Abstract
Background
This prospective study was designed to be the first to evaluate the toxicity of radiofrequency ablation (RFA) in patients with recurrent pediatric solid tumors.
Methods
From 2003 through 2008, we conducted a phase I/pilot study of RFA for recurrent pediatric solid tumors. A multidisciplinary cancer management team selected appropriate candidates for the study. Imaging-guided RFA was performed percutaneously. Repeat RFA was performed for recurrences when appropriate. Toxicity and imaging response was assessed at 1 and 3 months prospectively. Accrual stopped in 2006 and data collection stopped in 2008.
Results
Sixteen patients (age 4 – 33 years, median 15 years) and 56 tumor sites were treated in 37 RFA sessions including 38 pulmonary, 11 musculoskeletal, and 7 hepatic lesions (82 lesion-treatments). Post-procedural pain was moderate (median 5 on a scale from 1 to 10) and lasted a median of 9 days. Prolonged hospitalization (beyond 1 day) occurred 17 times (range 2–25 days, median 3 days). Hypoxia supported by supplemental oxygen occurred in 8 of 16 patients and resolved within one month after each RFA. No patient had tumor lysis syndrome but myoglobinuria/hemoglobinuria occurred in 6 of 16 patients all without renal damage. Serious complications from pulmonary RFA included two diaphragmatic hernias. Twenty-four of 82 (29%) lesions imaged remained ablated at the end of the study.
Conclusion
The toxicity from RFA of recurrent pediatric solid tumors is real but limited and RFA may offer a local tumor control alternative in carefully selected cases.
Keywords: childhood, pediatric, RF ablation, metastases, solid tumor, toxicity, pulmonary function
Introduction
Radiofrequency ablation (RFA) of solid tumors is an imaging-guided percutaneous or intra-operative procedure that uses a probe on the end of a sharp needle that is inserted directly into the tumor. The tumor is ablated by using electric current alternating at a radio frequency from the unshielded portion of the probe to raise the temperature of the tumor thereby causing irreversible cell death. RFA has become “state-of-the-art” for palliation of adult hepatic tumors and has shown curative potential, especially in lesions limited in number and size1. RFA has been used to treat primary and metastatic tumors in a variety of extra-hepatic sites including the lung2 and musculoskeletal system3 in adults. RFA has only been used routinely in a pediatric setting to treat children with osteoid osteoma4, which typically requires only a small burn volume for treatment. There have been a few case reports of RFA performed on children with Wilms tumor5, 6 and desmoplastic round cell tumor7. However, there have been no prospective studies on the safety and efficacy of this technique in patients with advanced pediatric malignancies. A method for predicting and controlling the core body temperature of patients undergoing RFA for malignant pediatric lesions has been reported8. There is clinical experience in using radiofrequency (RF) energy for other pediatric conditions including ablation of microcystic lymphatic malformations of the oral cavity9, dividing the liver during tumor resection10, 11, ablation of the lower esophageal sphincter12 and aberrant conduction pathways in the heart.
Methods
In January 2003, we initiated a phase I, IRB-approved protocol for RFA of solid tumors acquired during childhood. The primary aim of the study was to determine the safety of RFA for patients with recurrent pediatric solid tumors not amenable to other therapeutic approaches. Stopping rules for the phase I study were based on the first month observation period and accrual of 6 patients in each category: lung, musculoskeletal and liver. Toxicity was evaluated prospectively using Common Terminology Criteria for Adverse Events, version 2.0 for three months after each RFA.
Due to rapid accrual of lung ablation patients, accrual to this group was increased to 12 as a pilot study. Study accrual was stopped when the primary investigator who performed the RFA procedures left the institution in September 2006.
Response was prospectively assessed as a secondary aim up to three months post ablation. The study escalated through amendments to allow repeat ablation and review of late toxicity, response and outcome up to five years post RFA through April 2008.
Eligibility criteria included an original diagnosis of cancer or aggressive fibromatosis (desmoid tumor) at less than 21 years of age and recurrent or progressive disease at any age in the lung, liver, or musculoskeletal system. Each case was reviewed by a multidisciplinary cancer management team to ensure that observation, surgery, chemotherapy or radiation therapy was not more appropriate. Patients with uncorrectable coagulopathy or thrombocytopenia were not eligible. Pulmonary RFA candidates must have had prior pulmonary nodule resection, not require supplemental oxygen, and be expected to be free of dyspnea at rest in room air at one month following the ablation.
Baseline evaluations included tumor imaging, complete blood count, serum chemistries including liver function tests, and for pulmonary lesions positron emission tomography (PET) with computed tomography (CT) of the chest and pulmonary function testing including forced vital capacity (FVC). Pain service consultation was obtained prior to the first RFA.
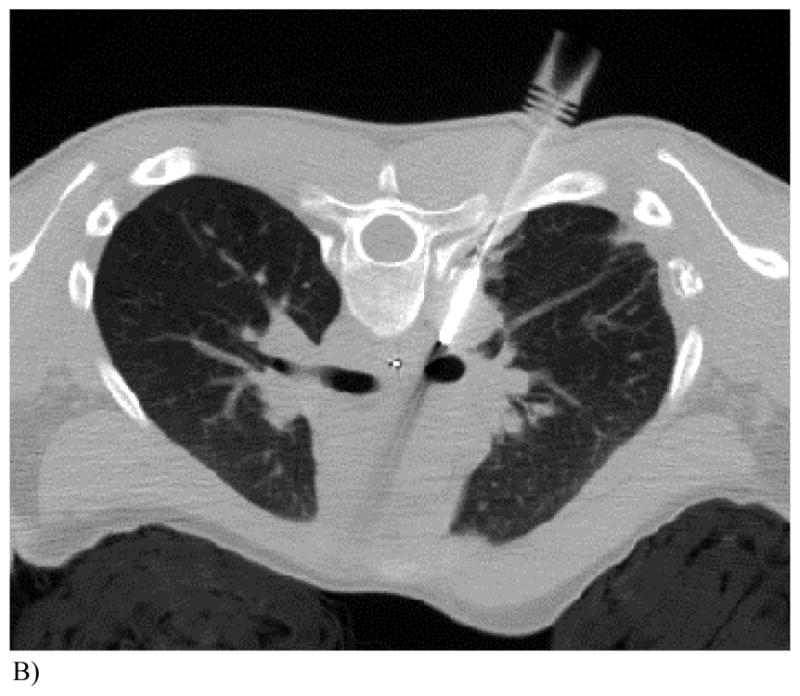
RFA was performed by one pediatric interventional radiologist. Pulmonary lesions (Fig. 1A) were ablated under CT guidance. The one osseous lesion was treated under fluoroscopic and ultrasound guidance. Soft tissue and hepatic lesions were ablated under ultrasound or CT guidance. General anesthesia was used for all ablations. Most patients undergoing pulmonary RFA were placed in a cooling blanket to control their core body temperature as previously described in a subset of 12 patients from this study8. Two to four grounding pads were placed on the patient, typically on the thighs, and connected to the generator. Electrical re-entry pathways that could result in skin burns were usually avoided by wrapping the extremities in pillowcases and towels. Skin burns over the RFA site were usually avoided by keeping the skin cool with a sterile ice-filled glove. If the spinal canal or ocular globe was within 1 cm of the RF probe, a percutaneous or esophageal temperature probe (Fig 1B) was placed to monitor and prevent nerve toxic temperatures (45°C).
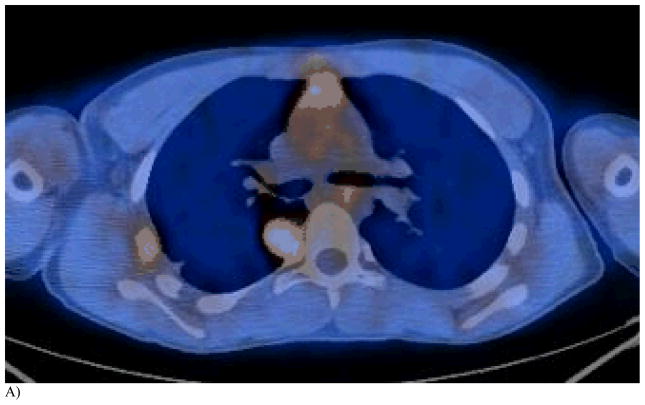
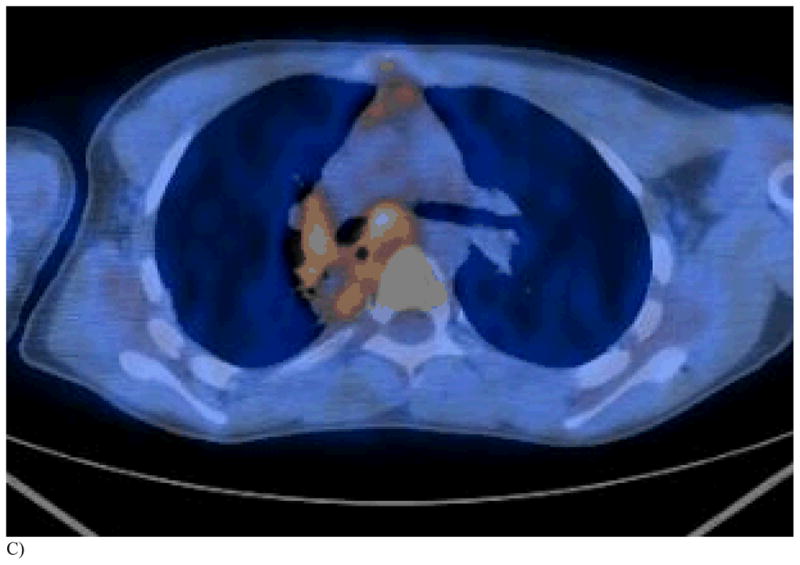
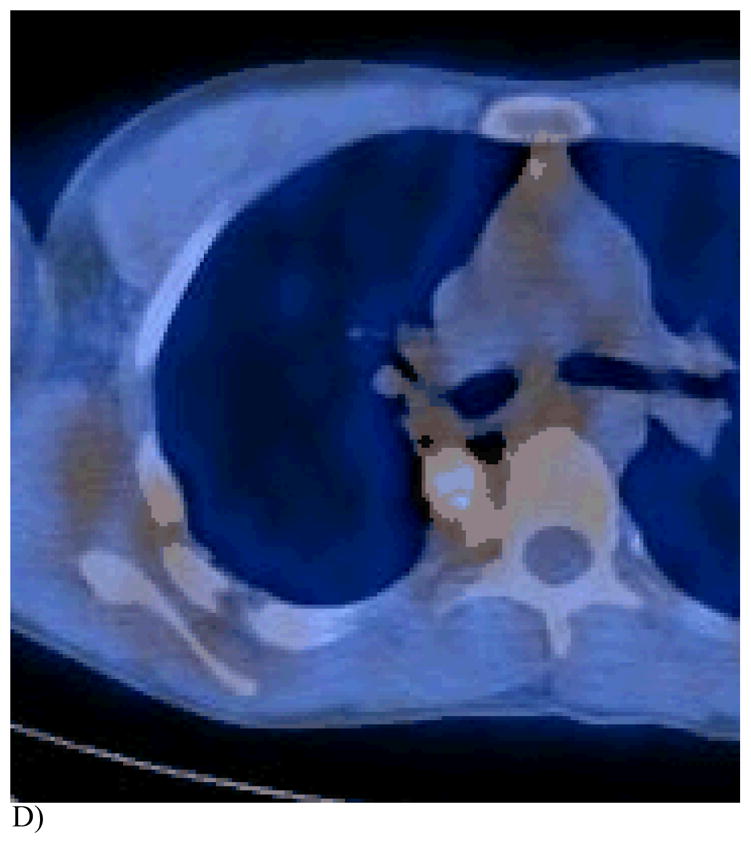
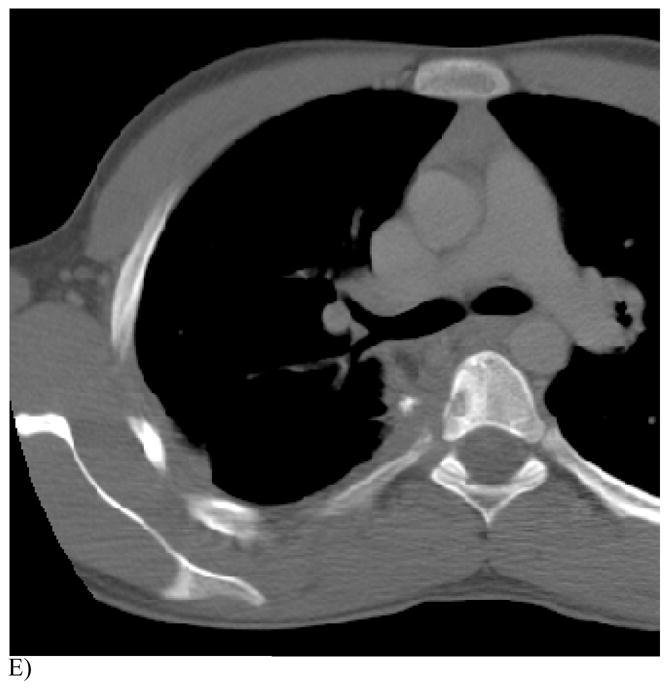
Figure 1. This 14 year old (patient 3#) with osteosarcoma had 2 thoracoscopic resections but refused further surgery. RFA had been performed 6 months earlier.
A) One lesion near the spinal canal recurred after RFA as noted on this FDG-PET/CT. The other lesion in lateral pleura was completely ablated by RFA but decreasing chest wall reaction remained.
B) Repeat RFA was performed with a cluster probe angled away from spinal canal. Note an esophageal temperature probe was equa-distant from the active cluster RF probe tip and the spinal canal and was used to estimate a safe temperature (<45°C) in the spinal canal. Maximum temperature within the tumor was 81°C and the maximum temperature registered within the esophagus was 38.6°C. The RF probe was repositioned 7 times for a total burn time of 54 minutes.
C) PET/CT 1 month after repeat RFA shows reactive hilar adenopathy and necrotic tumor with surrounding tissue reaction.
D) This FDG PET/CT 3 months post 2nd RFA demonstrates a tissue reaction in the region of the prior completely necrotic mass, mimicking a viable tumor.
E) Note the T5 vertebral body reaction on this CT portion of the FDG PET study. The patient is now alive and well without evidence of tumor recurrence 48 months after the first ablation.
Once the RF probe was placed in the tumor, an alternating electric current at the radio frequency was applied via a generator (Radionics Inc.; presently Valley Lab, Boulder, CO). The generator controlled the RFA usually under impedance control while the tip of the probe was hydraulically cooled to effectively distribute the tumor ablating heat throughout the whole tumor. The tumor temperature was monitored at the end of each application with a goal of achieving at least 50°C for cell death. Occasionally when effective tumor heating could not be achieved, manual control of the coagulator output was used without cooling the probe. Configuration varied for the single 17-gauge probe with a 1 to 3 cm active tip. Larger lesions required a cluster probe, with three 2.5 cm active tips (Fig. 1B). Each burn required 4 to 12 minutes to complete. Increased number of burns per tumor site was used for large lesions to create overlapping burn volumes. Up to 4 lesions were subject to RFA per session for a maximum of 4 hours per session. If the number or size of the lesions to be ablated did not allow complete ablation in the first session, one additional RFA session occurred 2 days to 2 weeks later after complete patient recovery from the first session.
After the procedure all patients were admitted, intravenous hydration was administered at twice the maintenance rate and continued until the urinalysis measured on the first voided urine or the next day was negative for blood (to rule out myoglobinuria or hemoglobinuria). Intravenous patient controlled analgesia (PCA) was utilized during the recovery period and patients were monitored until the post-ablation pain was controlled by oral opioids. Non-aspirin, non-steroidal anti-inflammatory agents or acetaminophen were prescribed for the fever of the post-ablation syndrome.
The study was to be stopped if 3 patients experienced any of the following serious complications potentially related to RFA during the 1-month observation period after the procedure: death, major bleeding, hepatic abscess, bile leak, diaphragmatic rupture, bowel perforation, peritonitis, skin burn requiring a graft, renal failure, permanent motor nerve deficit, fracture, osteomyelitis, septic arthritis, stroke, dyspnea at a normal level of activity for over one month, requirement for supplemental oxygen for over one month, assisted ventilation or pressure support for over 24 hour, lung abscess requiring resection, empyema, tracheal or esophageal disruption, and phrenic nerve paralysis. The investigators expected nearly all major toxicity to occur in the first month. Patients were expected to have transient pain after any RFA, transient pleural effusion, hypoxia or dyspnea after pulmonary RFA and transient hepatic enzyme release after hepatic RFA. Recurrent hypoxia and dyspnea after the one-month observation period were attributed to tumor progression and not RFA toxicity. These events were not used as stopping rules.
No other treatment (surgery, chemotherapy or radiation therapy) was given during this first month, however other treatment was allowed after this period (Table 1).
Table 1.
Patient history, management and outcome
| Patient # | Age (yrs) at first RFA | Tumor | Site of ablation | # of lesions treated | # lesion treatments (including repeat RFA) | Post RFA -therapy | Overall survival post first RFA (months) |
|---|---|---|---|---|---|---|---|
| 1 | 33 | synovial sarcoma | lung | 1 | 1 | Off protocol RFA Chemotherapy | 21 |
| 2 | 10 | osteosarcoma | lung | 2 | 2 | Radiotherapy | 8 |
| 3 | 14 | osteosarcoma | lung | 2 | 3 | none | 48* |
| 4 | 12 | osteosarcoma | lung | 4 | 4 | Chemotherapy | 12 |
| 5 | 18 | osteosarcoma | lung | 3 | 3 | Chemotherapy Cryotherapy | 7 |
| 6 | 16 | osteosarcoma | lung | 1 | 1 | Chemotherapy Surgery | 13 |
| 7 | 20 | osteosarcoma | lung | 2 | 3 | none | 5 |
| 8 | 4 | adrenocortical carcinoma | lung | 4 | 16 | Chemotherapy Surgery | 27 |
| 9 | 19 | hepatocellular carcinoma | liver | 7 | 9 | Chemotherapy Surgery | 60** |
| 21 | lung | 1 | 1 | ||||
| 10 | 16 | rhabdomyosarcoma | breast | 1 | 1 | Chemo- | 5 |
| 11 | 13 | rhabdomyosarcoma | maxilla | 1 | 2 | Chemotherapy | 5 |
| 12 | 16 | osteosarcoma | lung | 6 | 6 | Chemotherapy Radiotherapy | 12 |
| 16 | radius | 1 | 1 | ||||
| 13 | 13 | plantar fibromatosis | feet | 6 | 15 | none | 34** |
| 14 | 10 | Wilms tumor | lung | 9 | 9 | Chemotherapy | 33** |
| 15 | 12 | osteosarcoma | lung | 3 | 3 | none | 15 |
| 16 | 9 | leiomyosarcoma | ribs | 2 | 2 | Antiviral therapy | 26** |
Alive & well at end of study;
Alive with disease at end of study
Pain scores were obtained prior to, immediately after, the day after, and at 1 and 3 months after RFA. Pulmonary function testing was performed prior to, at 1 month and at 3 months after pulmonary RFA.
Tumor imaging was performed at 1 (Fig 1C) and 3 months (Fig 1D,E) after each ablation to determine completeness of ablation and tumor response. Complete ablation was defined as rim enhancement or no enhancement and incomplete ablation as asymmetric enhancement with contrast enhanced CT, MR, or FDG- PET at one-month post-RFA. The volume of the lesions in mL were determined by the product of the 3 orthogonal plane tumor lengths in cm x 0.52 and compared to baseline. Time to recurrence was determined only on RFA treated lesion as defined by nodular or asymmetric enhancement or progression of tumor size after the one month imaging. If the tumor was incompletely ablated or recurred, or new lesions appeared, RFA was repeated subject the same eligibility criteria, stopping rules and post procedure follow-up.
Kaplan-Meier (K-M) estimator was used to estimate the probability of no recurrence for ablated tumors at any time after treatment and the probability of patient survival. Survival times are defined as the time from first RFA treatment to the last follow-up or patient death. If patients were still alive at the end of the 5-year study period, the survival times were censored.
Results
The clinical features, treatment and outcome of the 16 patients enrolled are shown in Table 1. A total of 56 lesions: 38 pulmonary, 11 musculoskeletal including 1 breast lesion, and 7 hepatic lesions were treated with RF thermal energy over 37 sessions. Forty-two of the 56 lesions were treated once, and 14 lesions treated more than once, for 82 lesion-treatments in total including 52 pulmonary, 21 musculoskeletal, and 9 liver lesion treatments. The volume of the individual lesions treated range from 0.13 mL to 750 mL (median 7 mL, mean 46 mL).
Toxicity
The toxicity observed in the study did not meet the criteria for stopping the study and there were no procedure-related deaths. The accrual was halted temporarily while a third degree burn under a grounding pad was investigated by the IRB. The highest toxicity per patient, onset, duration and treatment are listed in Table 2. All Grade 3 and 4 toxicities possibly, probably or definitely related to RFA are listed in Table 3.
Table 2.
Worst RFA related toxicity per patient
| Patient | Highest Grade Toxicity | Nature | Onset post RFA | Resolution post RFA | Treatment |
|---|---|---|---|---|---|
| 1 | 3 | pain | 0 days | 14 days | supportive |
| 2 | 4 | FEV1 | 1 month | - | supportive |
| 3 | 2 | pain | 0 days | 42 days | symptomatic |
| 4 | 3 | hypoxia | 0 days | 4 days | supportive |
| 5 | 3 | hypoxia | 0 days | 4 days | supportive |
| 6 | 3 | bradycardia | 0 day | 2 minutes | vigorous |
| 7 | 3 | skin burn | 0 days | 2 months | supportive |
| 8 | 3 | diaphragmatic hernia | 6 days | 24 days | surgery |
| 9 | 4 | SGOT (AST) | 0 days | 1 month | none |
| 10 | 2 | pain | 0 days | 1 day | symptomatic |
| 11 | 1 | trismus | 0 days | - | none |
| 12 | 3 | FEV1 | 1 month | - | none |
| 13 | 2 | paresthesia | 0 days | - | none |
| 14 | 4 | diaphragmatic hernia | 5 months | 34 months | surgery |
| 15 | 4 | dyspnea | 2 days | 23 days | supportive |
| 16 | 3 | hypoxia | 9 days | 3 days | supportive |
Table 3.
All high-grade toxicities possibly to definitely related to RFA
| Toxicity | # of patients grade 3 | # of patients grade 4 |
|---|---|---|
| Diaphragmatic hernia | 1 | 1 |
| Hypoxia | 8 | |
| Dyspnea | 1 | 2 |
| FEV1 | 1 | 1 |
| Pleural effusion | 1 | |
| Bronchopleural fistula | 1 | |
| Pulmonary edema | 1 | |
| Skin burn | 1 | |
| Bradycardia | 1 | |
| Hyperthermia | 1 | |
| Pain | 2 | |
| Fatigue | 2 | |
| Leukocytosis | 1 | |
| SGOT (AST) (serum glutamic oxaloacetic transaminase) | 1 | |
| SGPT (ALT) (serum glutamic pyruvic transaminase) | 1 |
Pulmonary toxicity
The most prevalent toxicity was Grade 3 hypoxia that required supplemental oxygen only within the first month in 8 patients (Table 3). Grade 4 toxicities included dyspnea at rest that resolved within one month. Grade 4 toxicity by FEV1 measurement was also noted in patient #2 who had a prior pneumonectomy, a pre RFA FEV 1 of 34% of expected and a one month post RFA FEV1 of 17%.
The 11 of 12 patients that could be tested, baseline forced vital capacity (FVC) was 38 to 100% of normal (median, 61%). Of the 14 times FVC was tested at pre- and 1 month post-RFA, the median dropped from 58–61% to 48–53% normal (range 16–85% normal at one month). Of the 9 times FVC was tested pre-, 1 and 3 months post-RFA the median was 62, 48 and 49% respectively (range 31–81% of normal at 3 months). One patient (#8) was too developmentally delayed to be tested.
One patient (#14) underwent RFA of a 2 cm lesion abutting her left diaphragm, developed an “elevated left hemi-diaphragm” within 5 months. An emergent ICU admission for respiratory failure (Grade 4 toxicity) at 34 months prompted a large left diaphragmatic hernia repair. Pulmonary Grade 2 toxicities in this patient included development of a right upper lobe cavity that was eventually colonized with atypical mycobacteria. Another patient (#8) developed a quarter-sized diaphragmatic hernia (Grade 3 toxicity) after repeated RF ablation of a persistent left lower lobe pulmonary metastasis; this was discovered and repaired surgically during a surgical gastrostomy.
Bradycardia (Grade 3 toxicity) occurred in one patient (#6) during pulmonary RFA due to stimulation of the left vagal nerve and immediately responded to glycopyrrolate. One patient (#7) developed transient enlargement of his LUL lesion (listed as bronchopleural fistula, Grade 3 toxicity) as a result of necrotic debris. This necrotic material was drained percutaneously and lysed with instillation of tissue plasminogen activator reducing the volume of the lesion. After palliative RFA was attempted on a 750 mL R pulmonary lesion, he developed interstitial pulmonary edema (Grade 3 toxicity, Table 3) related to superior vena caval compression. Small self-limited pleural effusions were expected after pulmonary RFA but only one required chest tube placement (Grade 3 toxicity).
Hepatic Toxicity
RFA was performed on the hepatic lesions in the one patient (#9, Table 1) with multiple fibrolamellar hepatocellular carcinomas over 4 sessions in two years. The patient had toxicity limited to the first month post ablation including Grade 4 (Table 2) manifest by SGOT (serum glutamic oxaloacetic transaminase) elevation, Grade 3 by SGPT (serum glutamic pyruvic transaminase), pain, hypoxia and leukocytosis (Table 3), and Grade 2 by myoglobinuria or hemoglobinuria from grossly red urine.
Hyperthermia
Only one patient who weighed 25.8Kg and had centrally located pulmonary metastases RF ablated had Grade 3 toxicity of hyperthermia (core body temperature over 40° C) during the RFA. Some other patients who weighed 35 Kg or less had core temperatures over 39° C during ablation. The heaviest patient to have core body temperature elevation above 38.5° C during RFA weighed 54.7Kg. The specifics of this toxicity and its control in a subset of the study patients have been published8.
Pain
Pain was the most common adverse event occurring after 36 of 37 sessions. The worst procedural pain per patient after RFA ranged 0–10 on a scale of 0 to 10 (median, 5) and lasted 1 to 23 days (median 9 days). Only 2 patients had Grade 3 toxicity of post-procedural pain. After plantar RFA one patient (#13) had toe numbness and Grade 2 paresthesia that did not interfere with walking.
Hospitalization
In association with the 37 RFA procedures, hospitalization was planned for 1 day and was limited to 1 day on 20 occasions. Prolonged or recurrent hospitalization occurred 17 times (median 3 days, range 2–25 days); for pain control, respiratory difficulty related to pain control, or the above pulmonary complications.
Post-ablation syndrome
No patients in this series had blood chemistry abnormalities suggestive of tumor lysis (elevation of potassium, phosphate, and uric acid). Six patients developed myoglobinuria or hemoglobinuria as manifested by a rating of trace to +3 blood as measured through urinalysis within 24 hours. Only one had grossly bloody appearance (Grade 2 toxicity) after a 227 mL volume of hepatic ablation. All patients received twice-maintenance IV fluids for 24 to 48 hours. All hemo-myoglobinuria resolved within 48 hours and no patient developed renal insufficiency. Many patients presented with symptoms that could be related to necrotic tumor produced by RFA (post-ablation syndrome) including nausea, fatigue, and fever. The only Grade 3 toxicity from post-ablation syndrome was fatigue in 2 patients. These symptoms usually abated over 2 weeks allowing the patients to resume their normal activity.
Skin burns
Three patients developed localized skin burns at points of contact of the grounding pads. One third-degree burn (the only Grade 3 toxicity in this group) occurred with ulceration and some tissue loss at the site of the grounding pad placed over the anterior superior iliac spine superior to an above knee amputation. The bone was not exposed and the burned tissues healed spontaneously. Two other second-degree burns occurred in young patients over the groin area, and also healed spontaneously. One patient (#8) developed second-degree re-entry skin burns where a knuckle of one hand touched her thigh during a pulmonary RF ablation. Both areas healed spontaneously. One patient (# 13) developed skin burns over two of the plantar fibromatosis (desmoid tumors). This occurred on the third ablation session when the operator caused a re-entry circuit by touching the skin over the lesions and the opposite leg, conducting more energy through the skin to the grounding pads than intended. One, a 2nd degree burn, healed spontaneously. The other, a 3rd degree burn, broke down, unroofing the necrotic desmoid tumor under the skin. Once all necrotic material came out, the skin healed without intervention.
Response
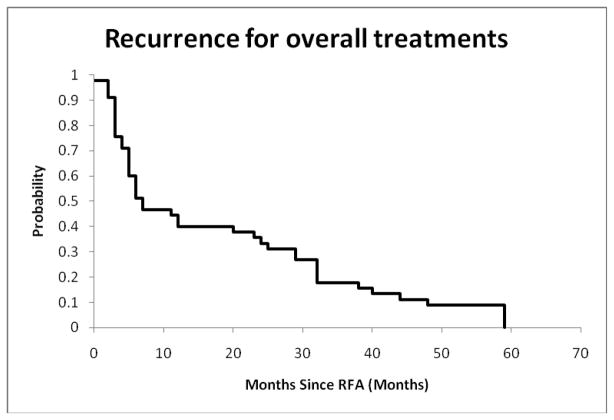
The ablation status is summarized in Table 4. Eighty-two lesion ablations (including repeat ablations) were performed; 51 (62%) were completely ablated and 31 (38%) were incompletely ablated upon examination at 1 month after procedure. Twenty-seven of the completely ablated tumors recurred (33%) by the end of the 5-year study period. For all treatments resulting in a complete ablation at 1 month, the probability of no recurrence of RFA treated lesions is 0.40±0.07 at 12 months, and is 0.33±0.07 at 24 months, as shown in Figure 2.
Table 4.
Ablation Status of Lesions-Treatments
| Type of Treatment and Lesion Location | Status of Ablation | Total | |||
|---|---|---|---|---|---|
| Incomplete | Complete | ||||
| No Recurrence | Recurrence | ||||
| First time treatment | Pulmonary lesions | 13 (34.2%) | 17 (44.7%) | 8 (21.1%) | 38 |
| Musculoskeletal lesions | 8 (72.7%) | 2 (18.2%) | 1 (9.1%) | 11 | |
| Hepatic lesions | 1 (14.3%) | 4 (57.1%) | 2 (28.6%) | 7 | |
| Last time treatment | Lesions that had multiple treatments | 1 (7.1%) | 2 (14.3%) | 11 (78.6%) | 14 |
| All treatments | All lesions | 31 (37.8%) | 24 (29.3%) | 27 (32.9%) | 82 |
Figure 2.
Probability of no recurrence after all complete ablations
The median volume of the pulmonary lesions treated the first time was 5 mL prior to the ablation. This increased to 43 mL during the ablation of the tumor and surrounding normal tissues, and then decreased to 17 mL at 1 month and further to 8 mL at 3 to 4 months. The virilization syndrome in patient #8 was controlled by pulmonary RFA. The most dramatic response in serum testosterone was from 23 ng/dL the morning before her last pulmonary lesion RF ablation, to 4 ng/dL two months later. The chest wall pain associated with the left upper lobe pulmonary osteosarcoma metastasis in patient #7 was reduced from a maximum of 10/10 severity to 6/10 by the pulmonary RFA within 24 hours.
Response after RFA for musculoskeletal lesions was variable as measured by completeness of ablation and recurrence (Table 4). The breast nodule and rib lesions were completely ablated although one rib lesion recurred. High impedance from distant grounding pad placement inhibited ablation of non-truncal lesions during cool perfusion of the RF probe tip. Effective heating of these facial or extremity lesions was accomplished without cool-tip perfusion limiting the effective size of the burn. The size of the foot lesions increased with each ablation but the lesions were softer and less tender to palpation. The maxillary mass in a patient with rhabdomyosarcoma progressed in size in spite of RF ablation and in spite of repeat RF ablation after transarterial embolization. The pain from the radial head osteosarcoma metastasis improved significantly and remained controlled by later radiation therapy. The pain from the plantar fibroses (desmoid tumor) improved after each of the three RFA sessions. The patient was wheel chair bound due to pain score of 8 on a 0–10 pain scale when attempting to walk before the first ablation, but was able to walk with a pain score of 5 when walking flatfooted after the last ablation.
Treatment for the partially ablated lesions and recurrences included repeat RFA ablation, other treatments beyond the first month observation period or no further treatment (Table 1). The rapidity of progression of the treated or untreated lesions is reflected by the outcome.
Outcome
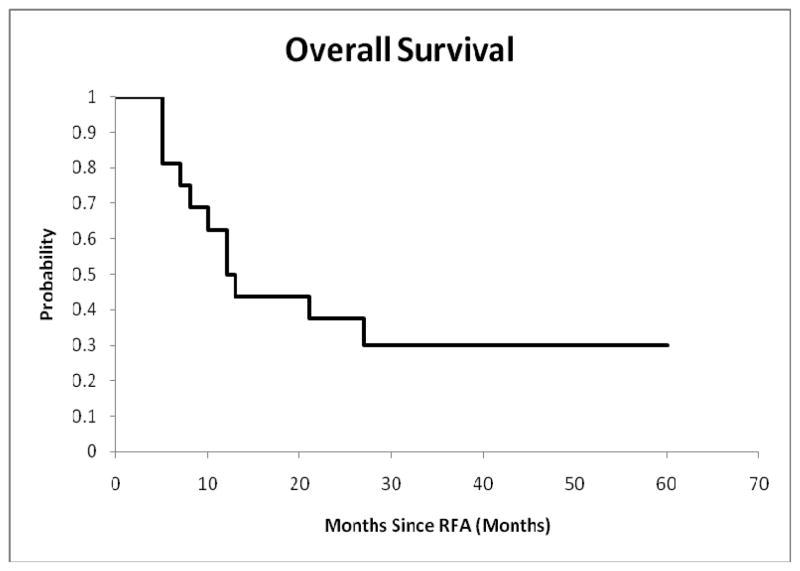
Eleven of 16 patients died during the 5-year study period including 10 as a result of underlying disease and 1 (patient #10) from pneumonia during post RFA chemotherapy (Table 1). The K-M estimates for the probabilities of patient survival are plotted in Figure 3. The probability of patient survival is 0.50±0.11, 0.38±0.12 and 0.30±0.18 at 12, 26 and 48 months respectively.
Figure 3.
Survival probability of patients who underwent RFA
Discussion
This phase I/pilot study mainly included children that could no longer have surgical resection of pulmonary metastases. The toxicity of the RFA of pulmonary metastases in these children and young adults with end-stage tumors was usually limited. Transient hypoxia or dyspnea was a common, expected complication requiring supplemental oxygen for less than one month in all patients. Pulmonary function did decrease with each RFA limiting the number of reablations that could be performed. Serious complications did occur including two diaphragmatic hernias.
Diaphragmatic hernia has not been previously identified as a complication of pulmonary RFA but is a known complication of hepatic RFA13 and may take 15 to 18 months to manifest as the liver slowly pushes through and enlarges the perforation. The frequency of diaphragmatic injury due to RFA of hepatic tumors is estimated to be 17%14. Our two diaphragmatic hernias were on the left, secondary to RFA of diaphragmatically based left lower lobe (LLL) pulmonary lesions. The diaphragmatic hernia in patient #8 could not have been avoided since that lesion was initially her largest tumor and was repeatedly ablated over 19 months. The diaphragmatic hernia in patient #14 could have been avoided since that lesion was only one of many slowly growing but FDG active metastases. One other patient (#12) had very aggressive but incomplete RFA of a pulmonary and pleural osteosarcoma on the right lateral diaphragm. He never developed a hernia. Avoiding RFA of pulmonary lesions on the diaphragm may be prudent, especially on the left side.
Pneumothoraces requiring chest tube drainage were avoided in our patients probably due to pleural adhesions from prior resections. When pulmonary RFA was performed on adults often without a prior thoracotomy, 28% developed pneumothoraces and 10% required chest tube placement2. Microwave ablation of adult lung malignancies was associated with a 39% incidence of pneumothoraces15.
Our relatively favorable results may be due to careful patient selection, avoiding treatment of patients with poorly compliant lungs, those on supplemental oxygen, and those in whom dyspnea at rest following RFA was expected. Obtaining pulmonary function tests in cooperative children can help determine how much RFA of pulmonary tissue will be tolerated. Those with FEV1 or FVC of 25 to 50% of normal should have conservative RFA aiming for cure of only a few small lesions or palliation of a large lesion. RFA in those with FEV1 or FVC less than 25% of normal should be avoided. As a cautionary note, we performed RFA on a child off protocol who had poorly compliant lungs and was recently weaned from supplemental oxygen. During the second RFA session, he developed a pulmonary vein to bronchial fistula with massive bleeding into the airway followed by manual ventilation and air embolism. He did not suffer a stroke and recovered from the acute event16.
The non-pulmonary toxicity from RFA in this series was limited. No patient experienced a procedure-related death. Hepatic toxicity from the hepatic RFA was severe but transient. Unlike the lung, the liver can regenerate. Skin burns under grounding pads have been previously reported11 and occurred in a few of this study group as a result of placement of grounding pads over the thin-skin portion of the groin in patients with an amputation or thin body habitus.
A recurrence rate of 50% within the pulmonary/pleural incision has been reported after surgical resection of pediatric pulmonary osteosarcoma metastases17. In comparison to surgery, initial RFA of residual or recurrent pediatric pulmonary lesions was complete with no recurrence in 45% of attempts (Table 3). Although a majority (66%) of the pulmonary lesions appear to be completely ablated after the first RFA treatment, residual microscopic viable tumor may persist near major vessels and major bronchi leading to local recurrence (21%). Also adhesions associated with each thoracotomy make it more difficult for the surgeons to remove the metastases subsequent times. Therefore RFA of pediatric pulmonary metastases should be considered after the first thoracotomy or thoracoscopic resection. A phase II RFA trial would be a fairer comparison to second time surgical resection of pulmonary metastases.
Therapeutic temperatures within lesions located in the head or extremities were difficult to achieve during RFA due high impedance secondary to the distance between the grounding pads and the RF probe. Our RFA controlled the pain of these lesions but they were incompletely ablated. Cryotherapy or microwave15 ablation does not require grounding pads and may be more therapeutic. Desmoid tumor is locally aggressive and tends to recur after excision and in our experience after RFA. Combined radiotherapy and heat therapy18, chemotherapy19, or chemical ablation with acetic acid20 are alternative treatments.
The limitations of this trial include very limited accrual for hepatic RFA and a limited number of young children enrolled. Stopping rules that covered only the first month and prospective analysis of toxicity only up to three months post RFA did not capture a late onset diaphragmatic hernia. The retrospective analysis of toxicity beyond three months was certainly not as inclusive. The common toxicity criteria are not well suited for thermal ablation. In addition, independent radiology review of response did not occur partly due to lack of understanding many diagnostic radiologists have of the post thermal ablation imaging. The classic measures of complete or partial response, stable disease or progressive disease as measured by tumor size are not well suited for thermal ablation. During RFA the tumor bulk is not removed but may be completely necrotic, the ablation causes an apparent increase size of the lesion because of destruction or reaction of normal marginal tissue and the bulk of the reaction in our experience returned to baseline size by three months or later. Therefore we relied on tumor viability or progression in size of the lesion after one month to determine response.
Although it is difficult to speculate about benefit in a phase I trial, the authors feel that the toxicity of RFA was worth the benefit in all 16 patients. This limited risk vs. benefit of RFA in childhood cancer has prompted the principal investigator and first author to open a phase II trial for RFA treatment of malignant and benign lesions in children.
Acknowledgments
Supported in part by the Cancer Center Support Grant CA 21765 from and by the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Drs. Victor Santana, Bhaskar Rao and Larry Kun for their support, Drs. Jeffrey S. Dome, Timothy P. Cripe and Stephen X. Skapek for their referrals, and Nikita Oigbokie for data management.
Footnotes
Informed consent: Informed consent has been obtained from all patients, parents, and/or guardians as per the Institutional Review Board policies of St. Jude Children’s Research Hospital.
References
- 1.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 2.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, Mayo-Smith WW. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiol. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 3.Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, Wong GY, Sloan JA, Novotny PJ, Petersen IA, Beres RA, Regge D, Capanna R, Saker MB, Gronemeyer DH, Gevargez A, Ahrar K, Choti MA, de Baere TJ, Rubin J. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–6. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 4.Torriani M, Rosenthal DI. Percutaneous radiofrequency treatment of osteoid osteoma. Pediatr Radiol. 2002;32:615–618. doi: 10.1007/s00247-002-0727-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown SD, Vansonnenberg E, Morrison PR, Diller L, Shamberger RC. CT-guided radiofrequency ablation of pediatric Wilms tumor in a solitary kidney. Pediatr Radiol. 2005;35:923–8. doi: 10.1007/s00247-005-1510-y. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi S, Meech SJ, Puthawala MA, Ferguson WS, Cardarelli GA, Dupuy DE. Combined computed tomography-guided radiofrequency ablation and brachytherapy in a child with multiple recurrences of Wilms’ tumor. J Pediatr Hematol Oncol. 2005;27:377–9. doi: 10.1097/01.mph.0000173846.87539.a9. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira-Filho AG, Miranda ML, Diz FL, et al. Use of radiofrequency for ablation of unresectable hepatic metastasis in desmoplastic small round cell tumor. Med Pediatr Oncol. 2003;41:476–7. doi: 10.1002/mpo.10386. [DOI] [PubMed] [Google Scholar]
- 8.Hoffer FA, Campos A, Xiong X, Wu S, Oigbokie N, Proctor K. Core body temperature regulation of pediatric patients during radiofrequency ablation. Pediatr Radiol. 2007;37:297–300. doi: 10.1007/s00247-006-0398-5. [DOI] [PubMed] [Google Scholar]
- 9.Grimmer JF, Mulliken JB, Burrows PE, Rahbar R. Radiofrequency ablation of microcystic lymphatic malformation in the oral cavity. Arch Otolaryngol Head Neck Surg. 2006;132:1251–6. doi: 10.1001/archotol.132.11.1251. [DOI] [PubMed] [Google Scholar]
- 10.Vaos G. Segmental liver resection in a child using intraoperative ultrasound-guided radiofrequency energy. J Pediatr Surg. 2007;42:E5–8. doi: 10.1016/j.jpedsurg.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Richtmyer JM. Electrosurgical burns in pediatric patients undergoing liver resection with saline-enhanced radiofrequency technology. AORN J. 2006;83:658–64. doi: 10.1016/s0001-2092(06)60193-2. quiz 665–7. [DOI] [PubMed] [Google Scholar]
- 12.Islam S, Geiger JD, Coran AG, Teitelbaum DH. Use of radiofrequency ablation of the lower esophageal sphincter to treat recurrent gastroesophageal reflux disease. J Pediatr Surg. 2004;39:282–6. doi: 10.1016/j.jpedsurg.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 13.di Francesco F, di Sandro S, Doria C, Ramirez C, Maurizio I, Navarro V, Silvestry S, Needleman L, Frank A. Diaphragmatic hernia occurring 15 months after percutaneous radiofrequency ablation of a hepatocellular cancer. Amer Surg. 2008;74:129–132. [PubMed] [Google Scholar]
- 14.Head HW, Dodd GD, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiol. 2007;243:877–884. doi: 10.1148/radiol.2433060157. [DOI] [PubMed] [Google Scholar]
- 15.Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiol. 2008;247:871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne LL, Pereiras LA, Laningham F, Shearer JR, Bikhazi GB, Hoffer FA. Near-fatal acute bronchovenous fistula in a child undergoing radiofrequency ablation of a metastatic lung tumor. Paediatr Anaesth. 2008;18:1131–3. doi: 10.1111/j.1460-9592.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 17.McCarville MB, Kaste SC, Cain AM, Goloubeva O, Rao BN, Pratt CB. Prognostic factors and imaging patterns of recurrent pulmonary nodules after thoracotomy in children with osteosarcoma. Cancer. 2001;91:1170–1176. doi: 10.1002/1097-0142(20010315)91:6<1170::aid-cncr1114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Oohata Y, Mibu R, Uehara S, et al. Regression of an aggressive abdominal desmoid tumor in a patient with familial adenomatous polyposis by hyperthermoradiotherapy. Am J Gastroenterol. 1997;92:156–8. [PubMed] [Google Scholar]
- 19.Skapek SX, Hawk BJ, Hoffer FA, Dahl GV, Granowetter L, Gebhardt MC, Ferguson WS, Grier HE. Combination chemotherapy using vinblastine and methotrexate for the treatment of desmoid tumor in children. J Clin Onc. 1998;16:3021–7. doi: 10.1200/JCO.1998.16.9.3021. [DOI] [PubMed] [Google Scholar]
- 20.Clark TW. Percutaneous chemical ablation of desmoid tumors. J Vasc Interv Radiol. 2003;14:629–34. doi: 10.1097/01.rvi.0000064861.87207.3e. [DOI] [PubMed] [Google Scholar]