Abstract
A 70-mer oligonucleotide-based microarray (1152 features) that emphasizes stress and immune responses factors was constructed to study transcriptomic responses of the snail Biomphalaria glabrata to different immune challenges. In addition to sequences with relevant putative ID and Gene Ontology (GO) annotation, the array features non-immune factors and unknown B. glabrata ESTs for functional gene discovery. The transcription profiles of B. glabrata (3 biological replicates, each a pool of 5 snails) were recorded at 12 hours post wounding, exposure to Gram negative or Gram positive bacteria (Escherichia coli and Micrococcus luteus, respectively), or infection with compatible trematode parasites (S. mansoni or E. paraensei, 20 miracidia/snail), relative to controls, using universal reference RNA. The data were subjected to Significance Analysis for Microarrays (SAM), with a false positive rate (FPR) ≤10%. Wounding yielded a modest differential expression profile (27 up/21 down) with affected features mostly dissimilar from other treatments. Partially overlapping, yet distinct expression profiles were recorded from snails challenged with E. coli (83 up/20 down) or M. luteus (120 up/42 down), mostly showing up-regulation of defense and stress-related features. Significantly altered expression of selected immune features indicates that B. glabrata detects and responds differently to compatible trematodes. Echinostoma paraensei infection was associated mostly with down regulation of many (immune-) transcripts (42 up/68 down), whereas S. mansoni exposure yielded a preponderance of up-regulated features (140 up/23 down), with only few known immune genes affected. These observations may reflect the divergent strategies developed by trematodes during their evolution as specialized pathogens of snails to negate host defense responses. Clearly, the immune defenses of B. glabrata distinguish and respond differently to various immune challenges.
Keywords: oligonucleotide microarray, immune challenges, comparative immunology, lophotrochozoan
Introduction
The planorbid snail Biomphalaria glabrata is an important intermediate host for the larval stages of the digenetic trematode Schistosoma mansoni, a parasite that infects nearly 100 million people in Africa and the neotropics (Morgan et al., 2001). In addition to S. mansoni, B. glabrata can be infected by pathogens including other trematode parasites such as Echinostoma paraensei (Loker et al., 1987), bacteria (Bean-Knudsen et al., 1988), and likely viruses (Rondelaud and Barthe, 1992). Commonly, B. glabrata counters pathogens with an effective immune response that involves soluble components and cell-mediated cytotoxicity (Adema et al., 1997; Bayne, 2009; Hahn et al., 2001). In case of infection by S. mansoni or E. paraensei, however, the snail immune response may fail to clear the infection, due in part to trematode-mediated avoidance or inhibition of snail defense mechanisms (Coustau and Yoshino, 1994; Douglas et al., 1993; Lie and Heyneman, 1977; Loker et al., 1986; Loker and Hertel, 1987; Noda and Loker, 1989a,b; Roger et al., 2008).
Current understanding of the immunity of B. glabrata and other molluscs is incomplete (Bayne, 2009), yet a number of recent studies have identified molecules that may be relevant to their defense (Bouchut et al., 2007; Bouchut et al., 2006a; Bouchut et al., 2006b; Guillou et al., 2007; Hanelt et al., 2008; Knight et al., 2009; Lockyer et al., 2007a; Lockyer et al., 2007b; Mitta et al., 2005; Raghavan et al., 2003; Stout et al., 2009; Vergote et al., 2005). Little is known about the specific functions of many of these molecules, but we can nonetheless gain valuable information about the roles of these molecules by assessing the snail response as a whole, documenting transcriptional trends displayed in response to specific stimuli. Of particular interest is to determine the extent to which snails, as representative lophotrochozoans, can mount responses that are tailored to specific groups of pathogens, as suggested by previous comparative analysis of ESTs profiles of B. glabrata exposed to bacteria or parasites (Mitta et al., 2005; Hanelt et al., 2008). A comprehensive microarray-based approach can reveal whether exposure to infection elicits a “one size fits all” type of defense response, or whether snails mount different kinds of responses depending on the stimulus. This is particularly of interest when the infectious agents such as digenetic trematodes, that have an intimate evolutionary association with snails, initiate complex developmental programs that result in long-term infections that are overtly deleterious to their snail hosts.
This study employed an oligo-based microarray to survey transcriptional responses of B. glabrata to wounding, exposure to bacteria (Gram negative Escherichia coli or Gram positive Micrococcus luteus) and to digenetic trematodes (S. mansoni, and E. paraensei). The design of the oligo-based array was targeted in the sense that it emphasizes features involved in immune or stress-related responses. The study of transcriptomic responses of snails to parasitism and other environmental stimuli is currently in its infancy, only a single other report featuring a B. glabrata cDNA microarray, different from the one employed here, has been published (Lockyer et al., 2008). Studies of other invertebrate host-parasite associations indicate the great potential for this approach to reveal immunological mechanisms critical to host defense or parasite survival (Abraham et al., 2004; Dimopoulos et al., 2002; Srinivasan et al., 2004; Xu et al., 2005; Baton et al., 2009). This paper aims to present and validate the oligo-based array, and to exemplify its use by comparative analysis of the responses of B. glabrata at a relatively early time point (12 hours) following the immune challenges noted above. The results indicate that B. glabrata snails mount different defense responses depending on the nature of the biological stimulus.
Materials and Methods
Live material and experimental treatments
The M line strain of the snail Biomphalaria glabrata used in these studies serves as intermediate host for both Echinostoma paraensei and Schistosoma mansoni (PR-1 strain). Both snails and trematodes were maintained at the University of New Mexico as previously described (Stibbs et al., 1979; Loker and Hertel, 1987). Seven groups of snails were used in this study. The first group consisted of un-manipulated snails (10–12mm shell diameter) that served as controls for snails of similar size in groups two through four. Snails in the second group were stab-wounded with a 27G hypodermic needle. Groups three and four were exposed to Escherichia coli or Micrococcus luteus, respectively, by injection in the headfoot with 50 µL of bacterial culture in LB medium (OD600 of 1.0 = 8 × 108 cells/ml) using a G27 hypodermic needle (Hanelt et al., 2008). These bacteria were selected because they are common in nature, their genomes have been characterized and they are frequently used as model infectious organisms in invertebrates (Hetru and Bulet, 1997). Groups five and six consisted of snails (4–8 mm) that were exposed to E. paraensei or S. mansoni, respectively. For both trematodes, snails were exposed individually to 15–20 miracidia per snail in the wells of a 24-well plate, in artificial spring water (ASW) (Loker and Hertel, 1987) for 12 hours. Size-matched snails (group seven) were sham exposed as controls. Snails from all groups were kept for 12 hours in 24-well plates before RNA was extracted from whole bodies of individual snails. For each group, three biological replicates consisting of pools of 5 snails were used.
Design and generation of a B. glabrata oligonucleotide-based microarray
The B. glabrata 70-mer oligoarray was designed at the Center for Evolutionary and Theoretical Immunology (CETI), University of New Mexico (UNM) and contains 1152 features. Target sequences were selected from the set of 4382 unique sequences identified by cluster analysis of B. glabrata ESTs in GeneBank (Blaxter, 2006, http://www.nematodes.org/NeglectedGenomes/MOLLUSCA/wwwPartiGene.php), combined with ORESTES many of which were recorded uniquely from B. glabrata after bacterial challenge (Hanelt et al., 2008; Lockyer et al., 2007b). The use as selection criteria of Gene Ontology terms related to immunity, stress response, phagocytosis, encapsulation, defense, lectin, lysosome, oxidant, radical, adhesion, apoptosis, cytoskeleton, kinase, and signal transduction yielded 557 targets for features on the array. An additional 502 features on the array represent novel sequences (i.e. no similarity to entries in GenBank databases) with unknown functions were incorporated with the goal of identifying new candidate factors important to the response of B. glabrata. Detection of coding regions using ESTscan v2.1 (Iseli et al., 1999) was used to infer 5’−3’ directionality for unknown ESTs. The array also includes nuclear rDNA sequences and genes of the mitochondrial genome of B. glabrata. An additional 37 features represent mitochondrial and rDNA genes of S. mansoni and E. paraensei, as well as transcripts expressed by intramolluscan larvae of these parasites.
Within the derived population of ESTs, unique 70-mer oligos were selected with the bioinformatics tools Yoda (Nordberg, 2005) and OligoArray v2.1 (Rouillard et al., 2003). Inclusion of 10 alien sequences (SpotReport® Alien® cDNA Array Validation System, Stratagene) facilitates normalization of relative signals for different probes. Sense 70 mer-oligonucleotides were obtained from Integrated DNA Technologies (IDT) and printing and quality control testing were performed at the Hollings Marine Laboratory Genomics Core Facility (HML-GCF)/MUSC in Charleston, SC. The GenePix Array List (GAL) file and details of the features on the array (feature ID, complete cDNA sequence, oligo sequence, BLAST similarity) are provided in supplementary table 1 and supplementary table 2. Note that many features were assigned putative identities based on BLAST results, pending validation by full experimental characterization.
For spotting on the microarray, the oligonucleotides (100µM in deionized water) were diluted into 50% water and 50% Epoxide Spotting Buffer (ESB, Integrated DNA Technologies) to a final concentration of 40 µM. Twenty microliters of each oligonucleotide was transferred to 384 well microarray plates (Genetix). Eight landing lights (consisting of 5 µM Cy5 labeled oligo, 5 µM Cy3 labeled oligo, 40 µM of unlabeled oligo (GCF3), and ESB) were printed on every subarray for orientation. A total of 300 Epoxide-coated glass slides (Corning Life Sciences) were spotted with two complete arrays, using the QArray Max (Genetix). The slides were dried for one hour at 80% humidity and overnight at 60% humidity. The testing of six slides across the printing batch revealed uniform shape and consistent presence of spotted features.
RNA isolation
After removal of the shell, whole body tissues of individual snails was homogenized in a 1.5 mL tube with a plastic pestle (Kimble/Kontes Glass Co.) in 500 µL Trizol (Invitrogen). The homogenate was extracted with 100 µL of chloroform and centrifuged (12,000 g, 15 min, 4°C). The aqueous phase was transferred to a new tube and the RNA was precipitated with 0.5 mL isopropanol, pelleted (12,000 g, 10 min, 4°C), washed (75% ethanol), air dried, and dissolved in 50 µL deionized water. The RNA samples were treated with TURBO DNA-FREE® (Ambion) to remove residual DNA, quantified spectrophotometrically, and evaluated using an Agilent 2100 Bioanalyzer.
Universal Reference RNA (URR)
A universal reference RNA (URR) sample was used for the normalization of gene expression data for all microarray experiments (Novoradovskaya et al., 2004). This URR was a mixture of RNA from control (non-infected) B. glabrata (95%), 1.25% from S. mansoni -infected B. glabrata, 1.25% from E. paraensei-infected B. glabrata, 1.25% from E. coli-exposed B. glabrata and 1.25% of M. luteus-exposed B. glabrata, in order to generate a positive signal for as many array features as possible.
Generation of labeled cDNA probes and microarray hybridization
cDNAs were generated from 1 µg of B. glabrata total RNA using a modified mRNA amplification reaction with template-switching PCR (Petalidis et al., 2003). RNA was mixed with 7 pg of each SpotReport Alien mRNA 1–10 (Stratagene), 20 pMol 3’ SMART CDS primer IIA (5′-AAGCAGTGGTATCAACGCAGAGTACT30VN-3′) and 20 pMol template switching primer [5′-d(AAGCAGTGGTATCAACGCAGAGTACGC)r(GGG)-3′] in a 12 µL volume, incubated at 72°C for 5 minutes, and placed on ice. Two µL of 10x ArrayScript™ buffer, 4 µL of 10mM dNTPs, 1 µL of RNase inhibitor (Applied Biosystems), 1 µL of ArrayScript™ Reverse transcriptase (Ambion) were added to generate first strand cDNA at 42°C for 2 hrs. Ten µL of the first-strand cDNA reaction was combined with 62 µL dH2O, 10 µL of 10x PCR buffer II, 10 µL of 25 mM MgCl2, 2 µL of 10 mM dNTPs, 4 µL of 10 µM 5’ PCR primer (5’-AAGCAGTGGTATCAACGCAGAGT-3’) and 2 µL of AmpliTaq® (40 U/ µL) (Applied Biosystems) for amplification of second-strand cDNA. Amplification conditions were 95°C for 1 min for one cycle, 95°C for 5s, 68°C for 6 mins for 15 cycles. Double stranded cDNA was purified (QIAquick® PCR Purification Kit, Qiagen) and quantified spectrophotometrically. For labeling (BioPrime® DNA Labeling System, Invitrogen) with Cy3-dCTP or Cy5-dCTP (GE Healthcare-Amersham), 200 ng of ds cDNA in 21 µL dH2O was combined with 20 µL of 2.5x random primer reaction buffer, incubated at 95°C for 5 min and placed on ice for addition of 5 µL low-C dNTP mix (5mM dATP, 5mM dGTP, 5mM dTTP, 2mM dCTP), 2 µL Cy3 or Cy5 dCTP (1 mM) and 1 µL Klenow enzyme (40U/ µL). The labeling reaction (37°C, 2 hrs) was stopped by adding 5 µL Stop Buffer. Labeled probes were purified separately (AutoSeq™ G-50 Dye Terminator Removal Kit, GE Healthcare). After spectrophotometric determination of labeling efficiency, the cDNA probes (Cy5-labeled expereimental, Cy3-labeled URR) were pooled, ethanol precipitated, resuspended in 43 µL hybridization solution (40% formamide, 5x SSC, 5x Denhardt’s solution, 1mM sodium phosphate, 50 mM Tris (pH 7.4) and 0.1% SDS) and incubated at 95°C for 5 min and 50°C for 5 min. Arrays were pre-hybridized for 12 hours at 42°C in a rotating hybridization oven. Labeled probes were added to the microarrays under lifterslips (22IX30-2-7059, Erie Scientific Company), one for each of the two array fields on each slide. Hybridization was performed in slide hybridization chambers (Corning) at 45°C for 16–18 hours. Three post hybridization washes were performed, (2x SSC, 0.1x SSC/0.1% SDS and 0.1x SSC, respectively) for 5 min each, at room temperature with agitation. Only the B. glabrata features on the array were considered in the analyses.
Microarray scanning and analyses
Microarray slides were recorded with a GenePix® 4000B scanner (Axon Instruments) with GenePix® Pro 6.0 (Axon Instruments) software using a modified protocol (Aragon et al., 2006). A preloaded B. glabrata grid was used to align and identify array spots. Alignment diameter limits ranged from 50% and 200%. Nearest negative control spots were selected for background subtraction. Using Acuity v4.0 (Axon Instruments), arrays were normalized using a ratio of medians. Signals were further normalized using the relative signal strength of SpotReport mRNA Alien sequence numbers 9 and 10. Microarray analysis was performed with raw expression data for each element, and signals expressed as a ratio of the experimental group (Cy5) to the URR (Cy3). The oligo-based array platform was validated by probing independent arrays with cDNA derived from each of the three biological replicate samples of unmanipulated B. glabrata (pools of 5 snails). The plotting of pair wise comparisons of the signals (ratio versus URR) recorded from all features for each biological replicate yielded graphs with signals falling closely along a 45° angle line, thus confirming functionality and reproducibility of the experimental methods (Fig. 1). Expression ratios obtained for features relative to size-matched control (unmanipulated) samples were then analyzed with Significance Analysis for Microarrays (SAM; Tusher et al., 2001) which utilized repeated permutations of the data to determine if the expressions of any genes are significantly related to the response. Transcripts expressed at the cutoff for significance of 10% false positive rate (FPR) and ± 1.0 log2 in all experimental groups were considered to be differentially expressed. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE16596 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16596).
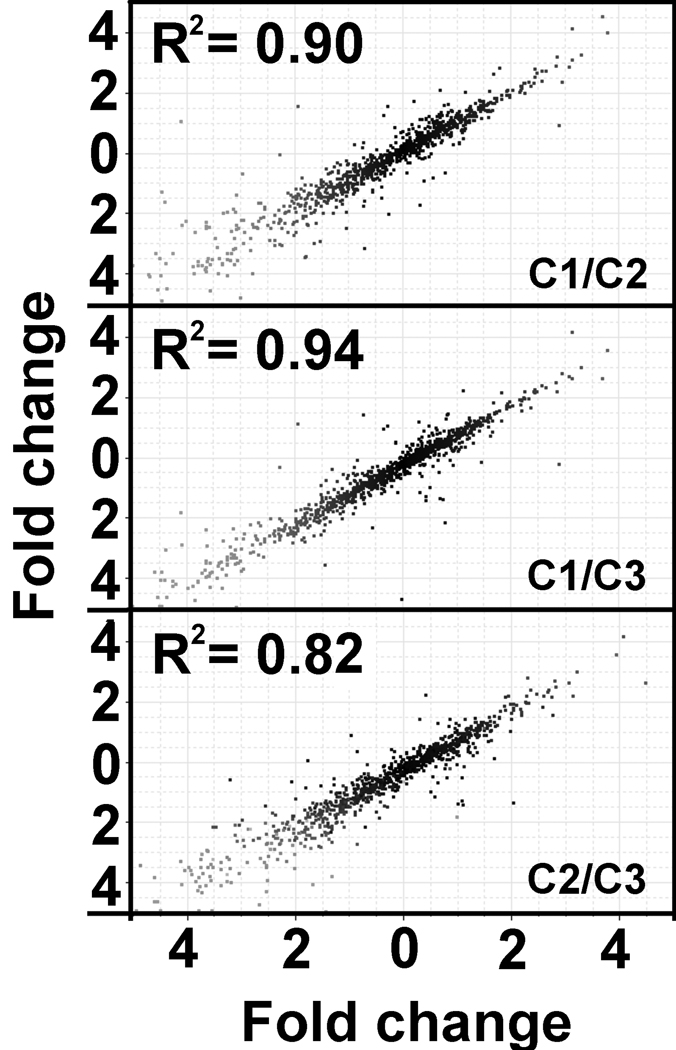
Figure 1. Reproducibility of the B. glabrata oligo-based microarray.
Three different arrays were hybridized with cDNA probes derived from RNA of three independent pools of untreated control B. glabrata (10–12mm shell diameter, n=5/pool). Pairwise comparisons showed that the signals obtained for features of the array (expressed as log2 ratio of experimental versus universal RNA reference) grouped closely along a 45° angle line (R2 values ranging from 0.82 to 0.94). This demonstrates that biological replicate samples yield highly similar signals between array hybridizations. C1, C2, C3 designate the cDNA probes produced with different biological replicates.
Quantitative PCR analysis
Quantitative PCR was used to validate the micro array results, employing cDNA templates that were generated from the same RNA samples as used to generate the array probes. For each target, three reactions were done for each of three biological replicate templates. Primers were designed with Primer Express 2.0 software (Applied Biosystems; Table 2) and used to perform qPCR reactions (SYBR Green PCR, Applied Biosystems) on a Sequence Detection System 7000 (Applied Biosystems), using 18S rDNA as an internal reference. The temperature profile was: 1 cycle of 95°C for 10 minutes; 40 cycles of 95°C for 15 seconds; 58°C for 1 minute. Dissociation curves were generated to check specificity of each amplification reaction.
Table 2.
Primers used for RT-qPCR.
Indicated are the names and sequences (5’–3’) of the transcript-specific primers.
| Primer name | Primer sequence |
|---|---|
| BgLBP/BPI-F | GTAGAGCGGCAAAGTGTCCAC |
| BgLBP/BPI-R | CAATGCATCGTTTTGTTACTTGG |
| Spondin-F | CTGTGGTCAAGCCTGGCTACT |
| Spondin-R | TCAAAGATATGAAGGATGAAATAAACAAG |
| BGCO3661-F | GGATGATTTAACAATGACAGACATGA |
| BGCO3661-R | AGACTATGTAAGTGCAAAGTCAATTCT |
| Lysozyme-F | ATATTATCATTTACTTTCTGCTTTGAATTGTAT |
| Lysozyme-R | CAAGTTATACTGTCCACGTTTCCA |
| C1q-like-F | CTGTAATCCTGAAACTGGCTAAAGAA |
| C1q-like-R | TCCGCGGTTTGCACG |
| F4Q3-F | TGATTCGCCGAATGATAATTGT |
| F4Q3-R | CGTTAAGGTTGACATCAGCACAGT |
Results
Comparison of wounding to bacterial and trematode challenge
Snails were wounded to ascertain the response to integumental breach, without deliberate introduction of pathogens, relative to un-manipulated control snails. The wounding treatment also served as a control for the two groups of snails exposed to bacteria using the same means of breaching the tegument. Wounding alone evoked less transcriptional response than exposure to either bacteria or trematodes (Fig. 2 and Fig. 3), resulting in an increase in expression for 27 transcripts (48% unknowns), and a decrease for 21 (57% unknowns). Of the transcripts with increased expression levels, 20 were also up-regulated following bacterial and trematode challenges (Fig. 3a). Specifically, 17 were up-regulated following exposure with E. coli, 19 with M. luteus, one with E. paraensei, and two with S. mansoni. None of the up-regulated transcripts occurred in all treatment groups. Transcripts involved in cell proliferation, including CDC7-related kinase, SMAD 4 and TGF-beta receptor 1, were common between bacterial injection and wounding (Table 3). Sixteen of the 21 transcripts with reduced expression were unique to the wounding treatment (Fig. 3b). The remaining five (all unknowns) were all down-regulated following injection with M. luteus, whereas two were shared with E. coli injection, none with E. paraensei infection, and one with S. mansoni infection.
Figure 2. Transcriptional responses of B. glabrata to experimental challenges.
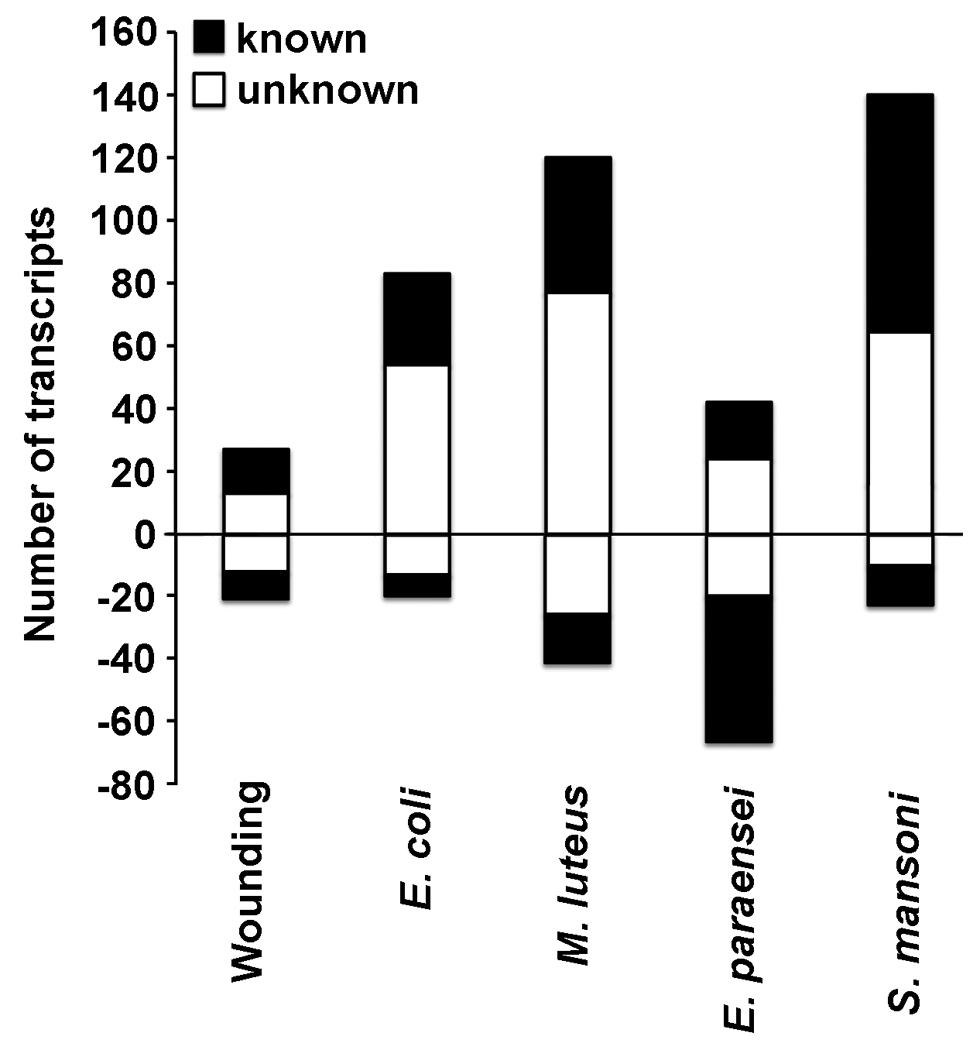
The number of features that were up- or down-regulated at 12 hours after challenge is shown for each experimental treatment. False Positive Rate ≤ 10%. Features with a (putative) ID are numbered in black bars, white bars indicate unknown (novel) sequences.
Figure 3. Response of B. glabrata to wounding compared to other challenges.
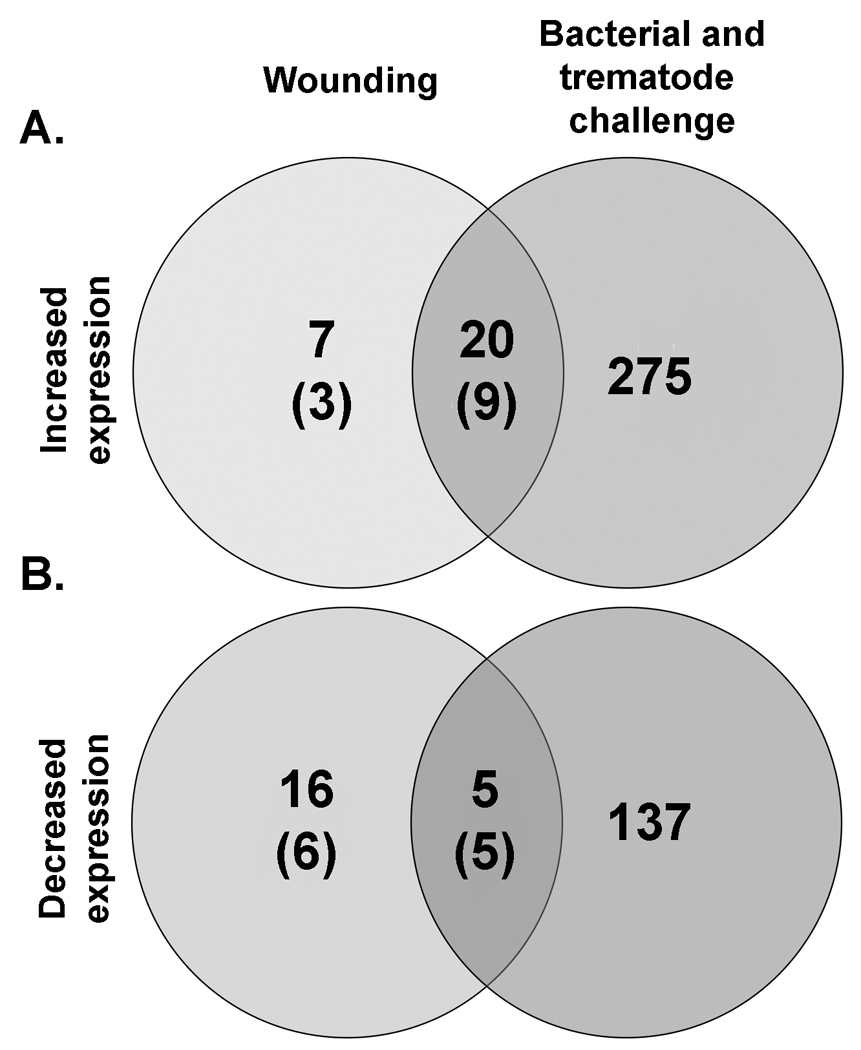
Transcripts significantly increased (a) or decreased (b) at 12 hours post wounding were modest in number and encompassed different sequences in relation to the features affected by challenge with bacteria and trematodes (shown combined). For wounding, the total number of differentially expressed features is shown with the number features with unknown function in brackets. False Positive Rate ≤ 10%.
Table 3.
Differentially expressed (putative) immune-relevant features,.
Listed for each experimental treatment are the affected transcripts (name, feature code from GAL file), fold change (negative = down-regulated, positive = up-regulated), and putative function.
| Fold Change |
Putative Function | |
|---|---|---|
| WOUNDING | ||
| Bg LBP/BPI, BGC03345 | 4.458 | Lipopolysaccharide-binding protein/Bactericidal permeability- increasing protein Anti-bacterial protein, binds LPS (Gonzalez et al., 2007). |
| Peptidoglycan-recognition protein-(PGRP) SC2, P073H12 |
−4.829 | Recognition of peptidoglycan. May protect against over-responding to bacterial infection (Bischoff et al., 2006). |
| SMAD4, BGC04177 | 6.812 | Transcription factor, Involved in TGF-B signaling pathway to induce proliferation and differentiation of cells (Brummel et al., 1999). |
| TGF-beta type I receptor (TGFR-1), BGC04521 |
2.667 | Likely involved in binding a TGF-beta-like molecule, possibly induces cell proliferation and differentiation (Dworkin and Gibson, 2006). |
| E. coli INJECTION | ||
| Bg LBP/BPI, BGC03345 | 2.351 | Lipopolysaccharide-binding protein/Bactericidal permeability- increasing protein Anti-bacterial protein, binds LPS (Gonzalez et al., 2007). |
| CDC7-related kinase, BGC01771 |
9.339 | Required for the initiation and progression of cell cycle (Masai et al., 1999). |
| C1q-like protein 4, BGC00252 |
2.331 | Contains C1q-like domain with lectin properties. Related sequence responds to bacteria in molluscs (Zhang et al., 2008a). |
| JNK-interacting protein 3, BGC02448 |
2.141 | Involved in signal transduction after TLR 4 activation in mammals. Presumed to also interact with Toll in Drosophila (Matsuguchi et al., 2003). |
| Low density lipoprotein (LDL) receptor, BGC04358 |
2.098 | Potentially involved in the recognition of oxidized lipoproteins. Cholesterol metabolism (Hazen, 2008). |
| LPS binding protein-like protein, BGC03011 |
2.708 | LBP/BPI (Lipopolysaccharide-binding protein/Bactericidal permeability-increasing protein) Anti-bacterial protein, binds LPS (sequence differs from BGC03345) (Gonzalez et al., 2007). |
| Lysozyme, BGC00466 | 2.013 | Bacteriolytic activity (Zavalova et al., 2006). |
| MAP kinase 2 (MPK2), BGC04690 |
3.677 | Involved in the p38 signaling pathway in Drosophila. This pathway is involved in cell responses to inflammatory and environmental stimuli (Zhuang et al., 2006). |
| Pulmonary surfactant- associated protein DSP-D, BGC03371 |
4.092 | C-type lectin domain, activities include binding and agglutination of pathogens (Haagsman et al., 2008). |
| Serpin B4, P075G03 | 2.294 | Promotes cell survival (Zou et al., 2009) |
| Serpin B6, BGC01370 | 2.009 | Regulates immune response by inhibiting function of proPO- activating proteinase-3 (Zou and Jiang, 2005) |
| SMAD4, BGC04177 | 4.607 | Involved in TGF-B signaling pathway to induce proliferation and differentiation of cells (Brummel et al., 1999). |
| Cu-Zn Superoxide dismutase 1 (SOD1), BGC03242 |
2.201 | Detoxification/production of superoxide radicals, especially from phagocytic/granulocytic cells (Marikovsky et al., 2003) |
| M. luteus INJECTION | ||
| Bg LBP/BPI, BGC03345 | 2.025 | Lipopolysaccharide-binding protein/Bactericidal permeability- increasing protein Anti-bacterial protein, binds LPS (Gonzalez et al., 2007). |
| Bg M-line FREP 4, BgMFREP4_1 |
2.348 | Lectin consisting of a single IgSF domain and fibrinogen domain. Evidence suggests involvement in host defense against parasite infection (Adema et al., 1997). |
| Bg M-line FREP 7, BgFibro7_1 |
2.056 | Lectin consisting of two IgSF domains and a fibrinogen domain. Likely involved in host defense (Zhang and Loker, 2004). |
| Low density lipoprotein (LDL) receptor, BGC04358 |
2.855 | Potentially involved in the recognition of oxidized lipoproteins. Cholesterol metabolism (Hazen, 2008). |
| Lysozyme, BGC00466 | 2.262 | Bacteriolytic activity (Zavalova et al., 2006). |
| MAP kinase 2 (MPK2), BGC04690 |
4.450 | Involved in the p38 signaling pathway in Drosophila. This pathway is involved in cell responses to inflammatory and environmental stimuli (Zhuang et al., 2006). |
| Multi-drug resistance- associated protein, P077E11 |
2.302 | Resembles ATP-binding cassette (ABC) drug resistance transporters. These proteins are involved in drug resistance (Sheps et al., 2004). |
| NF-kappa-B p105 subunit, 139917075 |
2.481 | Transcription factor of NF-kappa-B/Toll pathway, regulation inflammatory response (Chang et al., 2009). |
| Pulmonary surfactant- associated protein DSP-D, BGC03371 |
3.323 | C-type lectin domain, activities include binding and agglutination of pathogens (Haagsman et al., 2008). |
| Serpin B4, P075G03 | 2.471 | Promotes cell survival (Zou et al., 2009) |
| Serpin B6, BGC01370 | 2.772 | Regulates immune response by inhibiting function of proPO- activating proteinase-3 (Zou and Jiang, 2005) |
| SH3 domain-binding protein 5, BGC02605 |
2.361 | Binds to SH3 domains of intracellular proteins. May regulate signaling by SH3 binding (Kang et al., 2000). |
| TGF-beta type I receptor (TGFR-1), BGC04521 |
2.450 | Likely involved in binding a TGF-beta-like molecule, possibly induces cell proliferation and differentiation (Dworkin and Gibson, 2006). |
| Alkaline phosphatase, BGC04218 |
−4.613 | Lysosomal enzyme associated with granulocytic hemocytes of invertebrates (Xing et al., 2002). |
| ELP1 IKK complex- associated protein A, P077G11 |
−4.577 | Binds to NF-kappa-B inducing kinase and IKK which regulates 3 other kinases involved in inflammation (Strnad and Burke, 2007). |
| Peptidoglycan-recognition protein-(PGRP) SC2, P073H12 |
−4.829 | Recognition of peptidoglycan. May protect against over-responding to bacterial infection (Bischoff et al., 2006). |
| Multi-drug resistance protein 2, BGC01951 |
−2.563 | Resembles ATP-binding cassette (ABC) drug resistance transporters. These proteins are involved in drug resistance (Sheps et al., 2004). |
| S. mansoni INFECTION | ||
| Agrin precursor, BGC02479 | 10.283 | Important ECM protein involved in immunological synapse formation which is critical for immune cell activation (Zhang et al., 2006). |
| Bg M-line FREP 2, BgMFREP2_1 |
9.142 | Lectin consisting of a single IgSF domain and fibrinogen domain. Host response to parasite infection (Adema et al., 1997). |
| Bg M-line FREP 4, BgMFREP4_1 |
1.698 | Lectin consisting of a single IgSF domain and fibrinogen domain. Host response to parasite infection (Adema et al., 1997). |
| Bg M-line FREP 6, BgMFREP6 |
1.981 | Lectin consisting of a single IgSF domain and fibrinogen domain. Host response to parasite infection (Adema et al., 1997). |
| C1q TNF-related protein 3, BGC00252 |
3.951 | Induces the proliferation and migration of endothelial cells as well as the up-regulated expression of pro-inflammatory molecules (Paidassi et al., 2008). |
| C1q-like protein 4, BGC002524 |
3.851 | Contains C1q-like domain with lectin properties. Related sequence responds to bacteria in molluscs (Zhang et al., 2008a). |
| Epidermal growth factor (EGF)-related protein, BGC04033 |
4.312 | Regulates processes of cell proliferation, survival and plays an important role in wound healing and tissue remodeling (Schneider and Wolf, 2009) |
| Galectin-4, BGC00934 | 10.574 | Binds beta-galactosides, shown to be pro-inflammatory and have the ability to activate immune cells (Yang et al., 2008; Yoshino et al., 2008). |
| Glutathione S-transferase (GST), BGC02292 |
9.251 | Heat shock response and drug resistance, also has been shown to be produced by parasites to alter host immune cell activation and development (Ouaissi et al., 2002). |
| Histone H2A, BGC00456 | 4.591 | Host defense response through producing novel antimicrobial peptides (AMPs) from its N-terminus in vertebrates and invertebrates (Georgatos et al., 2009). |
| Histone H2AV H2A.F/Z, BGC02328 |
5.741 | Host defense response through producing novel antimicrobial peptides (AMPs) from its N-terminus in vertebrates and invertebrates (Georgatos et al., 2009) |
| Histone H3.3, BGC02445 | 43.017 | Host defense response through producing novel antimicrobial peptides (AMPs) from its N-terminus in vertebrates and invertebrates (Georgatos et al., 2009) |
| Lipopolysaccharide-binding prot LBP, BGC02348 |
7.658 | LBP/BPI (Lipopolysaccharide-binding protein/Bactericidal permeability-increasing protein) Anti-bacterial protein, binds LPS (sequence differs from BGC03345, BGC03011) (Gonzalez et al., 2007). |
| Cu-Zn Superoxide dismutase 1 (SOD1), BGC03242 |
6.495 | Enzyme involved in the detoxification/production of superoxide radicals, especially from phagocytic/granulocytic cells (Marikovsky et al., 2003) |
| E. paraensei INFECTION | ||
| Bg M-line FREP 4, BgMFREP4_1 |
3.324 | Lectin consisting of a single IgSF domain and fibrinogen domain. Host response to parasite infection (Adema et al., 1997)‥ |
| C1q TNF-related protein 3, BGC00252 |
1.896 | Induces the proliferation and migration of endothelial cells as well as the upregulated expression of pro-inflammatory molecules (Paidassi et al., 2008). |
| C1q-like protein 4, BGC002524 |
2.924 | contains C1q-like domain with lectin properties. Related sequence responds to bacteria in molluscs (Zhang et al., 2008a). |
| Epidermal growth factor (EGF)-related protein, BGC04033 |
18.866 | Regulates processes of cell proliferation, survival and plays an important role in wound healing and tissue remodeling (Schneider and Wolf, 2009). |
| Ras-related protein Rab-6.1, BGC01836 |
1.829 | Involved in organelle targetting, important for phagolysosome formation after initial phagocytic event (Kinchen and Ravichandran, 2008). |
| Serine/threonine-protein kinase Polo-like, P077E04 |
2.122 | Important for entry of the cell into M phase mitosis (Archambault and Glover, 2009). |
| Bg M-line FREP 8, BgMFREP8 |
−2.553 | Lectin consisting of a single IgSF domain and fibrinogen domain. Evidence suggests involvement in host defense against parasite infection (Zhang et al., 2008b). |
| CREB-binding protein, BGC02298 |
−1.753 | Important transcription factor involved in the acute and cronic inflammatory response after triggering the NF-kappa-B or AP-1 pathways (Matt, 2002). |
| Bg FREM, P079H03 | −2.203 | Fibrinogen-related domain (FReD) containing sequece wit two EGF domains which may be important for cell interactions or developmental processes (Zhang et al., 2008b). |
| Glutathione S-transferase (GST), BGC02292 OR BGC02321 OR P071F12 |
−2.411 | Important in the processes of heat shock response and drug resistance, also has been shown to be produced by parasites to alter host immune cell activation and development (Ouaissi et al., 2002). |
| Heat shock 70 kDa protein (HSP70) BGC02350 |
−2.166 | Enhances cell survival by preventing apoptotic signaling. Can inhibit inflammatory response if intracellular, enhance inflammatory response if extracellular (Giffard et al., 2008). |
| Histone H3.3, BGC02445 | −2.211 | Reported to participate in host defense response through producing novel antimicrobial peptides (AMPs) from its N-terminus in vertebrates and invertebrates (Georgatos et al., 2009). |
| LRP/Alpha-2-macroglobulin receptor, BGC04652 |
−2.193 | Variety of functions, lipid metabolism, protease degredation, activation of lysosomal enzymes. Important molecule in phagocytic cells (Correia Soeiro et al., 2001). |
| Macrophage migration inhibit factor (MIF), BGC02909 |
−1.888 | Involved in the inflammatory and immune responses, including phagocytosis, cell spreading and tumor suppression (Nishihira, 2000). |
| Mitogen-activated protein kinase-binding Mp1, BGC02379 |
−2.300 | Regulated MAP kinase signaling to control processes of cell proliferation, differentiation and activation (Sharma et al., 2005). |
| Peptidoglycan-recognition protein-(PGRP) SC2, P073H12 |
−2.227 | Recognition of peptidoglycan. May protect against over-responding to bacterial infection (Zhang et al., 2007). |
| Peroxiredoxin-4, BGC03555 | −2.233 | Reduces hydrogen peroxide, organic peroxides and peroxynitrite. Involved in reducing oxidative stress, and modulating signaling cascades involving nitric oxide (Knight et al., 2009). |
| Cu-Zn Superoxide dismutase 1 (SOD1), BGC03242 |
−1.234 | Enzyme involved in the detoxification/production of superoxide radicals, especially from phagocytic/granulocytic cells (Marikovsky et al., 2003). |
Profile of transcripts with increased expression after Gram positive or Gram negative bacterial challenge
The number of differentially expressed features in response to challenge with bacteria or trematode parasites is shown in Fig. 2 and Fig. 4. The proportion of unknown features amounted to 65% and 64% of the up-regulated transcripts and 65% and 62% of down regulated features for E. coli and M. luteus, respectively. Among the 52 transcripts that were elevated in response to both Gram negative and Gram positive bacteria are several factors that likely play a role in antibacterial immunity. These included MAP kinase 2, lipopolysaccharide binding protein/bacterial permeability increasing protein (LBP/BPI), lysozyme and serpins B4 and B6 (Table 3).
Figure 4. Comparison of transcription profiles of B. glabrata after challenge with bacteria or digenetic trematodes.
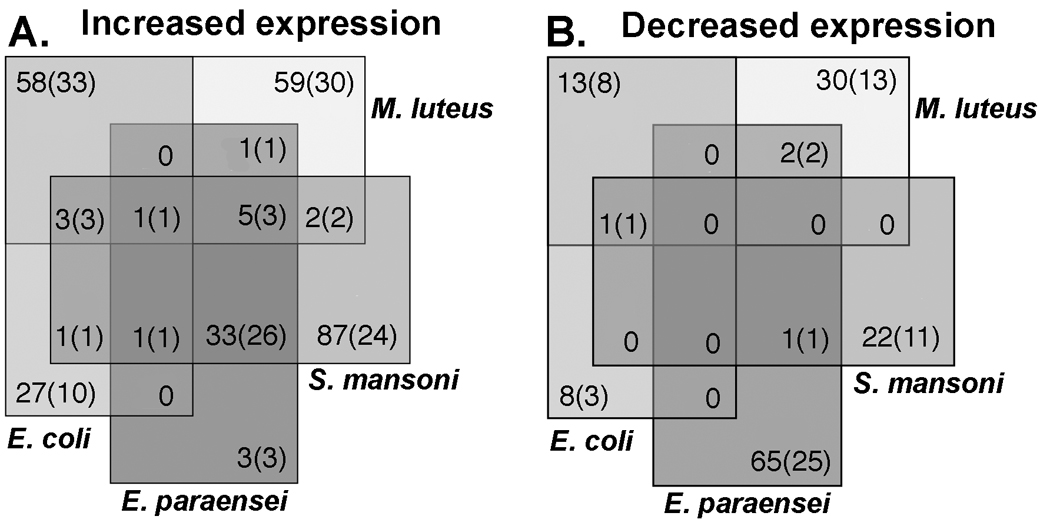
These Venn diagrams show the number of shared and unique features that were up-regulated (a) or down-regulated (b) at 12 hour post exposure to E.coli (Gram negative), M. luteus (Gram positive), E. paraensei and S. mansoni. Each challenge yielded a distinct transcriptome. Note the absence of overlap in features with decreased expression in response to E. paraensei and S. mansoni. The numbers in the Venn diagram represent total number of differentially expressed features with the number of features with unknown function shown in brackets. False Positive Rate ≤ 10%.
Escherichia coli increased expression of another 31 B. glabrata transcripts, four of which responded similarly to S. mansoni, and another that was up-regulated following infections with either trematode species. These latter five transcripts have unknown functions. Among the remaining 26 transcripts are several with immune functions: JNK interacting protein, SMAD 4, C1q-like protein (a lectin), LPS binding protein, and Cu-Zn superoxide dismutase (SOD) (Table 3).
Among the 68 features that yielded increased signals in response to M. luteus, but not to E. coli, were immune-relevant sequences such as fibrinogen-related protein (FREP) 7, NFκB subunit p105 (transcription factor), a TGF receptor homolog, multi-drug resistance-associated protein, and two SH3 domain binding proteins. FREP 4 and another nine features were also responsive to trematode infection (Table 3).
Transcripts with decreased expression following bacterial challenge
Fewer features were down-regulated following bacterial exposure compared to those with elevated expression levels (Fig. 2). The assembly of down-regulated transcripts with immune relevance suggest that bacteria do not appreciably suppress snail immunity (Fig. 4b). Transcripts encoding for immune proteins, peptidoglycan recognition protein (PGRP) SC2, multi-drug resistance protein 2, ELP1 IKK complex-associated protein and alkaline phosphatase were decreased in the presence of M. luteus. No known immune-relevant transcripts exhibited decreased expression after injection with E. coli. (Table 3).
Transcriptional responses of B. glabrata 12 h post exposure to S. mansoni or E. paraensei
Comparison of the response profiles of B. glabrata to the two trematode species disclosed remarkable differences. S. mansoni infection resulted in more features being up- than down-regulated (140 and 23, respectively), whereas E. paraensei yielded the opposite pattern (42 up/68 down). Of the up-regulated transcripts; 57% and 11% were unknown sequences for E. paraensei and S. mansoni, respectively. Of the down-regulated transcripts 29% and 43% were unknown sequences for E. paraensei and S. mansoni respectively (Fig 2). Schistosoma mansoni induced increased expression of more transcripts than any of the treatments tested. The 89 up-regulated transcripts unique to S. mansoni exposure included 7 factors with immune function: FREP 2, FREP 6, FREP 11, LPS binding protein, superoxide dismutase 1 copper chaperone, Cu-Zn SOD, and dual oxidase. Both parasite infections led to increased expression of transcripts resembling C1q TNF-related protein and C1q like protein 4 (Paidassi et al., 2008; Zhang et al., 2008a) (Table 3). All three of the B. glabrata transcripts uniquely up-regulated in the response to E. paraensei are of unknown function (Table 3).
None of the features uniquely down-regulated in the presence of S. mansoni have known immune relevance. However, the 66 transcripts uniquely down-regulated by E. paraensei infection include FREP 8, FReM, alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP/Alpha 2 MR), lysozyme, macrophage migration inhibition factor (MIF), MAP kinase binding protein Mp1, peptidoglycan recognition protein (PGRP SC2), SH3 containing growth factor receptor-bound protein 2 (GRB2)-like protein, and Cu-Zn SOD, that are all likely to have defense functions. No down-regulated transcripts were common between the two trematode infections. The transcriptional profile of B. glabrata at 12 hours post exposure, likely a composite of immune responses of the host and immuno-modulation efforts of the parasite is clearly particular to the species of infecting trematode.
Functional interpretation of challenge-specific transcript expression profiles
To facilitate overall interpretation of the different transcription profiles, immune-related features on the array were arbitrarily divided into the following categories: signal transduction, lectin, antimicrobial, cell adhesion, transcription, apoptosis, muscle, lysozyme, immune response, and proliferation/development. Also delineated were features related to structure and function of the cytoskeleton. Stress-related features were divided by general stress response and detoxification.
Considering these functional categories (Fig. 5), the wounding challenge caused up-regulation of cytoskeletal and proliferation/developmental transcripts in B. glabrata. The two antibacterial responses shared common features but the reaction to E. coli was distinct in consisting of increased expression of transcripts involved in signal transduction, anti-microbial and general immune response, in particular involving lectins. The transcriptomic response to M. luteus featured up-regulation of transcripts involved in signal transduction, but also down-regulation of transcription factors and anti-microbial factors (alkaline phosphatase and peptidoglycan recognition protein; PGRP). At 12 h post infection, the response of B. glabrata to E. paraensei was characterized largely by down-regulation of transcripts encoding lectins, anti-microbial factors, and features involved in signal transduction, cell proliferation, and general immune response. In contrast, S. mansoni infection led to down-regulation of some cytoskeletal and immune response transcripts, but was largely associated with a profile of up-regulation that included few known immune features except for several lectins.
Figure 5. Impact of different challenges on categories of immune or stress response features.
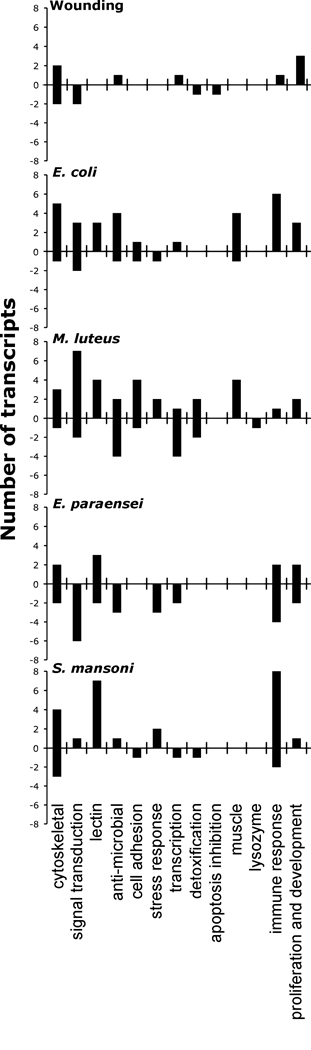
The number of differentially expressed features that was assigned to an immune or stress response category (based on putative ID and GO annotation) is presented for each separate experimental treatment. False Positive Rate ≤ 10%. Bacterial challenge evoked transcriptional responses with mostly increased expression. The responses of B. glabrata to the compatible digenean parasites E. paraensei and S. mansoni include many down-regulated features, likely reflecting less effective immune responses.
Each of the immune challenges also affected expression levels of so-called “unknown” B. glabrata transcripts. Because no functional context exists, however, the implication of differential regulation of these unknowns cannot be interpreted at this time. These unknowns are obvious candidates for additional study.
Confirmation of differential expression by qPCR
Differential expression as detected by the microarray approach was confirmed for a set of selected transcripts using qPCR (Fig. 6). Values for fold change in expression measured by qPCR analysis tended to be greater in magnitude when compared to the values obtained from the array. The qPCR results did correlate closely to the trends observed in transcript expression for all of the array experiments, however.
Figure 6. Differential expression validated by reverse transcripton quantitative PCR.
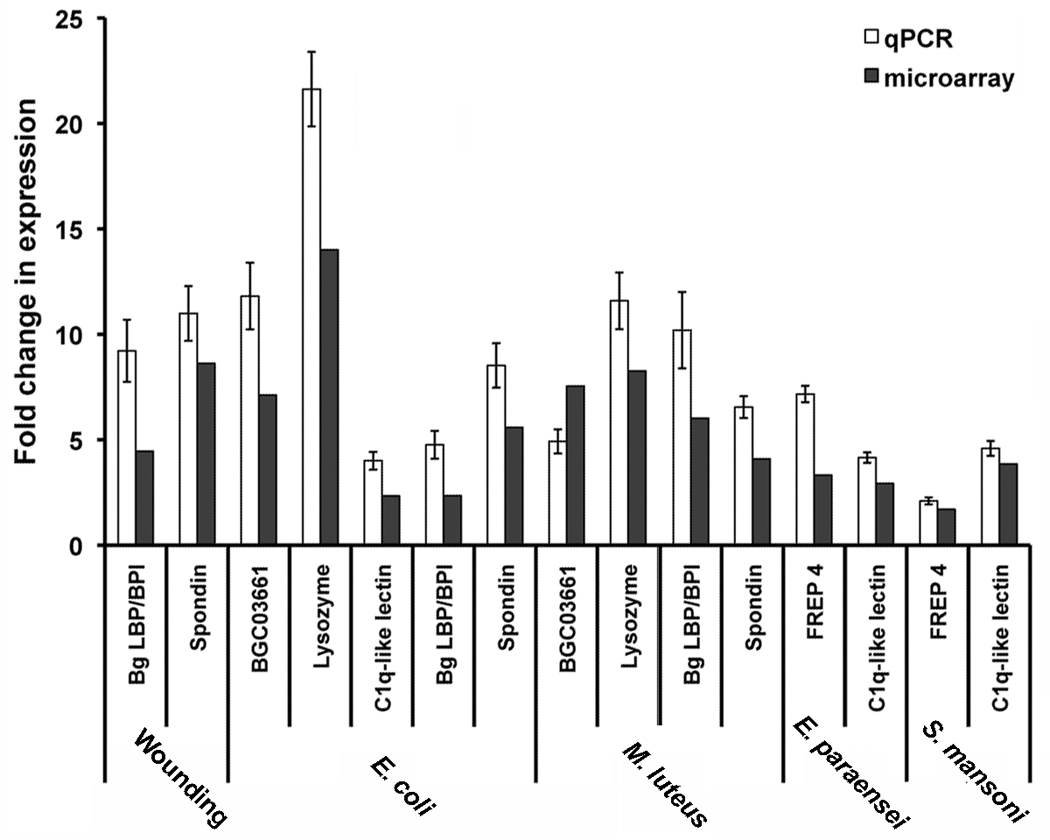
RT-qPCR was applied as independent means to validate differential expression as detected by the microarray approach. Primers were designed for selected features and qPCR was performed with the same templates that were used to generate the cDNA probes for the microarray experiments. While the results tended to indicate greater fold change in expression, RT-qPCR results did correlate closely to the trends observed in transcript expression for all the array treatments.
Discussion
This study provides the first microarray-based comparison of the defense responses of B. glabrata at 12 hours post exposure to a variety of immune challenges: wounding, Gram negative and Gram positive bacteria, and two different digenetic trematode species, S. mansoni and E. paraensei. The distinct responses that were generated to the different insults suggest that considerable complexity and discriminatory ability are associated with the immune response of B. glabrata.
The oligo-based microarray used in this study was designed with a focus on transcripts with relevance to defense and stress response. Our microarray results, validated by qPCR in this study as well as by transcriptome level responses for selected genes reported elsewhere e.g for FREPs, FReM (Zhang et al., 2008b), Cu-Zn SOD and Mn SOD (Guillou et al., 2007; Jung et al., 2005), are consistent with a functional role of homologs of known immune genes in responses of B. glabrata to challenge, and identify unknowns as candidate immune factors.
The wound response in B. glabrata, especially when compared to the reaction profiles generated in response to infectious agents, is relatively modest and distinct (Fig. 1), as it incorporates few known immune genes. This pattern, recorded from a lophotrochozoan, resembles that reported from the ecdysozoans Anopheles gambiae (Dimopoulos et al., 2002) and Drosophila melanogaster (Galko and Krasnow, 2004; Stramer et al., 2008). Larval D. melanogaster exhibit increased expression of many transcription factors and hematopoietic transcripts following injury (Pearson et al., 2009), consistent with production of an increased number of circulating hemocytes after wounding. However, other than melanization responses likely involved in repair (Stramer et al., 2008), wounded Drosophila larvae do not up-regulate a transcription profile indicative of an extensive immune response. Melanization responses play a much less conspicuous role in defense or repair in molluscs such as B. glabrata (Bahgat et al., 2002; Luna-Gonzalez et al., 2003), so it is not surprising that we did not observe up-regulated features involved in melanization cascades. In B. glabrata, transcripts resembling TGF-beta receptor 1 and SMAD 4 were both up-regulated after wounding. Given that homologs of these transcripts are important for fibroblast proliferation and function in vertebrates (Clark et al., 1997), we hypothesize that their increased expression after wounding in B. glabrata indicates activation of cell proliferation for tissue repair.
Invertebrates routinely contend with bacterial challenges and their immune cells swiftly engage in effective phagocytosis and nodulation responses accompanied by oxygen and nitrogen radical production to eliminate a majority of bacterial challenges (Bayne and Fryer, 1994; Hillyer et al., 2003; Molina-Cruz et al., 2008; Noda and Loker, 1989b; van der Knaap et al., 1981). Ecdysozoans like Drosophila (Ferrandon et al., 2007), and Anopheles gambiae (Dong et al., 2006; Heard et al., 2005) are capable of distinguishing between different types of bacterial pathogens. Our study demonstrated that B. glabrata also differentiates between Gram positive and Gram negative bacteria. Several features were in common between the snail’s responses to E. coli and M. luteus: for example, both included increased expression of classical anti-bacterial transcripts such as lysozyme, and LBP/BPI (Beamer et al., 1998; Gonzalez et al., 2007; Li et al., 2008a; Li et al., 2008b). It was evident however, that each bacteria provoked distinct subsets of features. A transcript that was up-regulated in response to E. coli was for Cu-Zn SOD 1, an enzyme involved in the regulation of production of oxygen radicals during the respiratory burst, an important process for killing of bacteria. This enzyme is a component of snail responses to Echinostoma caproni (Guillou et al., 2007). Biomphalaria glabrata is able to resolve E. coli infections quickly (Matricon-Gondran and Letocart, 1999), so the array results provide a better understanding of components involved in this successful response.
The response of B. glabrata to M. luteus encompassed both more uniquely up-regulated and down-regulated features. Notable among the down-regulated transcripts is a homolog of peptidoglycan recognition protein SC2 (PGRP SC2), a scavenger receptor that sequesters soluble peptidoglycan. High levels of circulating PGRP SC2 reduce the response to Gram positive bacteria by limiting the amount of free peptidoglycan available to bind directly to cell-surface PGRPs, an event that activates immune cells in the fruit fly (Charroux et al., 2009). The down-regulation of this transcript in snails suggests that there should be an enhanced capacity to respond to peptidoglycan, potentially increasing the efficiency of bacterial clearance.
Among the transcripts that were up-regulated in response to M. luteus were FREP 4 and FREP 7, these are lectins from B. glabrata that contain fibrinogen-related domains (FREDs). Such lectins have been recovered in surprising diversity from several invertebrates (Adema et al., 1997; Christophides et al., 2002; Dong and Dimopoulos, 2009; Hibino et al., 2006; Stout et al., 2009), and were identified as components of responses against bacteria and parasites of the immune systems of both invertebrates and vertebrates (Dong and Dimopoulos, 2009; Zhang and Loker, 2004, Azumi et al., 2003; Frederiksen et al., 2005). Our results suggest a role for FREPs in response to Gram positive but not Gram negative bacteria at 12 hours post exposure. FREP 3 and 7 were shown to bind both Gram positive and negative antigens (Zhang et al., 2008c), so they likely act as pattern recognition molecules for bacteria, as noted for other FRED-containing lectins in other invertebrates.
In addition to common pathogens such as viruses, bacteria and fungi, molluscs like B. glabrata must also contend with a major group of specialized pathogens, the digenetic trematodes. Almost all known digeneans have an obligatory dependence on molluscs, usually snails, for their larval development. Digenean infections typically are highly specific with respect to the molluscan host, are long-term and carry pronounced detrimental consequences, including parasitic castration (Crews and Yoshino, 1989). Biomphalaria glabrata can host multiple species of digeneans and study of these different parasite/host interactions is particularly relevant given that digeneans in their adult stages can cause significant disease in humans (Orihel and Ash, 1995). Some strains of B. glabrata are resistant to S. mansoni (Lie et al., 1979), and this remains an important topic for exploration (Bayne, 2009), as it may lead to novel methods of interrupting transmission of human schistosomaisis. Trematodes actively undertake efforts to avoid immune elimination by host defenses (Lie et al., 1981; Loker and Adema, 1994). Accordingly, gene expression profiles recorded from trematode infected snails will reflect an amalgam of anti-pathogen defense efforts mounted by the snail and of parasite-directed modifications of expression of host genes that may function in defense or other aspects of homeostasis.
In the case of S. mansoni infection, a number of defense-related lectin transcripts of B. glabrata were upregulated, including transcripts encoding for FREPs 2, 4 and 6 (Adema et al., 1997), galectin-4 (Yoshino et al., 2008), and two transcripts encoding lectins with sequence similarities to complement C1q-like lectins (Païdassi et al., 2008; Zhang et al., 2009). Also up-regulated were sequences encoding histones H2A, H2AV, and H3.3. Classically, histones play a role in chromatin remodeling during mitosis (Georgatos et al., 2009), however, in molluscs they have potential anti-microbial properties (Li et al., 2007). Interestingly, after S. mansoni infection histone H3.3 had the highest increase (43 fold) of any of the array features. Theories regarding successful S. mansoni infection in B. glabrata have centered on the concept of the parasite either acquiring host molecules or of producing host-mimicking molecules (Chacon et al., 2002) such that it evades detection by the snail’s immune response and ‘flies under the radar’ of the snail defenses (Loker and Adema 1995; Hanelt et al., 2008). On the contrary, at least at 12 h post-infection, this study suggests that the snail recognizes and responds to this parasite. A few known immune relevant features were up-regulated but since M line snails eventually succumb to S. mansoni infection, the immune response must in some way be inadequate or actively modulated by the parasite, it is also possible that immune compatibility is achieved later during this S. mansoni-B. glabrata interaction.
The cDNA array-based transcriptome study of Lockyer et al., (2008) showed that hemocytes from resistant B. glabrata transcribe many genes at 24 hours post exposure to S. mansoni, while few genes were differentially expressed in susceptible snails. We observed a number of upregulated transcripts in M line B. glabrata, which are also susceptible to S. mansoni. These apparently divergent observations likely stem from different experimental designs. Lockyer et al., (2008), directly comparing RNA expressed in hemocytes from susceptible versus resistant strains of B. glabrata. This highly valid approach is less likely to reveal transcripts that are similarly up- or down-regulated in both experimental groups: such genes will be identified from a comparison of the transcription profile of a susceptible B. glabrata infected with S. mansoni versus that of untreated control snails, as performed in this study. Additional differences may stem from the source of the cDNA probe (RNA from hemocytes versus whole body), bonafide changes in the parasite host interactions between time points, or a combination of these issues. Still, some transcripts were found up-regulated in this study and associated with resistant snails in their study (Lockyer et al., 2008), suggesting that heat shock protein 70, titin and multi-drug resistance associated protein do indeed play an important role in the internal defense system of B. glabrata.
In contrast to S. mansoni, infection with E. paraensei resulted in a predominance of down-regulated features, many of which have immune functions such as macrophage migration inhibition factor (MIF) (Nishihira, 2000), peroxiredoxin-4 (Diet et al., 2007; Knight et al., 2009), cAMP-responsive element modulator (CREM) binding protein (Matt, 2002) and Cu-Zn SOD1 (Marikovsky et al., 2003). Furthermore, FREP 8 and a transcript encoding a distinct category of fibrinogen related molecule (FReM) with an unusual N-terminal domain were also down-regulated. Previously, Zhang et al. (2008b) also observed decreased expression of FReM after E. paraensei infection. This strong trend of down-regulation of transcripts involved in host defense lends support to the hypothesis that E. paraensei actively interferes with the snail immune response (Lie et al., 1981; Loker et al., 1986; Loker et al., 1992).
Many of the features up-regulated by S. mansoni-infected snails were also expressed at elevated levels in response to E. paraensei. This included FREP 4, a plasma lectin that in our experience is the most consistently up-regulated feature accompanying trematode infection in B. glabrata, and that binds and precipitates secretory/excretory products from E. paraensei larvae (Adema et al., 1997). FREP 4 production increases dramatically upon E. paraensei infection, with maximum amounts of protein detected at 4–8 days post infection, followed by a decrease thereafter. These protein expression levels correlate with FREP 4 expression observed in this study. Infection of B. glabrata with E. paraensei also provokes an increase in the number of circulating hemocytes (Noda and Loker, 1989a). We observed an increase in expression of a transcript encoding a homolog of epidermal growth factor, the latter being a pivotal growth factor in vertebrates (Pastore et al., 2008). Given that many transcripts are up-regulated following exposure to E. paraensei, B. glabrata does detect this trematode and attempts to mount a response, but at least at 12 hours, E. paraensei is more capable than S. mansoni of down-regulating array features. This accords broadly with the conclusion of Lie et al., (1982), that the immunosuppressive/interference of E. paraensei is stronger than that of S. mansoni. Strikingly, there was no overlap in the features that were significantly down-regulated by B. glabrata in response to these two trematode species (Fig 3b).
Several recent studies revealed in invertebrates the capacity to recognize specific pathogens, to produce diversified recognition molecules, and even the potential for mounting responses of increased efficacy after prior exposure (Zhang and Loker, 2003; 2004; Kurtz and Armitage, 2006; Baton et al., 2009; Dong and Dimopoulos, 2009). This study demonstrates that juvenile and adult B. glabrata too mount different immune responses to different pathogens. Particularly with respect to E. paraensei, a parasite specifically adapted to colonize B. glabrata, there is evidence consistent with a strategy of parasite-mediated immuno-suppression. With a functional oligo-based microarray platform in hand, further studies are in progress to better define the immune responsiveness of B. glabrata to either S. mansoni or E. paraensei. These approaches will enable us to better understand how trematodes, including species of medical significance such as S. mansoni, continue to thrive in their natural environments, and provide valuable perspective on the immune capabilities of a prominent phylum of lophotrochozoan invertebrates, the Mollusca.
Supplementary Material
Table 1.
General description of features on the array.
| Category | Number | Comments |
|---|---|---|
| Biomphalaria (with putative ID) | 557 | Genes, ESTs, ORESTES (clusters, singletons) |
| Biomphalaria (unkown, novel) | 502 | Genes, ESTs, ORESTES (clusters, singletons) |
| Biomphalaria (other) | 25 | rDNA genes, mitochondrial genes |
| Echinostoma paraensei | 12 | ESTs, rDNA genes, mitochondrial genes |
| Schistosoma mansoni | 26 | ESTs, rDNA genes, mitochondrial genes |
| SpotReport Aliens | 20 | 10 sense, 10 antisense |
| Negative controls | 10 | Plant origin |
| Total: | 1152 |
Acknowledgements
Dr. Mark Blaxter provided us access to his cluster analysis of B. glabrata ESTs in aid of selecting features for the array. This study was supported in part by NIH grant number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources, NIH AI024340 (ESL), NIH AI062363 (CMA) and an NSERC PDF fellowship (PCH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Islam S, Srinivasan P, Ghosh AK, Valenzuela JG, Ribeiro JM, Kafatos FC, Dimopoulos G, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptional repertoire during ookinete development and midgut invasion. J Biol Chem. 2004;279:5573–5580. doi: 10.1074/jbc.M307582200. [DOI] [PubMed] [Google Scholar]
- Adema CM, Hertel LA, Miller RD, Loker ES. A family of fibrinogen-related proteins that precipitates parasite-derived molecules is produced by an invertebrate after infection. Proc Natl Acad Sci U S A. 1997;94:8691–8696. doi: 10.1073/pnas.94.16.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon AD, Quiñones GA, Thomas EV, Roy S, Werner-Washburne M. Release of extraction-resistant mRNA in stationary phase Saccharomyces cerevisiae produces a massive increase in transcript abundance in response to stress. Genome Biol. 2006;7:R9. doi: 10.1186/gb-2006-7-2-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- Azumi K, De Santis R, De Tomaso A, Rigoutsos I, Yoshizaki F, Pinto MR, Marino R, Shida K, Ikeda M, Arai M, Inoue Y, Shimizu T, Satoh N, Rokhsar DS, Du Pasquier L, Kasahara M, Satake M, Nonaka M. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: "waiting for Godot". Immunogenetics. 2003;55:570–581. doi: 10.1007/s00251-003-0606-5. [DOI] [PubMed] [Google Scholar]
- Bahgat M, Doenhoff M, Kirschfink M, Ruppel A. Serine protease and phenoloxidase activities in hemocytes of Biomphalaria glabrata snails with varying susceptibility to infection with the parasite Schistosoma mansoni. Parasitol Res. 2002;88:489–494. doi: 10.1007/s00436-002-0595-6. [DOI] [PubMed] [Google Scholar]
- Baton LA, Robertson A, Warr E, Strand MR, Dimopoulos G. Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics. 2009;10:257. doi: 10.1186/1471-2164-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: a 2009 assessment. Mol Biochem Parasitol. 2009;165:8–18. doi: 10.1016/j.molbiopara.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne CJ, Fryer SE. Phagocytosis and invertebrate opsonins in relation to parasitism. Ann N Y Acad Sci. 1994;712:162–177. doi: 10.1111/j.1749-6632.1994.tb33571.x. [DOI] [PubMed] [Google Scholar]
- Beamer LJ, Fischer D, Eisenberg D. Detecting distant relatives of mammalian LPS-binding and lipid transport proteins. Protein Sci. 1998;7:1643–1646. doi: 10.1002/pro.5560070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean-Knudsen DE, Uhazy LS, Wagner JE, Young BM. Systemic infection of laboratory-reared Biomphalaria glabrata (Mollusca: Gastropoda) with an acid-fast bacillus. J Invertebr Pathol. 1988;51:291–293. doi: 10.1016/0022-2011(88)90039-0. [DOI] [PubMed] [Google Scholar]
- Bender RC, Goodall CP, Blouin MS, Bayne CJ. Variation in expression of Biomphalaria glabrata SOD1: a potential controlling factor in susceptibility/resistance to Schistosoma mansoni. Dev Comp Immunol. 2007;31:874–878. doi: 10.1016/j.dci.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchut A, Coustau C, Gourbal B, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: new candidate genes evidenced by a suppressive subtractive hybridization approach. Parasitology. 2007;134:575–588. doi: 10.1017/S0031182006001673. [DOI] [PubMed] [Google Scholar]
- Bouchut A, Roger E, Coustau C, Gourbal B, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: potential involvement of adhesion genes. Int J Parasitol. 2006a;36:175–184. doi: 10.1016/j.ijpara.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Bouchut A, Sautiere PE, Coustau C, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: Potential involvement of proteins from hemocytes revealed by a proteomic approach. Acta Trop. 2006b;98:234–246. doi: 10.1016/j.actatropica.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O'Connor MB. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13:98–111. doi: 10.1101/gad.13.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon N, Losada S, Noya B, Alarcon de Noya B, Noya O. Antigenic community between Schistosoma mansoni and Biomphalaria glabrata: on the search of candidate antigens for vaccines. Mem Inst Oswaldo Cruz. 2002;97 Suppl 1:99–104. doi: 10.1590/s0074-02762002000900020. [DOI] [PubMed] [Google Scholar]
- Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun SC. NF-kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol. 2009;182:3131–3138. doi: 10.4049/jimmunol.0803637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B, Rival T, Narbonne-Reveau K, Royet J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170:69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Correia Soeiro MN, Paiva MM, Waghabi M, Meirelles MN, Lorent K, Araujo-Jorge TC, Van Leuven F. Differential expression of mRNA coding for the alpha-2-macroglobulin family and the LRP receptor system in C57BL/6J and C3H/HeJ male mice. Cell Struct Funct. 2001;26:161–167. doi: 10.1247/csf.26.161. [DOI] [PubMed] [Google Scholar]
- Coustau C, Yoshino TP. Schistosoma mansoni: modulation of hemocyte surface polypeptides detected in individual snails, Biomphalaria glabrata, following larval exposure. Exp Parasitol. 1994;79:1–10. doi: 10.1006/expr.1994.1053. [DOI] [PubMed] [Google Scholar]
- Crews AE, Yoshino TP. Schistosoma mansoni: effect of infection on reproduction and gonadal growth in Biomphalaria glabrata. Exp Parasitol. 1989;68:326–334. doi: 10.1016/0014-4894(89)90114-8. [DOI] [PubMed] [Google Scholar]
- Diet A, Abbas K, Bouton C, Guillon B, Tomasello F, Fourquet S, Toledano MB, Drapier JC. Regulation of peroxiredoxins by nitric oxide in immunostimulated macrophages. J Biol Chem. 2007;282:36199–36205. doi: 10.1074/jbc.M706420200. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Christophides GK, Meister S, Schultz J, White KP, Barillas-Mury C, Kafatos FC. Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc Natl Acad Sci U S A. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Dimopoulos G. Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem. 2009;284:9835–9844. doi: 10.1074/jbc.M807084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JS, Hunt MD, Sullivan JT. Effects of Schistosoma mansoni infection on phagocytosis and killing of Proteus vulgaris in Biomphalaria glabrata hemocytes. J Parasitol. 1993;79:280–283. [PubMed] [Google Scholar]
- Dworkin I, Gibson G. Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster. Genetics. 2006;173:1417–1431. doi: 10.1534/genetics.105.053868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol. 2005;62:462–473. doi: 10.1111/j.1365-3083.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos SD, Markaki Y, Christogianni A, Politou AS. Chromatin remodeling during mitosis: a structure-based code? Front Biosci. 2009;14:2017–2027. doi: 10.2741/3360. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109:339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Gueguen Y, Destoumieux-Garzon D, Romestand B, Fievet J, Pugniere M, Roquet F, Escoubas JM, Vandenbulcke F, Levy O, Saune L, Bulet P, Bachere E. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc Natl Acad Sci U S A. 2007;104:17759–17764. doi: 10.1073/pnas.0702281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou F, Mitta G, Galinier R, Coustau C. Identification and expression of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Dev Comp Immunol. 2007;31:657–671. doi: 10.1016/j.dci.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Haagsman HP, Hogenkamp A, van Eijk M, Veldhuizen EJ. Surfactant collectins and innate immunity. Neonatology. 2008;93:288–294. doi: 10.1159/000121454. [DOI] [PubMed] [Google Scholar]
- Hahn UK, Bender RC, Bayne CJ. Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. J Parasitol. 2001;87:778–785. doi: 10.1645/0022-3395(2001)087[0778:IONOIK]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hanelt B, Lun CM, Adema CM. Comparative ORESTES-sampling of transcriptomes of immune-challenged Biomphalaria glabrata snails. J Invertebr Pathol. 2008 doi: 10.1016/j.jip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard NA, Holmes CC, Stephens DA, Hand DJ, Dimopoulos G. Bayesian coclustering of Anopheles gene expression time series: study of immune defense response to multiple experimental challenges. Proc Natl Acad Sci U S A. 2005;102:16939–16944. doi: 10.1073/pnas.0408393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetru C, Bulet P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods Mol Biol. 1997;78:35–49. doi: 10.1385/0-89603-408-9:35. [DOI] [PubMed] [Google Scholar]
- Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, Buckley KM, Brockton V, Nair SV, Berney K, Fugmann SD, Anderson MK, Pancer Z, Cameron RA, Smith LC, Rast JP. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol. 1999:138–148. [PubMed] [Google Scholar]
- Jung Y, Nowak TS, Zhang SM, Hertel LA, Loker ES, Adema CM. Manganese superoxide dismutase from Biomphalaria glabrata. J Invertebr Pathol. 2005;90:59–63. doi: 10.1016/j.jip.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kang H, Freund C, Duke-Cohan JS, Musacchio A, Wagner G, Rudd CE. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 2000;19:2889–2899. doi: 10.1093/emboj/19.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Raghavan N, Goodall C, Cousin C, Ittiprasert W, Sayed A, Miller A, Williams DL, Bayne CJ. Biomphalaria glabrata peroxiredoxin: Effect of Schistosoma mansoni infection on differential gene regulation. Mol Biochem Parasitol. 2009 doi: 10.1016/j.molbiopara.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J, Armitage SA. Alternative adaptive immunity in invertebrates. Trends Immunol. 2006;27:493–496. doi: 10.1016/j.it.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Li C, Song L, Zhao J, Zhu L, Zou H, Zhang H, Wang H, Cai Z. Preliminary study on a potential antibacterial peptide derived from histone H2A in hemocytes of scallop Chlamys farreri. Fish Shellfish Immunol. 2007;22:663–672. doi: 10.1016/j.fsi.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Li H, Parisi MG, Toubiana M, Cammarata M, Roch P. Lysozyme gene expression and hemocyte behaviour in the Mediterranean mussel, Mytilus galloprovincialis, after injection of various bacteria or temperature stresses. Fish Shellfish Immunol. 2008a;25:143–152. doi: 10.1016/j.fsi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao J, Wang L, Qiu L, Zhang H, Dong C, Cong M, Song L. The polymorphism of lysozyme gene in Zhikong scallop (Chlamys farreri)and its association with susceptibility/resistance to Listonella anguillarum. Fish Shellfish Immunol. 2008b doi: 10.1016/j.fsi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lie KJ, Heyneman D. Studies on resistance in snails: interference by nonirradiated echinostome larvae with natural resistance to Schistosoma mansoni in Biomphalaria glabrata. J Invertebr Pathol. 1977;29:118–125. doi: 10.1016/0022-2011(77)90183-5. [DOI] [PubMed] [Google Scholar]
- Lie KJ, Heyneman D, Richards CS. Specificity of natural resistance to trematode infections in Biomphalaria glabrata. Int J Parasitol. 1979;9:529–531. doi: 10.1016/0020-7519(79)90008-0. [DOI] [PubMed] [Google Scholar]
- Lie KJ, Jeong KH, Heyneman D. Selective interference with granulocyte function induced by Echinostoma paraensei (Trematoda) larvae in Biomphalaria glabrata (Mollusca) J Parasitol. 1981;67:790–796. [PubMed] [Google Scholar]
- Lie KJ, Jeong KH, Heyneman D. Further characterization of acquired resistance in Biomphalaria glabrata. J Parasitol. 1982;68:529–531. [PubMed] [Google Scholar]
- Lockyer AE, Spinks J, Kane RA, Hoffmann KF, Fitzpatrick JM, Rollinson D, Noble LR, Jones CS. Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant- and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genomics. 2008;9:634. doi: 10.1186/1471-2164-9-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer AE, Spinks J, Noble LR, Rollinson D, Jones CS. Identification of genes involved in interactions between Biomphalaria glabrata and Schistosoma mansoni by suppression subtractive hybridization. Mol Biochem Parasitol. 2007a;151:18–27. doi: 10.1016/j.molbiopara.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer AE, Spinks JN, Walker AJ, Kane RA, Noble LR, Rollinson D, Dias-Neto E, Jones CS. Biomphalaria glabrata transcriptome: identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES) Dev Comp Immunol. 2007b;31:763–782. doi: 10.1016/j.dci.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loker ES, Adema CM. Schistosomes, echinostomes and snails: comparative immunobiology. Parasitol. Today. 1995;11:120–124. [Google Scholar]
- Loker ES, Bayne CJ, Yui MA. Echinostoma paraensei: hemocytes of Biomphalaria glabrata as targets of echinostome mediated interference with host snail resistance to Schistosoma mansoni. Exp Parasitol. 1986;62:149–154. doi: 10.1016/0014-4894(86)90018-4. [DOI] [PubMed] [Google Scholar]
- Loker ES, Cimino DF, Hertel LA. Excretory-secretory products of Echinostoma paraensei sporocysts mediate interference with Biomphalaria glabrata hemocyte functions. J Parasitol. 1992;78:104–115. [PubMed] [Google Scholar]
- Loker ES, Cimino DF, Stryker GA, Hertel LA. The effect of size of M line Biomphalaria glabrata on the course of development of Echinostoma paraensei. J Parasitol. 1987;73:1090–1098. [PubMed] [Google Scholar]
- Loker ES, Hertel LA. Alterations in Biomphalaria glabrata plasma induced by infection with the digenetic trematode Echinostoma paraensei. J Parasitol. 1987;73:503–513. [PubMed] [Google Scholar]
- Luna-Gonzalez A, Maeda-Martinez AN, Vargas-Albores F, Ascencio-Valle F, Robles-Mungaray M. Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bivalve molluscs. Fish Shellfish Immunol. 2003;15:275–282. doi: 10.1016/s1050-4648(02)00165-1. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O. Cu/Zn superoxide dismutase plays important role in immune response. J Immunol. 2003;170:2993–3001. doi: 10.4049/jimmunol.170.6.2993. [DOI] [PubMed] [Google Scholar]
- Masai H, Sato N, Takeda T, Arai K. CDC7 kinase complex as a molecular switch for DNA replication. Front Biosci. 1999;4:D834–D840. doi: 10.2741/masai. [DOI] [PubMed] [Google Scholar]
- Matricon-Gondran M, Letocart M. Internal defenses of the snail Biomphalaria glabrata. J Invertebr Pathol. 1999;74:248–254. doi: 10.1006/jipa.1999.4878. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Masuda A, Sugimoto K, Nagai Y, Yoshikai Y. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 2003;22:4455–4464. doi: 10.1093/emboj/cdg438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt T. Transcriptional control of the inflammatory response: a role for the CREB-binding protein (CBP) Acta Med Austriaca. 2002;29:77–79. doi: 10.1046/j.1563-2571.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Mitta G, Galinier R, Tisseyre P, Allienne JF, Girerd-Chambaz Y, Guillou F, Bouchut A, Coustau C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev Comp Immunol. 2005;29:393–407. doi: 10.1016/j.dci.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123 Suppl:S211–S228. doi: 10.1017/s0031182001007703. [DOI] [PubMed] [Google Scholar]
- Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J Interferon Cytokine Res. 2000;20:751–762. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- Noda S, Loker ES. Effects of infection with Echinostoma paraensei on the circulating haemocyte population of the host snail Biomphalaria glabrata. Parasitology. 1989a;98(Pt 1):35–41. doi: 10.1017/s0031182000059667. [DOI] [PubMed] [Google Scholar]
- Noda S, Loker ES. Phagocytic activity of hemocytes of M-line Biomphalaria glabrata snails: effect of exposure to the trematode Echinostoma paraensei. J Parasitol. 1989b;75:261–269. [PubMed] [Google Scholar]
- Nordberg EK. YODA: selecting signature oligonucleotides. Bioinformatics. 2005;21:1365–1370. doi: 10.1093/bioinformatics/bti182. [DOI] [PubMed] [Google Scholar]
- Novoradovskaya N, Whitfield ML, Basehore LS, Novoradovsky A, Pesich R, Usary J, Karaca M, Wong WK, Aprelikova O, Fero M, Perou CM, Botstein D, Braman J. Universal Reference RNA as a standard for microarray experiments. BMC Genomics. 2004;5:20. doi: 10.1186/1471-2164-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihel TC, Ash LR. Parasites in human tissues. Chicago, IL: ASCP Press; 1995. [Google Scholar]
- Ouaissi A, Ouaissi M, Sereno D. Glutathione S-transferases and related proteins from pathogenic human parasites behave as immunomodulatory factors. Immunol Lett. 2002;81:159–164. doi: 10.1016/s0165-2478(02)00035-4. [DOI] [PubMed] [Google Scholar]
- Paidassi H, Tacnet-Delorme P, Lunardi T, Arlaud GJ, Thielens NM, Frachet P. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS Lett. 2008;582:3111–3116. doi: 10.1016/j.febslet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- Pearson JC, Juarez MT, Kim M, McGinnis W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc Natl Acad Sci U S A. 2009;106:2224–2229. doi: 10.1073/pnas.0810219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petalidis L, Bhattacharyya S, Morris GA, Collins VP, Freeman TC, Lyons PA. Global amplification of mRNA by template-switching PCR: linearity and application to microarray analysis. Nucleic Acids Res. 2003;31:e142. doi: 10.1093/nar/gng142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan N, Miller AN, Gardner M, FitzGerald PC, Kerlavage AR, Johnston DA, Lewis FA, Knight M. Comparative gene analysis of Biomphalaria glabrata hemocytes pre-and post-exposure to miracidia of Schistosoma mansoni. Mol Biochem Parasitol. 2003;126:181–191. doi: 10.1016/s0166-6851(02)00272-4. [DOI] [PubMed] [Google Scholar]
- Roger E, Gourbal B, Grunau C, Pierce RJ, Galinier R, Mitta G. Expression analysis of highly polymorphic mucin proteins (Sm PoMuc) from the parasite Schistosoma mansoni. Mol Biochem Parasitol. 2008;157:217–227. doi: 10.1016/j.molbiopara.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Barthe D. [Epidemiological observations on iridovirosis of Lymnaea truncatula, host mollusca of Fasciola hepatica] C R Acad Sci III. 1992;314:609–612. [PubMed] [Google Scholar]
- Rouillard JM, Zuker M, Gulari E. OligoArray 2.0: design of oligonucleotide probes for DNA microarrays using a thermodynamic approach. Nucleic Acids Res. 2003;31:3057–3062. doi: 10.1093/nar/gkg426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- Sharma C, Vomastek T, Tarcsafalvi A, Catling AD, Schaeffer HJ, Eblen ST, Weber MJ. MEK partner 1 (MP1): regulation of oligomerization in MAP kinase signaling. J Cell Biochem. 2005;94:708–719. doi: 10.1002/jcb.20344. [DOI] [PubMed] [Google Scholar]
- Sheps JA, Ralph S, Zhao Z, Baillie DL, Ling V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004;5:R15. doi: 10.1186/gb-2004-5-3-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Abraham EG, Ghosh AK, Valenzuela J, Ribeiro JM, Dimopoulos G, Kafatos FC, Adams JH, Fujioka H, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J Biol Chem. 2004;279:5581–5587. doi: 10.1074/jbc.M307587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbs HH, Owczarzak A, Bayne CJ, DeWan P. Schistosome sporocyst-killing Amoebae isolated from Biomphalaria glabrata. J Invertebr Pathol. 1979;33:159–170. doi: 10.1016/0022-2011(79)90149-6. [DOI] [PubMed] [Google Scholar]
- Stout BA, Adema CM, Zhang S-M, Loker ES. Biology of FREPs: diversified lectins with fibrinogen-related domains from the freshwater snail Biomphalaria glabrata. In: Vasta GR, Ahmed H, editors. Animal lectins: a functional view. Boca Raton: CRC Press; 2009. pp. 475–491. [Google Scholar]
- Stramer B, Winfield M, Shaw T, Millard TH, Woolner S, Martin P. Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 2008;9:465–471. doi: 10.1038/embor.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap WP, Sminia T, Kroese FG, Dikkeboom R. Elimination of bacteria from the circulation of the pond snail Lymnaea stagnalis. Dev Comp Immunol. 1981;5:21–32. doi: 10.1016/s0145-305x(81)80004-3. [DOI] [PubMed] [Google Scholar]
- Vergote D, Bouchut A, Sautiere PE, Roger E, Galinier R, Rognon A, Coustau C, Salzet M, Mitta G. Characterisation of proteins differentially present in the plasma of Biomphalaria glabrata susceptible or resistant to Echinostoma caproni. Int J Parasitol. 2005;35:215–224. doi: 10.1016/j.ijpara.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Xing J, Zhan WB, Zhou L. Endoenzymes associated with haemocyte types in the scallop (Chlamys farreri) Fish Shellfish Immunol. 2002;13:271–278. doi: 10.1006/fsim.2001.0402. [DOI] [PubMed] [Google Scholar]
- Xu X, Dong Y, Abraham EG, Kocan A, Srinivasan P, Ghosh AK, Sinden RE, Ribeiro JM, Jacobs-Lorena M, Kafatos FC, Dimopoulos G. Transcriptome analysis of Anopheles stephensi-Plasmodium berghei interactions. Mol Biochem Parasitol. 2005;142:76–87. doi: 10.1016/j.molbiopara.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- Yoshino TP, Dinguirard N, Kunert J, Hokke CH. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene. 2008;411:46–58. doi: 10.1016/j.gene.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavalova LL, Yudina TG, Artamonova II, Baskova IP. Antibacterial non-glycosidase activity of invertebrate destabilase-lysozyme and of its helical amphipathic peptides. Chemotherapy. 2006;52:158–160. doi: 10.1159/000092904. [DOI] [PubMed] [Google Scholar]
- Zhang H, Song L, Li C, Zhao J, Wang H, Qiu L, Ni D, Zhang Y. A novel C1q-domain-containing protein from Zhikong scallop Chlamys farreri with lipopolysaccharide binding activity. Fish Shellfish Immunol. 2008a;25:281–289. doi: 10.1016/j.fsi.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Koh J, Lu J, Thiel S, Leong BS, Sethi S, He CY, Ho B, Ding JL. Local inflammation induces complement crosstalk which amplifies the antimicrobial response. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000282. e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang Y, Chu Y, Su L, Gong Y, Zhang R, Xiong S. Agrin is involved in lymphocytes activation that is mediated by alpha-dystroglycan. FASEB J. 2006;20:50–58. doi: 10.1096/fj.04-3303com. [DOI] [PubMed] [Google Scholar]
- Zhang SM, Loker ES. The FREP gene family in the snail Biomphalaria glabrata: additional members, and evidence consistent with alternative splicing and FREP retrosequences. Fibrinogen-related proteins. Dev Comp Immunol. 2003;27:175–187. doi: 10.1016/s0145-305x(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Zhang SM, Loker ES. Representation of an immune responsive gene family encoding fibrinogen-related proteins in the freshwater mollusc Biomphalaria glabrata, an intermediate host for Schistosoma mansoni. Gene. 2004;341:255–266. doi: 10.1016/j.gene.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Nian H, Zeng Y, Dejong RJ. Fibrinogen-bearing protein genes in the snail Biomphalaria glabrata: characterization of two novel genes and expression studies during ontogenesis and trematode infection. Dev Comp Immunol. 2008b;32:1119–1130. doi: 10.1016/j.dci.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Zeng Y, Loker ES. Characterization of immune genes from the schistosome host snail Biomphalaria glabrata that encode peptidoglycan recognition proteins and gram-negative bacteria binding protein. Immunogenetics. 2007;59:883–898. doi: 10.1007/s00251-007-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Zeng Y, Loker ES. Expression profiling and binding properties of fibrinogen-related proteins (FREPs), plasma proteins from the schistosome snail host Biomphalaria glabrata. Innate Immun. 2008c;14:175–189. doi: 10.1177/1753425908093800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZH, Zhou Y, Yu MC, Silverman N, Ge BX. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell Signal. 2006;18:441–448. doi: 10.1016/j.cellsig.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Zou Z, Jiang H. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8. cDNA cloning, protein expression, inhibition kinetics, and function elucidation. J Biol Chem. 2005;280:14341–14348. doi: 10.1074/jbc.M500570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Picheng Z, Weng H, Mita K, Jiang H. A comparative analysis of serpin genes in the silkworm genome. Genomics. 2009;93:367–375. doi: 10.1016/j.ygeno.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.