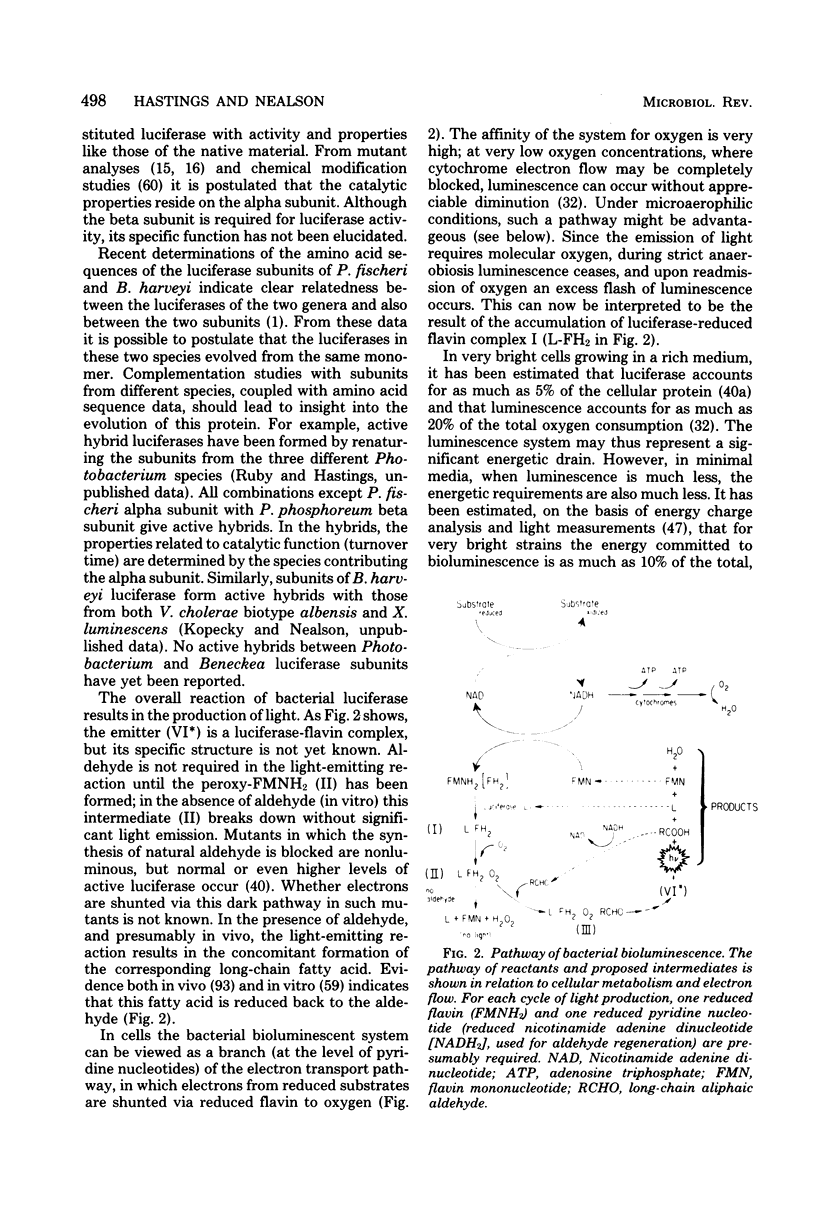
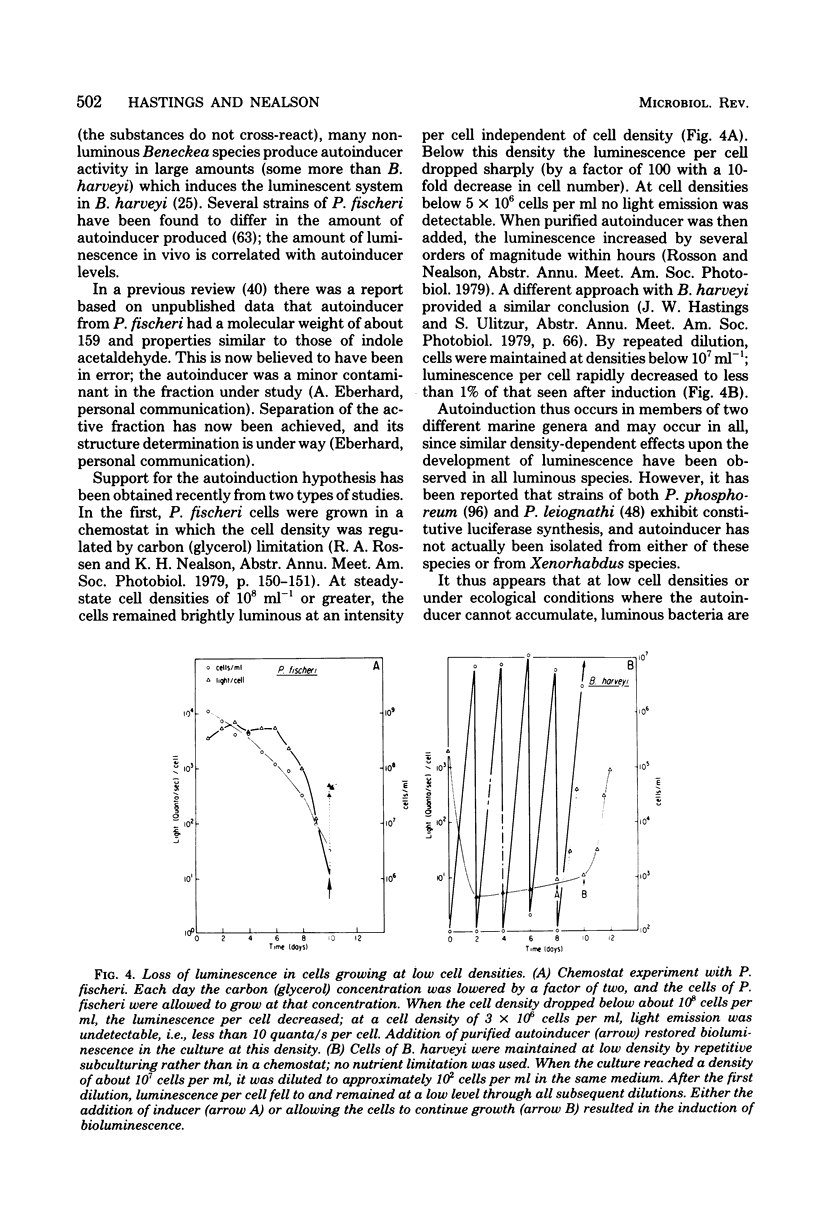
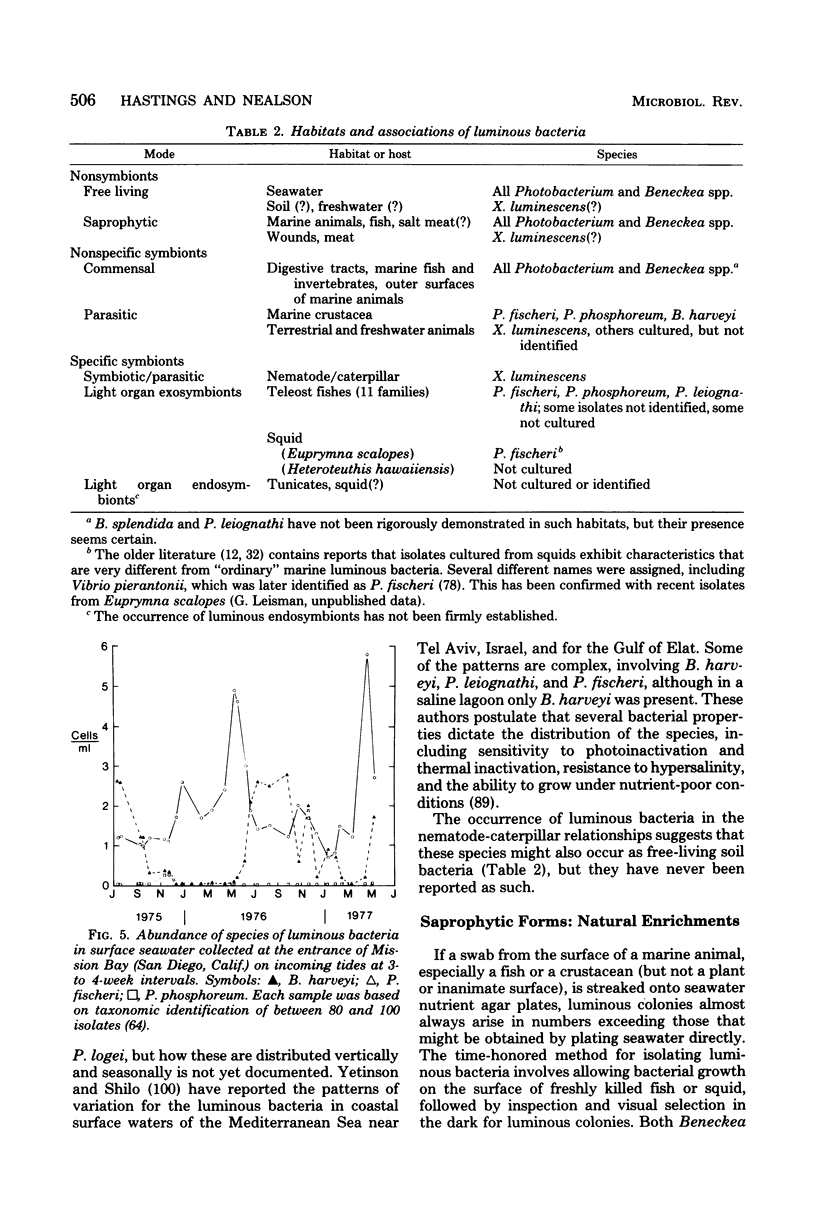
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balny C., Hastings J. W. Fluorescence and bioluminescence of bacterial luciferase intermediates. Biochemistry. 1975 Oct 21;14(21):4719–4723. doi: 10.1021/bi00692a024. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L. Biology of the marine enterobacteria: genera Beneckea and Photobacterium. Annu Rev Microbiol. 1977;31:39–61. doi: 10.1146/annurev.mi.31.100177.000351. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971 Jul;107(1):268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becvar J. E., Tu S. C., Hastings J. W. Activity and stability of the luciferase--flavin intermediate. Biochemistry. 1978 May 2;17(9):1807–1812. doi: 10.1021/bi00602a036. [DOI] [PubMed] [Google Scholar]
- Case J. F., Warner J., Barnes A. T., Lowenstine M. Bioluminescence of latern fish (Myctophidae) in response to changes in light intensity. Nature. 1977 Jan 13;265(5590):179–181. doi: 10.1038/265179a0. [DOI] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
- Cline T., Hastings J. W. Temperature-sensitive mutants of bioluminescent bacteria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):500–504. doi: 10.1073/pnas.68.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudoroff M. Studies on the Luminous Bacteria: I. Nutritional Requirements of Some Species, with Special Reference to Methionine. J Bacteriol. 1942 Oct;44(4):451–459. doi: 10.1128/jb.44.4.451-459.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galt C. P. Bioluminescence: dual mechanism in a planktonic tunicate produces brilliant surface display. Science. 1978 Apr 7;200(4337):70–72. doi: 10.1126/science.635574. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Hastings J. W., Favaudon V., Lhoste J. M. Structure of the oxygen adduct intermediate in the bacterial luciferase reaction: C nuclear magnetic resonance determination. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5860–5863. doi: 10.1073/pnas.75.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- HASTINGS J. W. Oxygen concentration and bioluminescence intensity. I. Bacteria and fungi. J Cell Physiol. 1952 Feb;39(1):1–30. doi: 10.1002/jcp.1030390102. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- HASTINGS J. W., SPUDICH J., MALNIC G. THE INFLUENCE OF ALDEHYDE CHAIN LENGTH UPON THE RELATIVE QUANTUM YIELD OF THE BIOLUMINESCENT REACTION OF ACHROMOBACTER FISCHERI. J Biol Chem. 1963 Sep;238:3100–3105. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda Y., Tsuji F. I. Light production in the luminous fishes Photoblepharon and Anomalops from the Banda Islands. Science. 1971 Jul 9;173(3992):143–145. doi: 10.1126/science.173.3992.143. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- Harvey E. N. A FISH, WITH A LUMINOUS ORGAN, DESIGNED FOR THE GROWTH OF LUMINOUS BACTERIA. Science. 1921 Apr 1;53(1370):314–315. doi: 10.1126/science.53.1370.314. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Balny C., Peuch C. L., Douzou P. Spectral properties of an oxygenated luciferase-flavin intermediate isolated by low-temperature chromatography. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3468–3472. doi: 10.1073/pnas.70.12.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Balny C. The oxygenated bacterial luciferase-flavin intermediate. Reaction products via the light and dark pathways. J Biol Chem. 1975 Sep 25;250(18):7288–7293. [PubMed] [Google Scholar]
- Hastings J. W. Light to Hide by: Ventral Luminescence to Camouflage the Silhouette. Science. 1971 Sep 10;173(4001):1016–1017. doi: 10.1126/science.173.4001.1016. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Henry J. P., Michelson A. M. Etudes de bioluminescence. Régulation de la bioluminescence bactérienne. C R Acad Sci Hebd Seances Acad Sci D. 1970 Apr 13;270(15):1947–1949. [PubMed] [Google Scholar]
- Katznelson R., Ulitzur S. Control of luciferase synthesis in a newly isolated strain of Photobacterium leiognathi. Arch Microbiol. 1977 Dec 15;115(3):347–351. doi: 10.1007/BF00446462. [DOI] [PubMed] [Google Scholar]
- Linton J. D., Harrison D. E., Bull A. T. Molar growth yields, respiration and cytochrome profiles of Beneckea natriegens when grown under carbon limitation in a chemostat. Arch Microbiol. 1977 Nov 18;115(2):135–142. doi: 10.1007/BF00406366. [DOI] [PubMed] [Google Scholar]
- Makemson J. C. Control of in vivo luminescence in psychrophilic marine Photobacterium. Arch Mikrobiol. 1973 Nov 19;93(4):347–358. doi: 10.1007/BF00427930. [DOI] [PubMed] [Google Scholar]
- Meighen E. A. Biosynthesis of aliphatic aldehydes for the bacterial bioluminescent reaction: stimulation by ATP and NADPH. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1080–1086. doi: 10.1016/s0006-291x(79)80018-2. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Nicoli M. Z., Hastings J. W. Functional differences of the nonidentical subunits of bacterial luciferase. Properties of hybrids of native and chemically modified bacterial luciferase. Biochemistry. 1971 Oct 26;10(22):4069–4073. doi: 10.1021/bi00798a009. [DOI] [PubMed] [Google Scholar]
- Ne'eman Z., Ulitzur S., Branton D., Hastings J. W. Membrane polypeptides co-induced with the bacterial bioluminescent system. J Biol Chem. 1977 Jul 25;252(14):5150–5154. [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Low oxygen is optimal for luciferase synthesis in some bacteria. Ecological implications. Arch Microbiol. 1977 Feb 4;112(1):9–16. doi: 10.1007/BF00446648. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Markovitz A. Mutant analysis and enzyme subunit complementation in bacterial bioluminescence in Photobacterium fischeri. J Bacteriol. 1970 Oct;104(1):300–312. doi: 10.1128/jb.104.1.300-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P., Baumann L. Study of genetic relationships among marine species of the genera Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch Microbiol. 1976 Oct 11;110(1):101–120. doi: 10.1007/BF00416975. [DOI] [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P. Effect of sodium chloride on growth of heterotrophic marine bacteria. Arch Microbiol. 1974 May 20;97(4):329–345. doi: 10.1007/BF00403071. [DOI] [PubMed] [Google Scholar]
- Reichelt J. L., Doelle H. W. The influence of dissolved oxygen concentration on phosphofructokinase and the glucose metabolism of Escherichia coli K-12. Antonie Van Leeuwenhoek. 1971;37(4):497–506. doi: 10.1007/BF02218520. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Morin J. G. Luminous enteric bacteria of marine fishes: a study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979 Sep;38(3):406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Pyruvate production and excretion by the luminous marine bacteria. Appl Environ Microbiol. 1977 Aug;34(2):164–169. doi: 10.1128/aem.34.2.164-169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Shilo M., Yetinson T. Physiological characteristics underlying the distribution patterns of luminous bacteria in the mediterranean sea and the gulf of elat. Appl Environ Microbiol. 1979 Oct;38(4):577–584. doi: 10.1128/aem.38.4.577-584.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Myristic acid stimulation of bacterial bioluminescence in "aldehyde" mutants. Proc Natl Acad Sci U S A. 1978 Jan;75(1):266–269. doi: 10.1073/pnas.75.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Yashphe J. An adenosine 3',5'-monophosphate-requiring mutant of the luminous bacteria Beneckea harveyi. Biochim Biophys Acta. 1975 Oct 9;404(2):321–328. doi: 10.1016/0304-4165(75)90339-6. [DOI] [PubMed] [Google Scholar]
- Warner J. A., Latz M. I., Case J. F. Cryptic bioluminescence in a midwater shrimp. Science. 1979 Mar 16;203(4385):1109–1110. doi: 10.1126/science.203.4385.1109. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Mimura N., Takimoto A., Nakamura T. Luminescence and respiratory activities of Photobacterium phosphoreum. Competition for cellular reducing power. J Biochem. 1975 Jun;77(6):1147–1155. [PubMed] [Google Scholar]
- Watanabe T., Nakamura T. Studies on luciferase from Photobacterium phosphoreum. II. Substrate specificity and stoichiometry of the reaction in vitro. J Biochem. 1972 Sep;72(3):647–653. doi: 10.1093/oxfordjournals.jbchem.a129942. [DOI] [PubMed] [Google Scholar]
- Waters C. A., Hastings J. W. Mutants of luminous bacteria with an altered control of luciferase synthesis. J Bacteriol. 1977 Aug;131(2):519–525. doi: 10.1128/jb.131.2.519-525.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetinson T., Shilo M. Seasonal and geographic distribution of luminous bacteria in the eastern mediterranean sea and the gulf of elat. Appl Environ Microbiol. 1979 Jun;37(6):1230–1238. doi: 10.1128/aem.37.6.1230-1238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. E., Roper C. F. Bioluminescent countershading in midwater animals: evidence from living squid. Science. 1976 Mar 12;191(4231):1046–1048. doi: 10.1126/science.1251214. [DOI] [PubMed] [Google Scholar]
- Zobell C. E., Rittenberg S. C. THE OCCURRENCE AND CHARACTERISTICS OF CHITINOCLASTIC BACTERIA IN THE SEA. J Bacteriol. 1938 Mar;35(3):275–287. doi: 10.1128/jb.35.3.275-287.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]