses to herbivory, pathogen attack, drought, cold, heat and osmotic stresses including salinity. At the interface between these two major signalling systems are the hormone abscisic acid (ABA) and signalling factors including protein kinases and transcription factors.
Scope
This briefing reviews links between ABA, stress and sugar signalling, focusing on the roles of sucrose non-fermenting-1-related protein kinases (SnRKs), SnRK1-activating protein kinases (SnAKs), calcium-dependent protein kinases (CDPKs) and ABA response element binding proteins (AREBPs, which are transcription factors). Links between stress and nitrogen / amino acid signalling are also described, including the roles of a protein kinase called general control non-derepressible (GCN)-2 in regulating protein synthesis through phosphorylation of the α-subunit of translation initiation factor-2 (eIF2α) in response not only to decreases in amino acid levels but also to a range of stresses. Evidence of a link between sugar and amino acid signalling is explored, with nitrate reductase being a target for regulation by both SnRK1 and GCN2 through different mechanisms; possible links between SnRK1 and GCN2 via a pathway including the protein kinase target of rapamycin (TOR)-1 are described. The significance of these interactions to the concept of signalling networks as opposed to simple cascades and pathways, and the importance of the subject in the context of the predicted increase in severity and range of stresses that plants will have to withstand as a result of global climate change are discussed.
Keywords: ABA (abscisic acid), AREBP (ABA response element binding protein), CDPK (calcium-dependent protein kinase), climate change, GCN2 (general control non-derepressible 2), metabolic signalling, protein kinase, signalling networks, SnAK (SnRK1-activating protein kinase), SnRK (sucrose non-fermenting-1-related protein kinase), stress signalling, transcription factor
INTRODUCTION
The ability of plants to tolerate herbivory, pathogen attack and abiotic stresses such as drought, cold, heat and osmotic stresses is a key factor in determining crop yield and quality. Cold, heat and water limitation all cause osmotic stress, so they have much in common with each other and with direct osmotic stress caused by, for example, exposure to high salt concentrations. Not surprisingly, therefore, the hormone abscisic acid (ABA), which is particularly associated with cellular osmotic stress, is also a common factor in mediating cold, heat and water stress responses. It is becoming clear that another common factor is metabolic regulation, partly perhaps because plants modify the balance between soluble and insoluble compounds in the cell in order to cope with osmotic stress. It is not surprising, therefore, that metabolic and stress signalling pathways interact and cross-talk, and a full understanding of plant stress responses will not be achieved without elucidation of the signalling networks that they form. The present briefing reviews what is known about the signalling factors that lie at the interface between metabolic and stress signalling.
LINKS BETWEEN ABA, STRESS AND SUGAR SIGNALLING
The first evidence of links between ABA, stress and sugar signalling pathways came from screens carried out by several independent groups to identify Arabidopsis mutants that were impaired in their response to sugar (sugar response mutants). Several of the mutants identified in these screens turned out to be ABA-related (reviewed by Halford, 2006). The discovery of these mutants led to the proposal of several hypotheses concerning cross-talk between ABA and metabolic signalling pathways. One proposed that sugar signalling could be directly mediated by ABA, a second that ABA could modulate sugar signalling by priming tissues to respond to sugars, and a third that ABA and sugar signalling, although essentially separate, could converge and cross-talk through specific factors. The identification of such factors became a key target because they would be expected to be involved in the control of developmental events such as germination that are sensitive to both ABA and sugars.
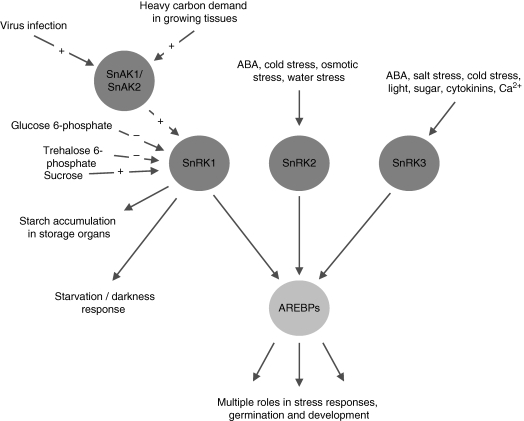
Further evidence of cross-talk between stress and sugar signalling pathways came with evidence that the protein kinase SnRK1 (sucrose non-fermenting-1-related protein kinase-1), a key metabolic regulator, is involved in stress signalling. SnRK1 regulates carbon metabolism through the modulation of enzyme activity and gene expression (Halford, 2006). It is closely related to the metabolic regulators 5′-AMP-activated protein kinase (AMPK) of mammals and protein kinase sucrose nonfermenting-1 (SNF1) of yeast (Saccharomyces cerevisiae), sharing 47 % amino acid sequence identity and showing similar substrate specificity. SnRK1 channels carbon into the starch biosynthetic pathway in storage organs through modulation of sucrose synthase and ADP-glucose pyrophosphorylase gene expression (McKibbin et al., 2006) and, in the case of ADP-glucose pyrophosphorylase, redox activation (Tiessen et al., 2003). Paradoxically, it is also required for the expression of α-Amy2 (α-amylase), a sugar-repressed gene that is involved in starch breakdown (Laurie et al., 2003). SnRK1 is involved in the re-allocation of carbon in response to herbivory (Schwachtje et al., 2006) and evidence from transcriptomic studies suggests that it is involved in a range of stress and darkness responses (Baena-González et al., 2007).
The metabolite or metabolites that are sensed by the SnRK1 system have been difficult to identify. However, glucose 6-phosphate and, more recently, trehalose 6-phosphate have been shown to inhibit SnRK1 (Toroser et al., 2000; Zhang et al., 2009), with trehalose 6-phosphate the more potent inhibitor of the two but requiring a hitherto unidentified intermediary factor to be present for inhibition to occur.
Evidence that ABA response element binding proteins are hubs linking metabolic and stress signalling pathways
One possible route by which SnRK1 could affect stress response pathways is through ABA response element binding proteins (AREBPs), a family of bZIP transcription factors. AREBPs contain highly conserved SnRK1 target sites, and peptides with amino acid sequences based on these sites are very good substrates for phosphorylation by SnRK1 (Zhang et al., 2008a).
AREBPs are unique to plants, so this activity for SnRK1 has evolved during plant evolution. Sub-families of protein kinases related to SnRK1 have also emerged during plant evolution: these are SnRK2 and SnRK3 (Halford and Hey, 2009), which are large and relatively diverse compared with SnRK1, with ten and 25 members, respectively, in Arabidopsis. There is now compelling evidence that members of the SnRK2 and SnRK3 sub-families of protein kinases are involved in ABA-mediated and other signalling pathways that regulate plant responses to drought, cold, salt and osmotic stresses. For example, an SnRK2-type protein kinase in Arabidopsis has been shown to control stress-responsive gene expression and improve drought tolerance when over-expressed (Umezawa et al., 2004), whilst expression of the entire SnRK2 sub-family of rice (ten members) is induced by osmotic stress and three of the sub-family members are also induced by ABA (Kobayashi et al., 2004). Similarly, PKABA1, an SnRK2 from wheat, mediates ABA-induced changes in gene expression in response to cold and other stresses (Gómez-Cadenas et al., 1999). Like SnRK1, SnRK2-type protein kinases have been shown to phosphorylate AREBPs (Kobayashi et al., 2005; Furihata et al., 2006).
SnRKs are closely related to calcium-dependent protein kinases (CDPKs); indeed, the SnRK3-type protein kinases are themselves likely to be calcium-dependent because they interact with calcineurin B-like (CBL) calcium-binding proteins (Guo et al., 2002). For this reason SnRK3s are also known as CBL-interacting protein kinases (CIPKs). The involvement of Ca2+ signalling in mediating responses to abiotic stress has been known and studied for many years, and several SnRK3-type protein kinases have been implicated in stress responses, although their substrates have not yet been identified. One of the 25 SnRK3-encoding genes of Arabidopsis [SnRK3·11, also known as CIPK24, and Salt Overly Sensitive 2 (SOS2)] is involved in conferring salt tolerance (Liu et al., 2000). Another SnRK3 (called SnRK3·1, CIPK15 or PKS3) functions as a global regulator of ABA responses, forming part of a calcium-responsive negative regulatory loop controlling ABA sensitivity (Guo et al., 2002). It is possible that SnRK3-type protein kinases phosphorylate AREBPs because a calcium-dependent activity from stressed Arabidopsis seedlings phosphorylates the same AREBP-based peptides that SnRK1 phosphorylates (Zhang et al., 2008a). This would make AREBPs potential convergence points for multiple signalling pathways (Fig. 1): hubs within an intricate signalling network (Halford and Hey, 2009).
Fig. 1.
ABA response element binding proteins (AREBPs) as hubs at the interface between metabolic and stress signalling networks, potentially targeted for phosphorylation by all three SNF1-related protein kinases (SnRK1 to 3) and with multiple roles in stress responses, germination and development.
SnRK3s have also been shown to interact with the type 2C protein phosphatases, abscisic acid insensitive-1 and -2 (ABI1 and ABI2), via a protein phosphatase interaction (PPI) domain in the regulatory region of the protein kinases and a protein kinase interaction (PKI) domain in the phosphatases (Guo et al., 2002; Ohta et al., 2003). Furthermore, part of the regulatory domain of SnRK3·11 is similar to that of the DNA repair and replication checkpoint protein kinase, CHK1, which also contains this conserved PPI domain (Ohta et al., 2003). CHK1 is required for cell cycle arrest in response to DNA damage and it has been reported that SnRK3·11 mutants show cell-cycle defects at the root meristem when subjected to salt stress (Liu et al., 2000). There is evidence to support a role for SnRK1 in cell-cycle control (Francis and Halford, 2006), and it is intriguing to consider the possibility that SnRK3s are also conduits for cross-talk between metabolic, stress and cell-cycle signalling.
SnRK2s and SnRK3s have diverged further from SNF1 and AMPK than has SnRK1. It is possible that they emerged as a result of duplication of a progenitor gene in ancestral eukaryotes before the divergence of plants, animals and fungi but were subsequently lost during animal and fungal evolution. However, we favour a hypothesis that they emerged only during plant evolution but then evolved rapidly as they took on new roles to enable plants to link metabolic and stress signalling.
CDPKs as conduits for cross-talk between metabolic and stress signalling
‘Classical’ CDPKs are also implicated in stress and ABA signalling (Hrabak et al., 2003). These protein kinases contain a calcium-binding, auto-regulatory domain, so do not require the interaction of a separate calcium-binding protein in the way that SnRK3 does. Binding of calcium ions to this domain increases kinase activity. Many CDPK genes themselves contain an ABA-response element in their promoter and, therefore not surprisingly, some have been shown to be induced at the transcriptional level by ABA.
SnRK1 and CDPKs have similar phosphorylation site requirements, with the minimal recognition motif for both generally given as a serine or threonine residue (SnRK1 much prefers serine) with a hydrophobic residue at position –5 and +4 with respect to the serine or threonine and a basic residue at –3 or (less favourably) –4 (Sugden et al., 1999b; Huang and Huber, 2001; Halford, 2006). However, a proline residue at position –4 selectively inhibits phosphorylation by CDPK relative to SnRK1, possibly by incorporating a tight turn in the substrate (Huang and Huber, 2001). This proline residue may also be involved in the recruitment of 14-3-3 proteins to the phosphorylation site, a prerequisite for regulation of some enzymes by SnRK1. The similarity in substrate specificities of SnRK1 and CDPK suggests that cross-talk could occur in both directions. In other words, CDPKs, which are usually associated with stress responses, could initiate signals that affect metabolic regulation, just as the metabolic regulator, SnRK1, has links with stress signalling factors. However, we are not aware of experimental evidence to support this hypothesis at present.
Evidence that biotic challenges initiate signals through metabolic signalling pathways
SnRK1, like its animal and fungal counterparts, AMPK and SNF1, is regulated in part by phosphorylation of a threonine residue in its so-called T-loop (Sugden et al., 1999a). Upstream kinases responsible for this activation remained elusive for many years, but two have been identified recently (Hey et al., 2007; Shen and Hanley-Bowdoin, 2006; Shen et al., 2009). These have been called SnRK1-activating kinase-1 and -2 (SnAK1 and SnAK2) (Hey et al., 2007) or geminivirus rep-interacting kinases (GRIK1 and GRIK2) (Shen and Hanley-Bowdoin, 2006). The GRIK name derives from the fact that they are expressed in response to geminivirus infection and interact with geminivirus replication protein AL1 (Shen and Hanley-Bowdoin 2006), suggesting that metabolic signalling pathways could respond to pathogen attack. They are also expressed in young tissues, and it has been suggested that they initiate metabolic responses to meet the demands of rapidly growing tissues and geminivirus-infected cells that have been induced to re-enter the cell cycle (Shen et al., 2009).
THE INTERFACE BETWEEN STRESS AND NITROGEN/AMINO ACID SIGNALLING
General regulation of the level of free amino acids and protein synthesis in all eukaryotic cells is achieved through the action of a group of regulatory protein kinases that bring about the reversible phosphorylation of the α-subunit of the eukaryotic translation initiation factor eIF2 (eIF2α). Four different eIF2α kinases are known to be able to phosphorylate eIF2α and thus to regulate protein synthesis in response to free amino acid levels and environmental stresses in this manner (reviewed by Halford, 2006). These are: double-stranded RNA-dependent protein kinase (PKR), PKR-like endoplasmic reticulum kinase (PERK), haem-regulated inhibitor (HRI) and general control non-derepressible-2 (GCN2). All four of these related eIF2α kinases contain a highly conserved kinase catalytic domain linked to a unique regulatory domain, and it is the regulatory domain that confers the ability of each eIF2α kinase to respond to a different, specific stimulus.
The distribution of eIF2α kinases varies within different groups of organisms; mammals, for example, contain all four different eIF2α protein kinases, whereas fungi and plants only contain one, namely GCN2, although there remains a degree of mystery over the existence of a PKR-like activity in plants that is discussed later.
GCN2 was originally characterized in yeast, where it functions to sense and respond to amino acid starvation. In this organism, amino acid starvation not only causes a general reduction in protein synthesis but also initiates a change in expression of a large number of genes involved in, amongst other things, amino acid biosynthesis; this response is known as general amino acid control (Hinnebusch, 2005). During general amino acid control, GCN2 physically senses the reduction of cellular amino acids through the interaction of uncharged tRNA with its regulatory domain, and is activated to phosphorylate eIF2α. eIF2 can bind either GDP or GTP, but only when bound to GTP is it able to attach methionyl–tRNA to the ribosome and transfer it to the 40S ribosomal subunit. Following attachment of the [eIF2.GTP.Met-tRNA] complex to the 40S subunit, the GTP is hydrolysed to GDP and inorganic phosphate. Phosphorylation of eIF2α inhibits the recycling of bound GDP to GTP and the rate of protein synthesis decreases.
There is a second aspect of general control of amino acid metabolism in yeast: GCN2-mediated phosphorylation of eIF2α under conditions of amino acid deprivation increases expression of a large set of genes, including many encoding enzymes of amino acid biosynthesis, through the action of a transcriptional activator, GCN4 (Hinnebusch, 2005). While phosphorylation of eIF2α by GCN2 represses translation of most transcripts, translation of the GCN4 transcript is increased through a ribosome re-initiation mechanism. This apparent paradox enables the de-novo production of amino acids during periods of starvation, thus helping the yeast cell to maintain homeostasis and survive during adverse conditions.
The molecular cloning of an Arabidopsis homologue of GCN2 (AtGCN2) was reported by Zhang et al. (2003). Recent experiments demonstrated that AtGCN2 does indeed function as an eIF2α kinase, phosphorylating Arabidopsis eIF2α at serine-52 in response to, for example, treatment with herbicides such as glyphosate, chlorsulfuron or IRL 1803 (Zhang et al., 2008b). This supports the hypothesis that general amino acid control is conserved, at least in part, in plants. However, no obvious candidate for a GCN4 homologue has been identified in plants, despite the extensive genome data now available (Halford, 2006). Furthermore, although genes encoding enzymes of amino acid biosynthesis in plants do respond to treatments that perturb amino acid metabolism, they appear to respond in similar fashion whether GCN2 is present or not (Zhang et al., 2008b).
It is possible that GCN2-dependent phosphorylation of eIF2α is involved in the regulation of expression of amino acid biosynthesis genes in plants but that other regulatory systems are able to compensate for GCN2 when GCN2 is not present. However, given that no plant GCN4 homologue has been identified, on balance the evidence to date suggests that the regulation of expression of genes of amino acid biosynthesis and other systems through a GCN4-like transcription factor does not occur in plants. Nevertheless, regulation of the translation of GCN4 mRNA in response to general amino acid control in yeast requires the presence of short open reading frames in the GCN4 transcript, upstream of the GCN4 coding sequence, and many plant genes contain similar upstream open reading frames (uORFs). These genes include a number that encode transcription factors (Kawaguchi and Bailey-Serres, 2005) and it is possible that, although these transcription factors do not have the same function as GCN4 in yeast, they are regulated in a similar fashion. Furthermore, promoter elements similar to that recognized by GCN4 are present in some plant genes, notably the prolamin storage protein genes of cereals in which they act as positive elements when nitrogen is in plentiful supply and negative elements when nitrogen is limiting.
GCN2-mediated phosphorylation of eIF2α as a general stress response
Studies in fungal and mammalian systems suggest that GCN2 may be activated and protein synthesis inhibited in response to stresses in addition to that of amino acid deprivation, including purine deprivation, exposure to UV-B, oxidative and osmotic stress, and glucose deprivation (Yang et al., 2000; Hinnebusch, 2005; Mascarenhas et al., 2008). In a recent study, the effect of some of these and other stresses on GCN2-dependent eIF2α phosphorylation was studied in Arabidopsis (Lageix et al., 2008): UV-B, cold-shock, wounding, methyl jasmonate, 1-aminocyclopropane-1-carboxylic acid (ACC), salicylic acid, and the purine analogue 8-azaadenine all caused GCN2-dependent phosphorylation of eIF2α, while treatment with salt, hydrogen peroxide or heat-shock did not. Methyl jasmonate and salicylic acid are signals of tissue injury and are involved in the activation of defence mechanisms in response to insect herbivores, so GCN2, like SnRK1, could be involved in the response to herbivory.
Links between carbon metabolic signalling and nitrogen/amino acid signalling
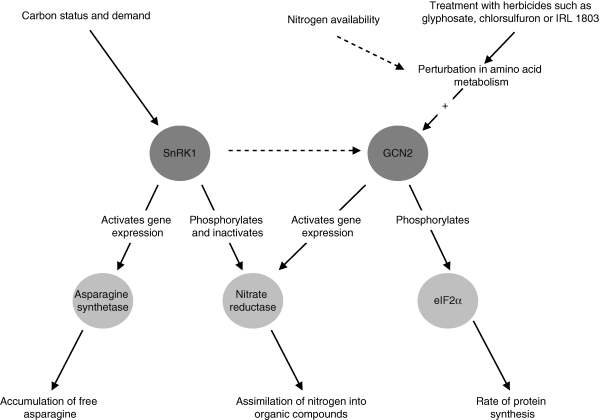
In plants, expression of many nitrogen-regulated genes is strongly influenced by sugar, suggesting an extensive interaction between sugar and nitrogen signalling pathways (Price et al., 2004). Such cross-talk seems logical because amino acids are, of course, based on carbon skeletons. SnRK1 may be known principally for its role in regulating carbon metabolism, but there are also routes through which it could affect nitrogen and amino acid metabolism (Fig. 2). Most obviously, nitrate reductase, the key enzyme for assimilation of inorganic nitrogen, is a substrate for SnRK1 in vitro (reviewed by Halford, 2006) and a recent study has provided strong evidence that it is regulated in part by SnRK1 in vivo (Polge et al., 2008). A CDPK has also been shown to phosphorylate nitrate reductase at the same target site (serine-543 in the spinach enzyme) (Douglas et al., 1998), and there is evidence that expression of a nitrate reductase gene, NIA1, of Arabidopsis is affected by GCN2 (its expression is reduced in a mutant lacking GCN2) (Zhang et al., 2008b), making nitrate reductase another convergence point for multiple signalling pathways (Fig. 2).
Fig. 2.
Interactions between carbon and nitrogen metabolic signalling networks. The link between SnRK1 and GCN2 and the effect of nitrogen availability on the GCN2 signalling pathway are hypothetical and therefore represented by dashed arrows. In animal and fungal systems the sugar signalling → GCN2 link comprises several factors, probably including the protein kinase TOR1.
Another route through which SnRK1 could affect nitrogen metabolism is through the regulation of asparagine synthetase gene expression: normal expression of one of the two SnRK1 genes of Arabidopsis has been shown to be required for sugar- and dark-responsive expression of an asparagine synthetase gene (Baena-González et al., 2007). Asparagine is required for protein synthesis, like any other amino acid, but free asparagine accumulates in response to a variety of abiotic and biotic stresses and to deficiencies in minerals, particularly sulphur, possibly acting as a nitrogen store when protein synthesis is impaired (Lea et al., 2007).
In other eukaryotic systems, GCN2 itself is a convergence point for carbon and nitrogen metabolic signalling pathways. In yeast, glucose limitation leads to an increase in GCN4 levels through the action of GCN2. This process is completely independent of the amino acid deprivation response (Yang et al 2000) and may involve TOR (target of rapamycin), a protein kinase that acts as a central regulator of cell growth in yeast in response to nutrient and growth factors (Schmelzle and Hall, 2000). In mammals, the SnRK1 homologue AMPK negatively regulates TOR (Avruch et al., 2006) and although more research is required in both yeast and mammalian systems to fill in gaps in the signalling pathways, this may be the route through which carbon metabolic signalling interacts with nitrogen/amino acid signalling in these systems.
A TOR homologue (AtTOR) has been identified in Arabidopsis and implicated in a number of plant processes including embryo development, meristem growth, osmotic stress responses and mRNA translation (Menand et al., 2002). Silencing AtTOR by RNA interference (RNAi) leads to an inhibition of translation initiation, with severe effects on shoot and root growth, while over-expressing it has the opposite effect (Deprost et al., 2007). TOR RNAi and over-expressing lines respond to herbicide-induced amino acid depletion in similar fashion, suggesting that TOR is not involved in the response to free amino acid depletion in Arabidopsis (Lageix et al., 2008). However, this does not mean that TOR and GCN2 do not interact in the response to other stimuli.
Phosphorylation of eIF2α in response to viral infection
AtGCN2 is the only eIF2α kinase-encoding gene in the Arabidopsis and rice genomes and it is present as a single copy (Halford, 2006). Screening of expressed sequence tag (EST) and genomic databases with the AtGCN2 nucleotide sequence has revealed the presence of similar genes and transcripts in wheat, barley, potato, soybean, sugar beet, sugarcane, Medicago, cotton, poplar, onion, lotus and Zinnia. In all cases there is no evidence to date of any plant species containing more than one copy of the GCN2 gene, or an additional gene encoding a different eIF2α kinase.
This is in stark contrast to the SnRK gene family, which, as described above, has burgeoned in plants and comprises 38 genes in Arabidopsis (Halford and Hey, 2009). It is also something of a mystery in that biochemical evidence of the existence of another eIF2α kinase, double-stranded RNA-dependent protein kinase (PKR), has been reported in plants. In humans and other mammals, PKR is activated in response to the accumulation of double-stranded RNA that is most commonly associated with viral infection, and a PKR-like activity (pPKR) was reported to be induced by viral infection of plants (Crum et al., 1988). Furthermore, a plant homologue of another protein kinase in the animal viral response pathway that involves PKR, P58IPK, has been identified (Bilgin et al., 2003). In animal cell cultures, P58IPK is active under normal conditions, suppressing PKR activity and thereby allowing protein synthesis to proceed normally. Under virus attack, P58IPK is switched off, PKR dimerizes and becomes active, eIF2α is phosphorylated and protein synthesis is shut down, leading eventually to programmed cell death (Lee et al., 1994).
One possible explanation of this was that the PKR and GCN2 activities had been consolidated in plants in a single protein kinase. However, in a recent study, phosphorylation of eIF2α could not be demonstrated in Arabidopsis plants infected with either Turnip crinkle virus or Turnip yellow mosaic virus, regardless of the presence or absence of AtGCN2 (Zhang et al., 2008b). The protein kinase responsible for the PKR-like activity reported in the earlier studies has therefore still to be identified, and the role of plant P58IPK requires further investigation.
CONCLUDING REMARKS
The hypothesis that signalling pathways often comprise multiple phosphorylation/dephosphorylation steps in order to allow amplification of a signal as it is passed down a protein kinase cascade has been replaced by one in which multiple steps in pathways have evolved to enable linking between pathways to form networks (reviewed by Halford and Hey, 2009). In these networks, key protein kinases, phosphatases and target transcription factors represent hubs on/from which multiple pathways converge and emerge. In this review we have described conduits through which carbon and nitrogen metabolic signalling networks can cross-talk with each other and with stress signalling networks. This is particularly pertinent in plants because plants are sedentary organisms that have to cope with stresses where they stand and have evolved sophisticated cellular response systems in order to do so. These responses often involve changes in metabolism.
Global climate change is predicted to change the nature and increase the severity of stresses that crops are exposed to, and lead to new pressures from weeds, pests and diseases. In the next few decades in high latitudes, for example, crops that have to tolerate cold stress in the early stages of their development may be faced with heat, water and osmotic stresses over the summer months that are much more severe than anything that they experience at present (Semenov and Halford, 2009). Elucidating and understanding metabolic and stress signalling networks and how they interact is therefore likely to become even more important as global climate change progresses.
ACKNOWLEDGEMENTS
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom and E.B. is supported by a studentship from the BBSRC.
LITERATURE CITED
- Avruch J, Hara K, Lin Y, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- Bilgin DD, Liu Y, Schiff M, Dinesh-Kumar SP. P58IPK, a plant ortholog of double-stranded RNA-dependent protein kinase PKR inhibitor, functions in viral pathogenesis and cell death. Developmental Cell. 2003;4:451–661. doi: 10.1016/s1534-5807(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Crum CJ, Hu J, Hiddinga HJ, Roth DA. Tobacco mosaic virus infection stimulates the phosphorylation of a plant protein associated with double-stranded RNA-dependent protein kinase activity. Journal of Biological Chemistry. 1988;263:13440–13443. [PubMed] [Google Scholar]
- Deprost D, Yao L, Sormani R, et al. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Reports. 2007;8:864–870. doi: 10.1038/sj.embor.7401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P, Moorhead G, Hong Y, Morrice N, MacKintosh C. Purification of a nitrate reductase kinase from Spinacea oleracea leaves, and its identification as a calmodulin-domain protein kinase. Planta. 1998;206:435–442. doi: 10.1007/s004250050419. [DOI] [PubMed] [Google Scholar]
- Francis D, Halford NG. Nutrient sensing in plant meristems. Plant Molecular Biology. 2006;60:981–993. doi: 10.1007/s11103-005-5749-3. [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proceedings of the National Academy of Sciences of the USA. 2006;103:1988–1993. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho T-HD, Walker-Simmons MK. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proceedings of the National Academy of Sciences of the USA. 1999;96:1767–1772. doi: 10.1073/pnas.96.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Song C-P, Gong D, Halfter U, Zhu J-K. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Developmental Cell. 2002;3:233–244. doi: 10.1016/s1534-5807(02)00229-0. [DOI] [PubMed] [Google Scholar]
- Halford NG. Regulation of carbon and amino acid metabolism, roles of sucrose nonfermenting-1-related protein kinase-1 and general control nonderepressible-2-related protein kinase. Advances in Botanical Research Including Advances in Plant Pathology. 2006;43:93–142. [Google Scholar]
- Halford NG, Hey SJ. SNF1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochemical Journal. 2009;419:247–259. doi: 10.1042/BJ20082408. [DOI] [PubMed] [Google Scholar]
- Hey S, Mayerhofer H, Halford NG, Dickinson JR. DNA sequences from Arabidopsis which encode protein kinases and function as upstream regulators of Snf1 in yeast. Journal of Biological Chemistry. 2007;282:10472–10479. doi: 10.1074/jbc.M611244200. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annual Review of Microbiology. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- Hrabak EM, Chan CWM, Gribskov M, et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiology. 2003;132:666–680. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J-Z, Huber SC. Phosphorylation of synthetic peptides by a CDPK and SNF1-related protein kinase. Influence of proline and basic amino acid residues at selected positions. Plant and Cell Physiology. 2001;42:1079–1087. doi: 10.1093/pcp/pce137. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Bailey-Serres J. mRNA sequence features that contribute to translational regulation in Arabidopsis. Nucleic Acids Research. 2005;33:955–965. doi: 10.1093/nar/gki240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori H. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Murata M, Minami H, et al. Abscisic acid-activated SnRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant Journal. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- Lageix S, Lanet E, Pouch-Pelissier MN, et al. 2008. Arabidopsis eIF2 alpha kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biology8: Article Number: 134. [DOI] [PMC free article] [PubMed]
- Laurie S, McKibbin RS, Halford NG. Antisense SNF1-related (SnRK1) protein kinase gene represses transient activity of an α-amylase (α-Amy2) gene promoter in cultured wheat embryos. Journal of Experimental Botany. 2003;54:739–747. doi: 10.1093/jxb/erg085. [DOI] [PubMed] [Google Scholar]
- Lea PJ, Sodek L, Parry MA, Shewry PR, Halford NG. Asparagine in plants. Annals of Applied Biology. 2007;150:1–26. [Google Scholar]
- Lee TG, Tang N, Thompson S, Miller J, Katze MG. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Molecular and Cellular Biology. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ishitani M, Halfter U, Kim C-S, Shu J-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proceedings of the National Academy of Sciences of the USA. 2000;97:3730–3734. doi: 10.1073/pnas.060034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas C, Edwards-Ingram LC, Zeef L, Shenton D, Ashe MP, Grant CM. 2008. Gcn4 is required for the response to peroxide stress in the yeast Saccharomyces cerevisiae. Molecular Biology of the Cell 19; pp. 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin RS, Muttucumaru N, Paul MJ, et al. Production of high starch, low glucose potatoes through over-expression of the metabolic regulator, SnRK1. Plant Biotechnology Journal. 2006;4:409–418. doi: 10.1111/j.1467-7652.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Menand B, Desnos T, Nussaume L, et al. Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proceedings of the National Academy of Sciences of the USA. 2002;99:6422–6427. doi: 10.1073/pnas.092141899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Guo Y, Halfter U, Zhu J-K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proceedings of the National Academy of Sciences of the USA. 2003;100:11771–11776. doi: 10.1073/pnas.2034853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polge C, Jossier M, Crozet P, Gissot L, Thomas M. β-Subunits of the SnRK1 complexes share a common ancestral function together with expression and function specificities; physical interaction with nitrate reductase specifically occurs via AKINβ1-subunit. Plant Physiology. 2008;148:1570–1582. doi: 10.1104/pp.108.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell. 2004;16:2128–2150. doi: 10.1105/tpc.104.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences USA. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MA, Halford NG. Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. Journal of Experimental Botany. 2009;60:2791–2804. doi: 10.1093/jxb/erp164. [DOI] [PubMed] [Google Scholar]
- Shen W, Hanley-Bowdoin L. Geminivirus infection up-regulates the expression of two Arabidopsis protein kinases related to yeast SNF1- and mammalian AMPK-activating kinases. Plant Physiology. 2006;142:1642–1655. doi: 10.1104/pp.106.088476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Reyes MI, Hanley-Bowdoin L. Arabidopsis protein kinases GRIK1 and GRIK2 specifically activate SnRK1 by phosphorylating its activation loop. Plant Physiology. 2009;150:996–1005. doi: 10.1104/pp.108.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant Journal. 1999a;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Sugden C, Donaghy P, Halford NG, Hardie DG. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase and sucrose phosphate synthase in vitro. Plant Physiology. 1999b;120:257–274. doi: 10.1104/pp.120.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, et al. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant Journal. 2003;35:490–500. doi: 10.1046/j.1365-313x.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Toroser D, Plaut Z, Huber SC. Regulation of a plant SNF1-related protein kinase by glucose-6-phosphate. Plant Physiology. 2000;123:403–411. doi: 10.1104/pp.123.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA. 2004;101:17306–17311. doi: 10.1073/pnas.0407758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Wek SA, Wek RC. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Molecular and Cellular Biology. 2000;20:2706–2717. doi: 10.1128/mcb.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dickinson JR, Paul MJ, Halford NG. Molecular cloning of an Arabidopsis homologue of GCN2, a protein kinase involved in co-ordinated response to amino acid starvation. Planta. 2003;217:668–675. doi: 10.1007/s00425-003-1025-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andralojc PJ, Hey SJ, et al. Arabidopsis SNF1-related protein kinase-1 and calcium-dependent protein kinase phosphorylate conserved target sites in ABA response element binding proteins. Annals of Applied Biology. 2008a;153:401–409. [Google Scholar]
- Zhang Y, Wang Y, Kanyuka K, Parry MAJ, Powers SJ, Halford NG. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2α in Arabidopsis. Journal of Experimental Botany. 2008b;59:3131–3141. doi: 10.1093/jxb/ern169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, et al. Inhibition of Snf1-related protein kinase (SnRK1) activity and regulation of metabolic pathways by trehalose 6-phosphate Plant Physiology. 2009;149:1860–1871. doi: 10.1104/pp.108.133934. [DOI] [PMC free article] [PubMed] [Google Scholar]