Abstract
Interferon gamma (IFNγ) is important for immune resistance to herpes simplex virus (HSV) infection. To examine the influence of IFNγ on the development of HSV-specific immune responses and test for IFNγ-independent adaptive immune mechanisms of protection, IFNγ-deficient mice (IFNγ−/−) were immunized with thymidine kinase-deficient HSV-2 (HSV-2 333tk−). HSV-specific cellular and humoral responses were elicited in immunized IFNγ−/− mice resulting in increased resistance relative to non-immune C57BL/6J (B6) mice following challenge with fully virulent HSV-2. CD8+ T cells from IFNγ−/− mice displayed cytotoxic activity and secreted TNFα. HSV-specific CD4+ T cells from immunized IFNγ−/− mice secreted IL-4, TNFα, and IL-17, but unlike T cells from HSV-immune B6 mice, could not clear virus from genital tissue following adoptive transfer. HSV-immune IFNγ−/− mice produced predominantly IgG1 HSV-specific antibodies while immune B6 mice produced predominantly IgG2c antibodies. Transfer of equivalent amounts of HSV-specific antibodies from either strain to naïve mice imparted equivalent early resistance against infection of the genital epithelia. However, protection against neurological symptoms mediated by immune B6 antibodies was superior late in infection. Taken together, these results demonstrate that the limited resistance of HSV-immune IFNγ−/− mice to HSV-2 infection resulted from the action of HSV-specific Ab rather than IFNγ-independent effector functions of T cells. Further, protection against neurological manifestations of HSV-2 infection was superior in mice receiving Ab from immune B6 mice suggesting that Ab-mediated protective mechanisms involving IFNγ-induced IgG subclasses were more effective once virus had spread to neural tissues.
Keywords: HSV-2, sensory ganglia, antibody, female genital tract, IFNγ, IL-17
1. Introduction
Herpes simplex virus type 2 (HSV-2) is an important sexually transmitted pathogen. Severe disease may occur in infected immune-compromised individuals or newborns and HSV-induced genital ulcerations may increase the risk of acquiring HIV (Wald & Link, 2002; Corey et al., 2004). HSV-2 spreads from the genital epithelium to sensory neurons and following brief viral replication, life-long latent infection is established. During reactivation, virus is transported to and shed from the epithelia near the original infection site in the presence or absence of disease symptoms (Wald et al. 2000).
Antibody clearly plays a role in resistance to HSV infection and spread of virus within the nervous system in animal models (Dix et al., 1981; Kohl et al., 1990). Re-infection of immune animals despite high HSV-specific Ab titers has been reported (Kuklin et al., 1998; Milligan et al., 1998), although sterilizing immunity has also been observed (Parr & Parr, 1998). Mechanisms of Ab-mediated protection are not fully understood.
HSV-specific T cells clear infectious virus from epithelial sites of recurrent infection and resolve lesions (Posavad et al., 1997; Koelle et al., 1998). Consistent with this, HSV-specific T cells reside in epithelial/sensory neuron junctions and skin near previous lesions (Zhu et al., 2007). In animal models, CD4+ and CD8+ T cells secreting primarily type 1 cytokines and exhibiting cytolytic function have been detected at the genital site of HSV-2 infection (Milligan & Bernstein, 1995; Milligan et al., 2004). HSV-specific T cell mechanisms of clearance are not completely understood, although evidence suggests requirements for virus-specific cytolytic activity (Dobbs et al., 2005) and involvement of numerous cytokines including IFNγ, TNFα, type I IFNs, and IL-15 (Ito & O-Malley, 1987; Rossol-Voth et al., 1991; Smith et al., 1994; Bouley et al., 1996; Milligan & Bernstein, 1997; Tsunobuchi et al., 2000; Ashkar & Rosenthal, 2003; Pierce et al., 2005). IFNγ is important in protection against HSV infection. Studies involving IFNγ-deficient mice or in vivo neutralization of IFNγ demonstrated significant alteration of outcome including delayed virus clearance from the genital epithelium (Smith et al., 1994; Bouley et al., 1995; Milligan & Bernstein, 1997; Parr & Parr, 1999; Milligan et al., 1998; Harandi et al., 2001a). Rapid resolution of HSV-2 from the female genital tract requires IFNγ stimulation of parenchymal rather than hematopoietic cells (Bird et al., 2007; Ijima et al., 2008).
We previously detected delayed virus clearance in the absence of IFNγ or its receptor (Bird et al., 2007) suggesting alternative, IFNγ-independent clearance mechanisms. T cell-mediated IFNγ-independent protective mechanisms have also been reported during cutaneous HSV-1 infections (Yu et al., 1996). In the present study, IFNγ-deficient mice were immunized with HSV-2 333tk− to examine and evaluate the elicited IFNγ-independent adaptive immune mechanisms in resistance against genital HSV-2 infection.
2. Materials and Methods
2.1. Virus
HSV-2 333tk− (McDermott et al., 1984) was obtained from Mark McDermott (McMaster University, Ontario, Canada). HSV-2 strain 186 was utilized as the challenge virus as described previously (Milligan et al., 2004).
2.2. Virus inoculation and quantification
Mice received 2.0 mg medroxyprogesterone acetate (SICOR Pharmaceuticals, Inc., Irvine, CA) s.c. six days prior to intravaginal inoculation to prepare the genital epithelium for infection (Linehan et al., 2004, Kaushic et al., 2003). Mice were inoculated as described previously (Chu et al., 2008). Mice were not pre-swabbed in Ab passive-transfer experiments to prevent perturbation of Ab-containing vaginal secretions.
To quantify HSV-2 from the vaginal tract, swabs were obtained and titered as described previously (Chu et al., 2008). Infectious virus from homogenized lumbosacral ganglia and adjacent spinal cord was quantified as described previously (Johnson et al., 2008).
2.3. Mice
C57BL/6J (B6), B6.129S7-Ifngtm1Ts/J (IFNγ−/−), and T-cell receptor (TCR)-transgenic C57BL/6-Tg (TcraTcrb) 425Cbn/J (OT-II, chicken ovalbumin peptide OVA323–339-specific CD4+ T cells) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in the AAALAC-approved facility at the University of Texas Medical Branch, Galveston, TX. Experiments were approved by the Institutional Animal Care and Use Committee with oversight by staff veterinarians and followed NIH guidelines on the care and use of laboratory animals.
2.4. Quantification of HSV-specific IgG
HSV-specific IgG was quantified as described previously (Dudley et al., 2000). OD490 was determined on a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA) and compared to standard (purified mouse IgG) values over the linear portion of the standard curve. Ab concentrations were calculated using Softmax software (Molecular Devices). IgG subclass endpoint titers were obtained by plating serial dilutions of serum followed by addition of HRP-conjugated IgG subclass-specific antibodies to detect specific mouse IgG subclasses. Endpoint dilution was defined as the reciprocal of the final dilution resulting in an OD490 greater than 0.1 and greater than twice the values from diluent only controls.
Virus neutralization assays were performed by a previously described plaque reduction method, utilizing rabbit complement (Accurate Chemical and Scientific, Westbury, NY; Milligan et al., 1998).
2.5. Quantification of HSV-specific T lymphocytes
Cytokine-secreting T lymphocytes were quantified by ELISPOT by a modification of the method described previously (Milligan & Bernstein, 1995). CD4+ or CD8+ populations were isolated using magnetic isolation kits (Miltenyi Biotec, Inc., Auburn, CA) and incubated 40 h with mitomycin C-treated (Sigma-Aldrich) B6 splenocytes pulsed with UV-irradiated HSV-2 (CD4+ cells) or gB498–505 (CD8+ cells) on nitrocellulose plates coated with capture Ab for IL-4, IL-17, TNFα, or IFNγ (BD Pharmingen, San Diego, CA). Plates were developed as described previously (Milligan & Bernstein, 1995). Spot-forming cells (SFC) were quantified using an ImmunoSpot reader and analyzed with ImmunoSpot software (Version 4.0, Cellular Technology Ltd, Cleveland, OH).
2.6. Passive transfer of HSV-specific IgG
B6 or IFNγ−/− mice were inoculated intravaginally with HSV-2 333tk− (HSV-immune) twice within four weeks. Immune sera were collected at three weeks after the second inoculation, precipitated with ammonium sulfate and dialyzed extensively against PBS as described previously (Chu et al., 2008). Mice received 23.0 μg of HSV-specific Ab i.p. yielding a serum concentration of approximately 8.0 μg/ml (within the range of HSV-specific IgG concentrations normally detected in B6 mice after intravaginal HSV-2 333tk− inoculation). Mice receiving immune IgG, or undiluted normal mouse serum as a control, were challenged three days later with 104 PFU HSV-2 186.
2.7. Adoptive transfer of CD4+ or CD8+ T cells from HSV-immune B6 or IFNγ−/− mice
HSV-immune B6 and IFNγ−/− mice were challenged with 5 × 105 PFU HSV-2 186. Four days later, lymphocytes from spleen and iliac lymph nodes were cultured at 2 × 108 cells per 175 cm2 flask for three days with UV-inactivated HSV-2 (CD4+ T cells) and 100 U/ml recombinant IL-2. Control cultures of OT-II splenocytes were established similarly using 2 × 107 mitomycin C-treated syngeneic spleen cells pulsed with 100 μM OVA323–339 peptide. CD4+ T cells were purified from day 4 cultures using isolation kits (Miltenyi Biotec, Inc.). Irradiated recipient mice (650 cGy) were reconstituted with 2.25 × 106 purified, activated CD4+ T cells and challenged with 104 PFU HSV-2 333tk−.
2.8. Measurement of in vivo Cytotoxic T-Lymphocyte (CTL) activity
CTL activity was quantified by a modification of the method used previously (Dobbs et al., 2005). HSV-immune B6 or IFNγ−/− mice were challenged with 106 PFU HSV-2 186. B6 splenocyte target cells were split into fractions: one pulsed with gB498–505 peptide and labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes, Eugene, OR), and the second incubated with media only and labeled with 0.25 μM CFSE. On the indicated days post-challenge, 107 cells from each fraction were injected i.v. After 4 hours, single-cell suspensions of spleens were fixed with 1% formaldehyde and analyzed by flow cytometry. Percent specific lysis = (1 - ratio of naïve cell recipient / ratio of activated CD8+ T cell recipient) × 100, where ratio = % CFSElow / % CFSEhigh.
2.9. Statistics
Survival curves were compared by Logrank test. HSV-specific T cell quantification and HSV-2 titers were analyzed by Student’s t test when two groups were compared, or ANOVA with Bonferoni correction for multiple groups. Analyses were performed using GraphPad Prism Version 3.0 for Windows (GraphPad Software, Inc., San Diego, CA). P values less than 0.05 were considered to indicate statistical significance.
3. Results
3.1. Immunization and HSV-2 genital challenge of B6 and IFNγ−/− mice
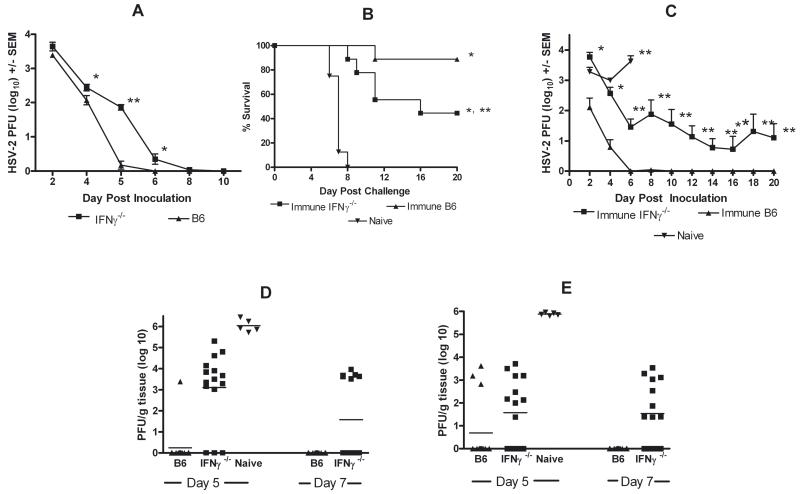
B6 and IFNγ−/− mice were inoculated intravaginally with HSV-2 333tk−. B6 mice were found to clear genital HSV-2 by day 6 post-inoculation (Fig. 1A). Clearance in IFNγ−/− mice was delayed 2 days and titers were significantly higher on days 4 through 6 post-inoculation compared to B6 mice. Resolution of HSV-2 333tk− vaginal infection allowed immunization of IFNγ−/− mice and testing for IFNγ-independent cell- or Ab-mediated mechanisms of protection against HSV-2 challenge. HSV-immune B6 and IFNγ−/− mice were challenged with fully virulent HSV-2 186. All (10/10) naïve B6 mice died by day 8 post-challenge whereas 9/10 HSV-immune B6 mice survived (Fig. 1B). HSV-immune IFNγ−/− mice survived through day 8, but approximately half succumbed by day 20 (P < 0.05 compared to immune B6). As reported previously (Milligan et al., 2004), high HSV-2 titers were detected in naïve B6 mice whereas HSV-immune B6 mice rapidly cleared HSV-2 by day 6 post-challenge (Fig. 1C). Titers in HSV-immune IFNγ−/− mice were initially high (P < 0.001 compared to immune B6), falling rapidly through day 6 before stabilizing through day 20. In a separate experiment of identical design, infectious virus in sensory ganglia (Fig. 1D) and spinal cords (Fig. 1E) of naïve mice reached high levels on day 5 post-challenge (P < 0.001 compared to immune mice) and all naïve mice died prior to day 7 sampling. Titers were below detection levels in sensory ganglia and spinal cords in 11/14 HSV-immune B6 mice on day 5 and all immune B6 mice (14/14) on day 7 post-challenge. HSV-2 titers in HSV-immune IFNγ−/− mice were significantly lower than naïve mice (P < 0.001). Although titers in neural tissues were below detection levels for several HSV-immune IFNγ−/− mice on days 5 and 7 post-challenge, infectious virus was detected in either sensory ganglia or spinal cords in 9/14 animals.
Fig. 1.
HSV-2 challenge of HSV-immune B6 and IFNγ−/− mice. A) Clearance of HSV-2 333tk− from the genital epithelium non-immune of B6 or IFNγ−/− mice. Mice were inoculated intravaginally with 2 × 105 PFU HSV-2 333tk− (n = 9; * P < 0.05; ** P < 0.0001; ANOVA). B) Survival of HSV-immune B6, -immune IFNγ−/−, and naïve B6 mice after intravaginal challenge with 105 PFU HSV-2 186 (n = 10; * P < 0.0001 compared to naïve B6 mice; ** P < 0.05 compared to immune B6; Logrank test). C) Failure of HSV-immune IFNγ−/− mice to clear HSV-2 from the vagina. HSV-immune B6, -immune IFNγ−/− mice, and naïve B6 mice were challenged with HSV-2 186. Vaginal titers were obtained on the indicated days (n = 9; * P < 0.001; ** P < 0.0001 compared to immune B6 mice; ANOVA). HSV-2 titers in lumbosacral ganglia (D) and adjacent spinal cords (E) of HSV-immune B6, -immune IFNγ−/−, and naïve control mice after challenge with 104 PFU HSV-2 186. Mice were sacrificed on days 5 or 7 after challenge and infectious HSV-2 was quantified from the sensory ganglia and spinal cords. Day 5 lumbosacral ganglia and spinal cord titers from naïve B6 mice were significantly greater than HSV-immune B6 and -immune IFNγ−/− mice (P < 0.001; ANOVA).
3.2. Cell-mediated immune responses in HSV-immune B6 and IFNγ−/− mice
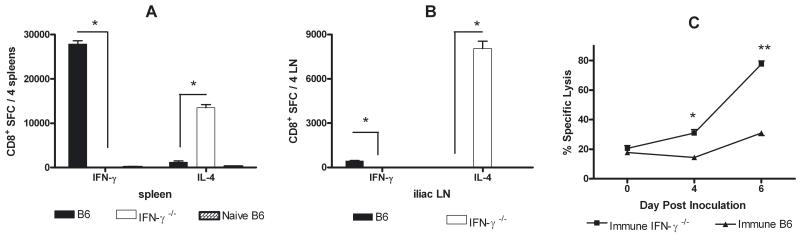
CD8+ T cells from HSV-immune B6 mice predominantly secreted IFNγ, although a smaller IL-4-secreting response was detected in spleen (Fig. 2A) and iliac lymph nodes (Fig. 2B). Predictably, CD8+ T cells from HSV-immune IFNγ−/− mice produced IL-4, but not IFNγ. Both strains of immune mice displayed a cytolytic response upon challenge (Fig. 2C). HSV-specific lysis on days 4 and 6 post-challenge in immune IFNγ−/− mice was significantly higher than B6 mice (P < 0.005, P < 0.001, respectively), likely reflecting continued presence of viral antigen in these mice or higher CD8+ T cell numbers resulting from a dysregulated contraction phase in the absence of IFNγ (Badovinac et al., 2001). Adoptive transfer of activated CD8+ T cells from HSV-immune B6, but not IFNγ−/− mice cleared virus from the genital tract (not shown).
Fig. 2.
Cytokine secretion by HSV-specific CD8+ T cells from HSV-immune B6 and IFNγ−/− mice. HSV-immune B6, -immune IFNγ−/−, and naïve B6 mice were challenged intravaginal with 104 PFU HSV-2 186. Six days later, CD8+ T cells isolated from spleens and iliac lymph nodes were stimulated with syngeneic spleen cells pulsed with HSV gB498–505 peptide, and IFNγ-secreting cells and IL-4-secreting cells from the spleen (A) or iliac lymph nodes (B) were quantified by ELISPOT (*P < 0.001; ANOVA). (C) An in vivo CTL assay was performed on the indicated days after challenge of HSV-immune B6 and IFNγ−/− mice (*P < 0.005; ** P < 0.0001; Student’s t test). Results are representative of 2 experiments performed.
3.3. Inability of HSV-specific CD4+ effector T cells from immune IFNγ−/− mice to clear HSV-2 333tk− from the genital tract
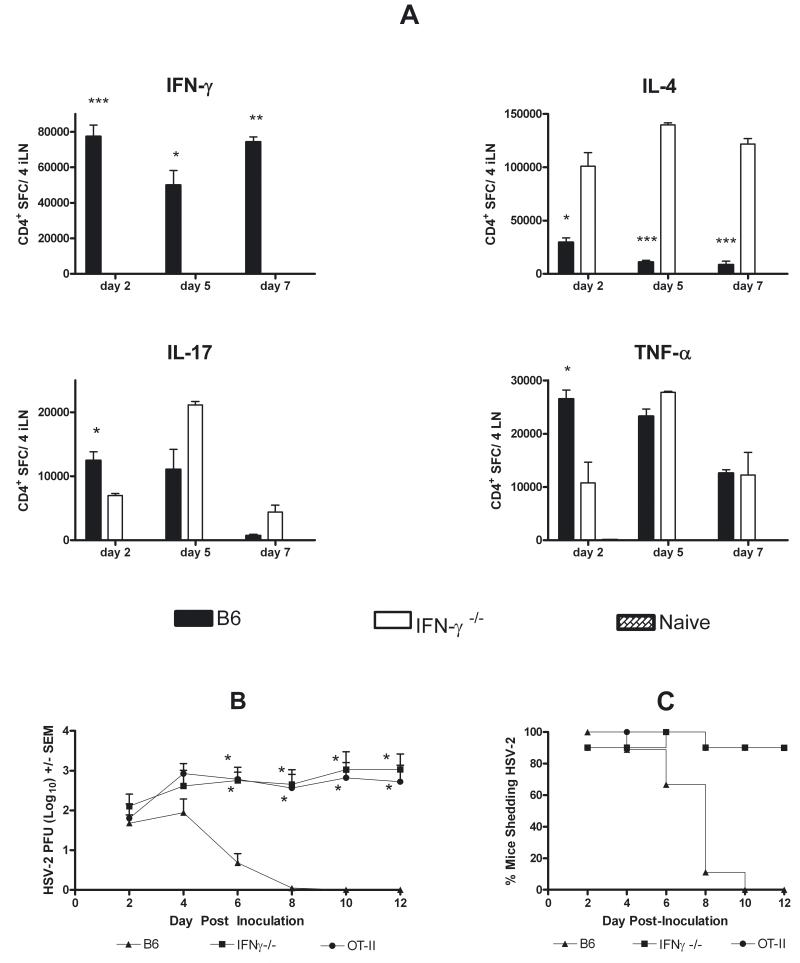
CD4+ T cells from iliac lymph nodes of HSV-immune B6 mice predominantly produced IFNγ, with lower magnitude IL-4, IL-17, and TNFα responses (Fig. 3A). Numbers of IL-4-secreting CD4+ T cells from HSV-immune IFNγ−/− mice were significantly higher than B6 mice (P < 0.05, day 2; P < 0.001, day 5, 7), whereas numbers of IL-17- and TNFα-secreting cells in draining lymph nodes were comparable in immune IFNγ−/− and B6 mice on days 5 and 7 post-challenge. Comparable numbers were obtained from analysis of genital tract-infiltrating T cells (not shown).
Fig. 3.
Cytokine secretion by HSV-specific CD4+ T cells from HSV-immune B6 and IFNγ−/− mice (A). CD4+ T cells were isolated and pooled from iliac lymph nodes and vaginal tracts of HSV-immune B6, -immune IFNγ−/− and naïve B6 mice on the indicated day after challenge with 104 PFU HSV-2 186. Results are expressed as the total number of cells obtained from the tissues from 4 mice and are representative of 3 experiments performed (*P < 0.05; ** P < 0.01; *** P < 0.001 for comparisons between immune B6 and immune IFNγ−/− mice; Student’s t test). Adoptive transfer of CD4+ T cells from HSV-immune B6, but not HSV-immune IFNγ−/− mice, results in clearance of HSV-2 333tk− from the genital epithelium (B). Lymphocytes from HSV-immune B6, -immune IFNγ−/−, and naïve OT-II mice were activated in culture for 4 days with specific antigen. CD4+ T cells were isolated and transferred to irradiated B6 recipients as described in Methods. Mice were immediately challenged with 5 × 103 PFU HSV-2 333tk− and virus was quantified on the indicated days. (B) Virus titers following HSV-2 challenge (*P < 0.001 compared to HSV-immune B6 mice; ANOVA). (C) Percent of mice shedding virus. Results are pooled from 2 experiments of identical design.
CD4+ T cells are the predominant T cell subset for clearing HSV from the genital epithelium of immune mice (Milligan et al., 1998; Kuklin et al., 1998; Harandi et al., 2001b). Transfer of activated HSV-immune B6 CD4+ T cells to irradiated recipients resulted in clearance of HSV-2 333 tk− by day 10 post-challenge (Fig. 3B, C) whereas control mice receiving activated OT-II cells failed to clear virus through day 12 post-challenge. Titers remained high in HSV-immune IFNγ−/− CD4+ T cell-recipients and were comparable to OT-II-recipients through day 12 post-challenge. Ultimately, 9/10 HSV-immune IFNγ−/− T cell-recipients failed to clear virus.
3.4. Resistance to HSV-2 challenge provided by HSV-specific Ab from B6 or IFNγ−/− mice
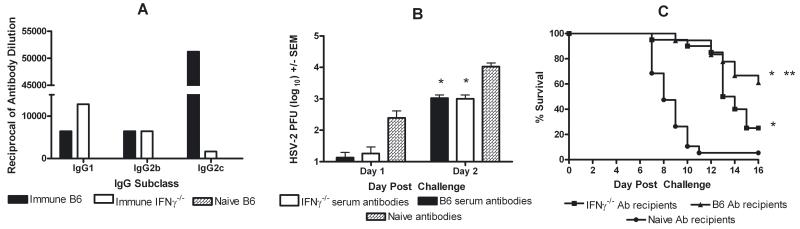
The mean HSV-specific serum-IgG titer from immune B6 mice (12.2 +/− 2.0 μg/ml, range: 3.1 to 36.7 μg/ml; n = 23) was not significantly different from immune IFNγ−/− mice (16.3 +/− 2.2 μg/ml, range: 2.3 to 41.6 μg/ml; n = 25). The mean neutralizing serum Ab titer was approximately 1.5-fold higher in immune IFNγ−/− mice (1:216.3 (+/− 85.7) for immune B6 and 1:319.5 (+/− 97.3) for immune IFNγ−/− mice). To compare efficacy, 23.0 μg of HSV-specific IgG isolated from these two strains of immune mice was passively transferred to naïve B6 recipients prior to challenge. IgG Ab from immune B6 mice was predominantly IgG2c whereas HSV-immune IFNγ−/− mice produced predominantly IgG1 (Fig. 4A), mirroring the subclass distribution of HSV-specific IgG serum Ab from immune serum donors (not shown). HSV-specific Ab from either strain did not prevent infection. However, initial vaginal HSV-2 titers were reduced on days 1 and 2 post-challenge, prior to the development of the HSV-specific T cell response, compared to non-immune serum-recipients (Fig. 4B). Approximately 95% of non-immune serum-recipients died by day 11 post-challenge (Fig. 4C). Protection was nearly identical through day 12 in immune B6 and IFNγ−/− Ab-recipients. Resistance waned in immune IFNγ−/− Ab-recipients by day 13 and was significantly lower compared to immune B6 Ab-recipients (P = 0.039).
Fig. 4.
Differential protection by passive transfer of HSV-specific antibodies from HSV-immune B6 and IFNγ−/− mice. B6 mice received 23.0 μg HSV-specific IgG from immune B6 or IFNγ−/− mice or non-immune mouse serum and were challenged 3 days later with 105 PFU HSV-2 186. Mice were swabbed for virus on days 1 and 2 post-challenge and were observed 16 days for disease symptoms. A) HSV-specific IgG subclasses in IgG-enriched Ab preparations from HSV-immune B6, -immune IFNγ−/−, or naïve mice. B) HSV-2 titers in the genital tracts of HSV-immune B6 IgG-, -immune IFNγ−/− IgG-, or non-immune serum recipients (*P < 0.01 compared to non-immune serum-recipients; ANOVA). Results are representative of 2 experiments performed. C) Survival of mice following transfer of HSV-immune IgG preparations or non-immune serum (*P < 0.0001 compared to non-immune serum recipients; ** P = 0.039 compared to immune IFNγ−/− serum recipients; Logrank test). Results are pooled from 2 experiments of identical design.
4. Discussion
In murine models, IFNγ is important for resistance to primary HSV-1 and HSV-2 infections (Smith et al., 1994; Bouley et al., 1995, Milligan & Bernstein, 1997; Harandi et al., 2001a). In HSV-immune mice, IFNγ appears critical for resistance despite HSV-specific Ab and CTL responses (Milligan et al., 1998; Parr & Parr, 1999; Milligan et al., 2004). IFNγ impacts infectious virus titer in genital tracts of HSV-immune mice within 24 hours of intravaginal HSV-2 re-challenge (Milligan et al., 1998; Parr & Parr, 1999). Rapid virus clearance by CD8+ or CD4+ T lymphocytes requires IFNγ interaction with parenchymal rather than hematopoietic cells (Bird et al., 2007; Ijima et al., 2008). IFNγ produced by HSV-immune T cells has been suggested to synergize with type I IFNs to inhibit HSV replication (Mikloska & Cunningham, 2001; Sainz & Halford, 2002). In the present study, clearance of an attenuated HSV-2 virus by IFNγ−/− mice and development of adaptive immune responses allowed testing for IFNγ-independent mechanisms of immune protection. Using a similar approach, Yu et al. (1996), determined IFNγ−/− mice could be fully immunized by HSV-1 inoculation and T lymphocytes from immune mice protected recipients from developing HSV-1 cutaneous lesions in a zosteriform challenge model. In the current studies, immunization rendered IFNγ−/− mice partially resistant to intravaginal HSV-2 challenge. Transferred HSV-immune CD4+ or CD8+ T cells from IFNγ−/− mice failed to clear genital HSV-2 333tk− while transferred immune serum conferred resistance. Discrepancies between our results and Yu et al. may reflect use of HSV-1 versus HSV-2 or different murine genetic backgrounds.
Naïve CD4+ T cells differentiate into distinct effector cell subsets based on cytokines present during activation. Antigenic stimulation in the absence of IFNγ results in development of T cell populations secreting type 2 cytokines. In addition to IL-4-secreting T cells detected previously in HSV-immune IFNγ−/− mice (Bouley et al., 1995; Yu et al., 1996), we detected numerous CD4+ T cells secreting TNFα and IL-17 in secondary lymphoid and vaginal tissues of both strains of immune mice. Although TNFα plays a role in HSV clearance in vitro and in vivo (Ito & O-Malley, 1987; Rossol-Voth et al., 1991) and IL-17 has been shown to elicit a granulocyte response which may function to limit viral spread (Tumpey et al., 1996; Milligan et al., 2001), adoptive transfer of HSV-specific T cells from immune IFNγ−/− mice secreting these cytokines did not result in virus clearance. Thus, we detected no evidence that the T cell response of immune IFNγ−/− mice conferred resistance to HSV-2 challenge despite secretion of potentially antiviral cytokines.
Comparable HSV-specific serum IgG levels were detected in HSV-2 333tk−-immunized B6 and IFNγ−/− mice. IgG subclass expression reflected the presence or absence of IFNγ during B cell priming. Passive transfer of IgG1-rich HSV-specific Ab from IFNγ−/− mice was as protective early in infection as IgG2c-rich Ab from immune B6 mice, manifested as resistance to initial infection with lower day 1 and 2 vaginal titers and greater protection against mortality through day 12 post-challenge relative to non-immune IgG-recipients. Given comparable neutralization activity of Ab preparations, early protection was likely conferred by HSV neutralization. Lack of protection by HSV-specific IgG from IFNγ−/− mice after day 13 suggests the Ab was less effective in preventing virus spread and replication within the nervous system, perhaps reflecting diminished ability of IgG1 to activate complement or mediate Ab-dependent cell-mediated cytotoxicity. Since Ab from immune B6 mice (primarily IgG2c) provided superior protection during this time, Ab-mediated protection at neural sites may require biologic functions associated with this IFNγ-influenced IgG subclass. IgG1 Ab binds less well to Fcγ receptors compared to IgG2b or IgG2c. Superior protection afforded by IgG2c-rich HSV-specific IgG is consistent with previous studies demonstrating a role for the Fcγ receptor in Ab-mediated protection against genital HSV-2 infection (Chu et al., 2008).
In conclusion, HSV-immune IFNγ−/− mice were more resistant to HSV-2 challenge than non-immune mice. Partial resistance was not T cell-mediated, but could be transferred by HSV-specific Ab from immune mice. Our results suggest HSV-specific Ab of any IgG subclass may provide initial resistance to HSV-2, though prolonged resistance in neural tissues may require subclasses expressed as a result of IFNγ presence. Different Ab-mediated mechanisms may be involved in protection of genital and neural tissues against HSV-2 infection.
Acknowledgements
We thank Nigel Bourne and Jane Strasser for critical reading of the manuscript. This work was supported by NIH grants AI42815 and AI05444. AJJ was supported by a Vale-Asche Predoctoral Fellowship, MHN by a Sealy Center for Vaccine Development Predoctoral Fellowship, and MDB by a McLaughlin Predoctoral Fellowship.
Abbreviations
- CTL
cytotoxic T-lymphocyte
- HSV
herpes simplex virus
- SFC
spot-forming cell
- TCR
T cell receptor
- tk−
thymidine kinase-deficient
- intravaginal
intravaginal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2001;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Bird MD, Chu C-F, Johnson A,J, Milligan GN. Early resolution of herpes simplex virus type 2 infection of the murine genital tract involves stimulation of genital parenchymal cells by gamma interferon. J. Virol. 2007;81:423–426. doi: 10.1128/JVI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type -1 infection and herpetic stromal keratitis development in IFNγ knockout mice. J. Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- Chu C-F, Meador JG, Young CG, Strasser JE, Bourne N, Milligan GN. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J. Reprod. Immunol. 2008;78:58–67. doi: 10.1016/j.jri.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L, Wald S, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 2004;35:435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Dix RD, Pereira L, Baringer JR. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect. Immun. 1981;34:192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs ME, Strasser JE, Chu C-F, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin-or Fas-mediated cytolytic mechanisms. J. Virol. 2005;79:14545–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley KL, Bourne N, Milligan GN. Immune protection against HSV-2 in B-cell-deficient mice. Virology. 2000;270:454–463. doi: 10.1006/viro.2000.0298. [DOI] [PubMed] [Google Scholar]
- Harandi AM, Svennerholm B, Holmgren JH, Eriksson K. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 2001a;75:6705–6709. doi: 10.1128/JVI.75.14.6705-6709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFNgamma-secreting CD4 (+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 2001b;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- Ijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, O-Malley JA. Antiviral effects of recombinant human tumor necrosis factor. Lymphokine Res. 1987;6:309–318. [PubMed] [Google Scholar]
- Johnson AJ, Chu CF, Milligan GN. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J. Virol. 2008;82:9678–9688. doi: 10.1128/JVI.01159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Strynadka NCJ, Hodges RS, Pereira L. Analysis of the role of antibody-dependent cellular cytotoxicity antibody activity in murine neonatal herpes simplex virus infection with anti bodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J. Clin. Invest. 1990;86:273–278. doi: 10.1172/JCI114695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklin NA, Daheshia M, Chun S, Rouse BT. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 1998;160:5998–6003. [PubMed] [Google Scholar]
- Linehan MM, Richman S, Krummenacher C, Eissenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 1984;51:747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 2001;75:11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–189. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- Milligan GN, Bourne N, Dudley KL. Role of polymorphonuclear leukocytes in resolution of HSV-2 infection of the mouse vagina. J. Reprod. Immunol. 2001;49:49–65. doi: 10.1016/s0165-0378(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Dudley-McClain KL, Young CG, Chu C-F. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J. Reprod. Immunol. 2004;61:115–127. doi: 10.1016/j.jri.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 1998;72:5137–5145. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J. Gen. Virol. 2005;86:2421–2432. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol-Voth R, Rossol S, Schutt KH, de Cian W, Falke D. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J. Gen. Virol. 1991;72:143–147. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- Sainz B, Jr., Halford WP. Alpha/Beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 2002;76:11541–11550. doi: 10.1128/JVI.76.22.11541-11550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Wolcott RM, Chervenak R, Jennings SR. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ. Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- Tsunobuchi H, Hishimura H, Goshima F, Daikoku T, Suzuki H, Nakashima I, et al. A protective role of interleukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology. 2000;275:57–66. doi: 10.1006/viro.2000.0455. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 2002;18:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- Yu A, Manickan E, Rouse BT. Role of interferon-γ in immunity to herpes simplex virus. J. Leuk. Biol. 1996;60:528–532. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]