Abstract
Women comprise almost 50% of the population of people living with HIV and the majority of these women contracted the virus through sexual transmission in monogamous relationships in the developing world. In these environments, where women are not empowered to protect themselves through the negotiation of condom use, effective means of preventing HIV transmission are urgently needed. In the absence of an approved and effective vaccine, microbicides have become the strategy of choice to provide women with the ability to prevent HIV transmission from their infected partners. Topical microbicides are agents specifically developed and formulated for use in either the vaginal or rectal environment that prevent infection by sexually transmitted infectious organisms, including pathogenic viruses, bacteria and fungi. Although a microbicidal product will have many of the same properties as other anti-infective agents and would be similarly developed through human clinical trials, microbicide development bears its own challenges related to formulation and delivery and the unique environment in which the product must act, as well as the requirement to develop a product that is acceptable to the user. Herein, perspectives based on preclinical and clinical microbicide development experience, which have led to an evolving microbicide development algorithm, will be discussed. This article forms part of a special issue of Antiviral Research marking the 25th anniversary of antiretroviral drug discovery and development, Vol 85, issue 1, 2010”.
2. Introduction
The latest UNAIDS report on the global AIDS epidemic has restated the global emergency status of the epidemic. Over 25 million people have died since the first case of AIDS was identified in 1981, and the number of people living with HIV worldwide numbered 33 million at the end of 2007. Almost 2.5 million people worldwide became newly infected with HIV and an estimated 2.1 million human deaths were attributed to AIDS in 2007 (UNAIDS/WHO 2007). The rate of HIV infection and AIDS related deaths is projected to increase over the course of the next decade with rapid expansion in Asia, Africa, and Eastern Europe. The epidemic is not limited to underdeveloped and low to middle income countries, as the number of infected individuals living with HIV/AIDS has also risen and the rates of HIV infection have not declined in the United States and Western Europe (UNAIDS 2004).
Nucleoside and non-nucleoside reverse transcriptase (RT) inhibitors and protease inhibitors have been effectively used for the past decade in highly active antiretroviral therapies (HAART) to significantly reduce HIV virus load in infected individuals for prolonged periods of time (Fischl et al. 1987). The utilization of HAART has dramatically changed the therapeutic landscape of HIV treatment and the application of cocktails of antiretroviral agents is now the standard of care for HIV patients (Bonfanti et al. 1999; Yeni et al. 2004). The dramatic reduction in viral load and clinical improvements achieved with HAART is a rigorous validation of the ability of anti-HIV drugs to contain and manage HIV disease, and demonstrates that a combination of three or more anti-HIV agents - even when directed against only two of the putative 10 viral targets - is superior to single or two drug chemotherapy. Thus, the prevailing belief is that the addition of new anti-HIV agents to HAART regimens will provide additional clinical benefits (Mocroft et al. 1998; Palella et al. 1998). Over the last several years, inhibitors of HIV integrase and HIV entry (CCR5 antagonist) have been added to the portfolio of approved drugs available to HIV-infected individuals and additional RT and protease inhibitors with greater potency continue to be developed. Despite its success, HAART suffers from the emergence of multi-drug resistant virus strains, toxicity, difficult treatment regimens, and inadequate pharmacology, bioavailability and tissue distribution (Richman 1996; Carpenter et al. 2000; Trabattoni et al. 2002). In the developing world, many of these therapeutic strategies are unavailable due to the prohibitively high cost of the drugs. In these areas, the absence of an effective vaccine and the lack of effective therapy, means that sub-Saharan Africa and Southeast Asia remain epicenters for the spread of HIV, especially among heterosexual women (Letvin 2004). In these areas of extremely high HIV transmission rates, the opportunities to derail the AIDS pandemic rests on the processes of education and behavioral prevention and the development of effective prophylaxis, including specific HIV prevention strategies employing chemical agents to prevent the sexual transmission of HIV (Turpin 2002; Lard-Whiteford et al. 2004).
Topical microbicides represent an important strategy with clear potential for preventing the transmission of HIV through sexual intercourse, the predominant mode of HIV transmission worldwide. The latest statistics indicate that the number of women with HIV infection and AIDS has been increasing steadily worldwide and according to the World Health Organization, women accounted for 50% of adults living with HIV at the end of 2007 (UNAIDS/WHO 2007). Thus, the dynamics of the epidemic demand the development of safe, effective, and acceptable female-controlled chemical and physical barrier methods including topical microbicides, to reduce HIV transmission. The development of microbicidal agents has gained significant focus and momentum during the past few years due to the realization that suppression of HIV transmission in the developing world can have a great impact on the HIV pandemic. It has been estimated that a single microbicide with 60% effectiveness could prevent millions of new cases of HIV infection each year throughout the world (Watts et al. 2002).
Topical microbicides consist of products that attack cellular or viral targets and prevent the infection of target cells or the replication of the virus, resulting in decreased virus transmission and acquisition of HIV. Microbicide strategies may or may not include those effective against sexually transmitted infections (STIs) that affect the acquisition or course of HIV infection and may or may not be contraceptive. A major challenge in the development of topical microbicides revolves around the actual biology of HIV infection in the vagina and/or rectum and full understanding of the actual molecular and cellular events which occur during transmission and infection. Infectious virus, supplied via the ejaculate, contains both cell-free and cell-associated virus (Gupta et al. 1997; Quayle et al. 1997; Coombs et al. 1998). Infectious virus can be recovered from mononuclear cells in seminal fluid, but endogenous antiviral factors in semen make quantification and recovery of infectious cell-free virus highly variable. Results from non-human primate models argue strongly for cell-free virus as the source of infection, and it has been shown that viral load correlates with transmission (Pilcher et al. 2004; Cohen et al. 2005). Once deposited in the vaginal or rectal vaults, the virus or virus infected cell(s) must penetrate the epithelium of the tissues in order to reach their target cells (monocytes, dendritic cells, and/or T cells) in the sub-mucosa. In the case of the vagina and ectocervix, the squamous epithelium is keratinized and can be up to 50 cell layers thick. In the endocervix, the epithelia transitions to a single layer of columnar cells. Additional defenses may include the barrier properties of cervical mucus, antiviral factors secreted by the innate immune system, and protective factors from naturally occurring microflora such as Lactobacilli sp. (Miller et al. 2003; Cole 2006). The mechanism by which HIV evades these host defenses is unknown, but micro-trauma resulting in access to the sub-mucosa from intercourse and/or STI-induced lesions have been identified as potential routes of entry. Once access to susceptible cells in the sub-mucosa is obtained, a number of studies have suggested that infection potentially occurs in a two-stage process with local infection of these susceptible cells in the tissue followed by rapid dissemination from the genital tract-associated mucosa to regional lymph nodes (Zhang et al. 1999; Pope et al. 2003; Haase 2005; Miller et al. 2005). The identity of the initially infected cell is still unknown. Some studies identify resting T cells as the first cells to be infected. Others show strong evidence for capture of virus by dendritic and Langerhans cells and/or direct infection of dendritic cells, either of which would facilitate infection of resting T cells through cell-to-cell interactions (Frank et al. 2002). Obviously a microbicide would need to protect against viral infection, possibly at multiple stages of infection.
The National Institutes of Health (NIH) has recently indicated their perspective that research also needs to be conducted on the use of combinations of microbicides to determine if protective efficacy of the products is increased when microbicides with two or more different mechanisms of action are used together. Thus, there is a critical need to promote the discovery and development of safe, novel microbicides and combination microbicide therapies and to provide support for translational studies to advance new candidates and combinations that have proven safe in preclinical studies into early clinical trials. At present the precise characteristics of the development pathway for microbicides has not been ascertained since the IND-directed preclinical data cannot yet be compared with clinical experience as can be done with systemic inhibitors of HIV. Several compounds have reached Phase III human clinical trials and three products (Nonoxynol-9, Carraguard and cellulose sulfate) have failed in those trials either due to damage to the vaginal epithelium, resulting in increased infection in women using the product (2000; Hillier et al. 2005; Tao et al. 2008; Van Damme et al. 2008), or lack of efficacy (Skoler-Karpoff et al. 2008). Recently, PRO2000 (Indevus, Inc.) has successfully completed a Phase 2B trial demonstrating a 30% reduction in transmission rates among women participating in the trial (2009). The safety and efficacy of a variety of diverse microbicides are currently being evaluated in clinical trials and preclinical development algorithms as both single and combination products (www.microbicide.org). Combinations of topical microbicides are just now beginning to be evaluated in advanced preclinical and early clinical studies.
Although the development of antiretroviral agents for prevention of infection share many challenges with the development of systemic inhibitors, a variety of additional hurdles must be overcome prior to approval of a microbicidal product. The significant differences are related to the highly specialized environment and conditions in which the microbicide product will be used (Turpin 2002; Lard-Whiteford et al. 2004). Some of the more important considerations that must be added to the typical HIV drug development plan involve (1) the need to assure that the microbicide product will remain active in the presence of vaginal fluid, cervical mucous, semen and at vaginal pH (Turpin 2002; Lard-Whiteford et al. 2004), (2) the need to effectively formulate the microbicide product for use in an environment significantly different from that required for systemic inhibitors (Garg et al. 2003; Garg et al. 2003), (3) the need to protect the normal vaginal microenvironment from harm (Hillier 1998; Martin et al. 1999), (4) the requirement to develop products that are safe for both the vaginal and rectal environments using variations in formulation design (D’Cruz et al. 2004), (5) the ability to utilize higher concentrations of the microbicide product since continuous exposure as required for therapeutic agents is not expected to be necessary, (6) the need to evaluate the extent of absorption of the product from the vaginal or rectal vaults into systemic circulation (Van Damme 2000; Lard-Whiteford et al. 2004) and (7) the need to evaluate the acceptability of a product to the end users (Woodsong 2004). It also must be understood that these products will be used in both the developed and undeveloped world, necessitating the development of products with excellent stability characteristics, low cost, and ease of application. Finally, microbicide development must take into account the fact that the goal is to prevent, not treat, infection and thus the microbicide prevention strategies should also include virucidal agents that directly inactivate virus in semen or vaginal fluids or that act prior to the integration of the virus in the target cells of the vagina or rectum (Mathijs et al. 1988; Martin et al. 1999). The therapeutic environment of the vagina or rectum is at present not well understood (D’Cruz et al. 2004; Balzarini et al. 2005) and therefore the product must be able to effectively suppress infection by virus contained in different inoculum formats (cell-free virus or cell-associated virus), in different target cells (CD4-expressing, CD4-non-expressing), for unknown periods of interaction time following the introduction of the infectious inoculum, and in the presence of concurrent vaginal infections with other viral, bacterial and fungal organisms (Tan et al. 1993; Tan et al. 1996; Bomsel 1997; Ibata et al. 1997; Hu et al. 1998; Stahl-Hennig et al. 1999; Zhang et al. 1999; Collins et al. 2000; Hu et al. 2000; Di Fabio et al. 2001; Carreno et al. 2002).
3. Existing Classes of Topical Microbicides Under Development
A variety of different classes of inhibitors are currently being developed as topical microbicides (Turpin 2002). The first generation of microbicides included highly sulfated molecules and detergent-based approaches to prevent virus attachment or directly inactivate infectious virus. Additionally, indirect microbicide strategies such as buffering the pH of the vagina to maintain the naturally occurring low pH have been used in an attempt to prevent infection. Subsequently, more specific anti-retroviral agents (ARVs), including attachment inhibitors and reverse transcriptase inhibitors, have entered development and the evaluation of these specific classes of antiretroviral inhibitors is now being expanded to include compounds with greater potency and selectivity. More recently, strategies have evolved to include the evaluation of molecules that target cellular proteins or stimulate innate or adaptive immune responses, including NK cells, defensin-type molecules, and neutralizing antibodies in the mucosa. Table 1 provides an overview of agents currently being evaluated experimentally or in human clinical trials.
Table 1.
| Table 1A: Microbicide and PrEP Candidates in On-Going Clinical Trials | ||
|---|---|---|
| Product | Mechanism of Action | Stage of Development |
| PRO2000 (Gel) | Virus Entry | Phase 3 |
| Truvada (Oral) | NRTI/NtRTI | |
| Viread (Oral) | NtRTI | |
| Truvada (Oral) | NRTI/NtRTI | |
| Viread (Oral) | NtRTI | Phase 2/3 |
| Tenofovir | NtRTI | Phase 2B |
| Tenofovir (Gel) | NtRTI | Phase 2 |
| Viread (Oral) | ||
| Viread (Oral) | NtRTI | |
| Dapivirine (Gel) | NNRTI | Phase1/2 |
| VivaGel (Gel) (SPL7013) | Virus Entry | |
| Acidform | Vaginal Defense Enhancer | Phase 1 |
| Device | ||
| HEC/CS/N-9 Gels (Assessment of markers of inflammation after vaginal product use) | N/A | |
| PRO2000 (Gel) | Virus Entry | |
| Tenofovir (Gel) | NtRTI | |
| UC-781(Gel) | NNRTI | |
| VivaGel (Gel) (SPL7013) | Virus Entry | |
| Table 1B: Microbicide and PrEP Candidates in Planned and/or Funded Clinical Trials | ||
|---|---|---|
| Products | Mechanism of Action | Planned Stage of Development |
| BufferGel (Barrier Method and Gel) | Vaginal Defense Enhancer | Phase 3 |
| Dapivirine (Gel and Ring) | NNRTI | |
| Tenofovir (Gel) | NtRTI | |
| Invisible Condom | Entry/Fusion | Phase 2/3 |
| Tenofovir (Gel) | NtRTI | Phase 2/2B |
| Dapivirine (Gel) | NNRTI | Phase ½ |
| Dapvirine (Ring) | NNRTI | |
| CAP (Vaginal Tablet) | Virus Entry | Phase 1 |
| Dapivirine (Gel) | NNRTI | |
| Dapivirine (Ring) | NNRTI | |
| MIV-150 (Gel) | NNRTI | |
| MIV-150 (Ring | NNRTI | |
| Tenofovir (Gel) | NtRTI | |
| Table 1C: Microbicide Candidates in Preclinical Development | ||
|---|---|---|
| Product | Mechanism of Action | Development Phase |
| CAP | Virus Entry/Fusion | Advanced Preclinical |
| Cyanovirin-N (CV-N) | Virus Entry/Fusion, Vaginal Defense | |
| D-Peptides | Virus Entry/Fusion | |
| 5P12-RANTES | Virus Entry/Fusion | |
| Mapp66 | CCR5 and HSV Ab combinations | |
| Nisin | Virus Entry | |
| Octylglycerol Gel | Surfactant | |
| Opuntia spp(Osp) | Virus Entry/Fusion, Virus replication | |
| PEHMB | Virus Entry/Fusion | |
| Polycarboxylated aryl oligomer (PPCM) | Virus Entry/Fusion | |
| Retrocyclins | Virus Entry/Fuison | |
| IQP-0528 (SJ-3991) | NNRTI/Virus Entry | |
| C5A | Vaginal Defense Enhancer | Discovery/Early Preclinical |
| CADA | Entry/Fusion Inhibitor, Uncharacterized mechanism | |
| CAP and combinations with NNRTIs and ZFIs | Multiple Mechanisms | |
| Combinations (Entry and Replication Inhibitors) | Multiple mechanisms | |
| Diterpene | Integrase | |
| DS003/BMS-599793 | Virus Entry – gp120 | |
| DS004/L-860/872 | Virus Entry – CCR5 | |
| DS005/L860,882 | Virus Entry – CCR5 | |
| EBd peptides | Virus Entry/Fusion | |
| Flavonoids | Virus Entry/Fusion | |
| Glycerol monolaurate | Uncharacterized mechanism | |
| HHA, KRV2110, T20 Combinations | Virus Entry/Fusion | |
| ISIS 5320 | Virus Entry – gp120 | |
| K5-N, OS(H), K50SH | Virus Entry/Fusion | |
| KP1, KP17 | Replication inhibitor | |
| L644 Peptide | Fusion inhibitor – gp41 | |
| Maraviroc | Entry inhibitor – CCR5 | |
| MIV-150 Vaginal Ring | Virus NNRTI | |
| Nanobodies | Entry/Fusion | |
| NCp7 Inhibitors (Thioesters) | Viral Inactivators | |
| Novasomes | Virus Entry/Fusion, Uncharacterized mechanism | |
| Optimised dendrimers | Virus Entry/Fusion | |
| PC-710 | VirusEntry/Fusion and Zinc | |
| PSC-RANTES | Virus Entry – CCR5 | |
| Pyrimidinediones | NNRTI/Virus Entry | |
| Pyrimidinediones + ISIS 5320 | NNRTI/Virus Entry – gp120 | |
| RANTES peptides | Virus Entry | |
| Recombinant Lactobacillus (LAB) | Virus Entry | |
| REP 9C, REP 9AC | Virus Entry | |
| sCD4-17b | Virus Entry | |
| Single-chain ICAM | Virus Entry | |
| siRNA | Virus Entry/Fusion | |
| Sodium Rutin Sulfate (SRS) | Virus Entry/Fusion | |
| Soluble DC-SIGN | Virus Entry/Fusion | |
| Syndecan | Virus Entry – CCR5 | |
| Talactoferrin | Virus Entry/Fusion, Uncharacterized mechanism | |
| TATC-D peptides | Virus Entry/Fusion | |
| Unipron | Vaginal Defense Enhancer | |
| x-REPLAB | Vaginal Defense Enhancer | |
| ZCM (PC-1005) | Virus Entry/Fusion, Zinc and NNRTI | |
| Zinc tetra-ascorbocamphorate derivative “C14” | Virus Entry and integrase | |
Several microbicide products have thus far been evaluated in advanced human clinical trials to monitor their safety and efficacy. Trials with the surfactant-based products SAAVY (C31G) and nonoxynol-9 were stopped due to lower than expected HIV infection rates in the trial cohort and because of enhanced rates of infection in trial participants, respectively. The surfactant-based products directly inactivated HIV by damaging the membrane coat of HIV, rendering the virus noninfectious, but the products appear to have had exerted detrimental toxicities on the vaginal epithelium by the same mechanism, creating micro-lesions resulting in enhanced opportunity for infection with HIV. A retrospective evaluation of preclinical data obtained with nonoxynol-9 suggests that this clinical result should have been anticipated (Beer et al. 2006). Trials with cellulose sulphate and Carraguard, sulfated polysaccharides which inhibit the binding of HIV to CD4(+) target cells, also resulted in failure in human clinical trials. Continued retrospective evaluation of trial data and additional mechanistic studies are being performed to further understand the lack of efficacy of the polyanion class of inhibitors. However, based on the results obtained with both the surfactants and polyanionic compounds, additional product development in these areas has been suspended.
The first product found to be effective in human clinical microbicide trials is PRO2000, a cationic naphthalene sulfonate polymer which targets an epitope on CD4 and prevents HIV attachment. PRO2000 has also been found to be active against other STIs and viruses. In standard HIV microbicide efficacy assays PRO2000 demonstrated 50% inhibition of HIV replication (EC50) at concentrations ranging from 0.3 μg/mL to 4.3 μg/mL (reviewed in (Lackman-Smith et al. 2008)). Against HSV-2, the EC50 concentration was defined as 11.4 μg/mL (Fletcher et al. 2006). PRO2000 exhibited a good safety profile in multiple early stage clinical trials (Poynten et al. 2009). Initial results from the Phase 2/2B HPTN 035 trial, which was a multi-center clinical trial evaluating the safety and effectiveness of two different candidate microbicides, BufferGel® and PRO 2000, in 3100 sexually active HIV-negative women at seven sites in Africa and the United States, indicated 30% effectiveness with the 0.5% PRO2000 gel (unpublished data). Although the initial results are promising for PRO2000 and the microbicide field in general, additional data needs to be obtained to comprehensively evaluate the effectiveness of PRO2000. MDP-301 is a Phase III human clinical trial that evaluated the safety and effectiveness of 0.5% and 2% PRO2000 gels. One of the first of its kind, this trial enrolled over 9,000 women and involved 6 research centers. In February of 2008, 2% PRO2000 gel arm was discontinued by the Data Safety Monitoring Board due to a finding of futility since there was little chance it would prove effective. The 0.5% PRO2000 gel arm was continued. The trial ended in August of 2009. The data have not yet been reported.
4. Development of Microbicide Inhibitors
Detailed algorithms, based on the Food and Drug Administration (FDA) Points to Consider (PTC) guidance documents for the development of systemic HIV inhibitors to treat HIV infection, have been defined to expeditiously progress compounds through the preclinical and clinical evaluation of HIV therapeutic agents. The PTC guidance addresses issues critical to providing a rationale for the clinical development of a therapeutic anti-HIV agent, including the evaluation of (1) compound efficacy and toxicity in relevant cell-based systems, (2) the range and mechanism of action of candidate compounds, (3) the interactions of the compound in combination with other approved drugs, and (4) resistant virus selection upon long term exposure of virus to the experimental agent. In general, the current development algorithm for topical microbicides is identical to that described for systemic HIV inhibitors and can be summarized as shown in Table 2. For microbicide development it is important to keep in mind the primary characteristics or requirements for an effective agent, namely activity against clinical strains of virus, the ability to inhibit early pre-integration steps in virus replication to prevent infection of target cells, and lack of toxicity at high concentrations to normal vaginal flora and cells and tissue representative of the vaginal, cervical and/or rectal epithelium (Mathijs et al. 1988; Patterson et al. 1998; Martin et al. 1999). Ultimately, the inhibitor must be formulated in an appropriate manner (gels, creams, rings, films, tablets, etc) for vaginal or rectal application (Garg et al. 2003; Garg et al. 2003). The consensus opinion is that a microbicide product should be inexpensive, colorless, odorless and tasteless, and should be available in forms that may or may not be contraceptive (Turpin 2002). In addition the microbicide should be easy to use, amenable to covert use and should enhance or at least not interfere with sexual pleasure (Bentley et al. 2000; Mason et al. 2003; Morrow et al. 2003; El-Sadr et al. 2006; Rosen et al. 2008). Much of the microbicide research and development currently being performed is targeted at preventing transmission of HIV in sub-Saharan Africa and Southeast Asia, but an effective microbicide will undoubtedly be used worldwide to prevent the sexual transmission of HIV and other sexually transmitted infectious organisms (STIs).
Table 2.
Stages of Preclinical Microbicide Development
| Stage 1: In vitro efficacy and toxicity assays |
| Efficacy versus CCR5-tropic virus strains |
| PBMC efficacy and toxicity assays (clinical subtypes B, C, and/or E) |
| Efficacy in monocytes and dendritic cells |
| Efficacy in presence of mucin |
| Efficacy in presence of synthetic vaginal fluid and seminal plasma |
| Efficacy at vaginal pH |
| Stage 2: Transmission inhibition assays |
| Cell-free and cell-associated virus transmission assays |
| CD4-dependent and CD4-independent transmission assays |
| Transmission inhibition and sterilization assay (MTSA) |
| Stage 3: Mechanistic assays |
| Attachment inhibition |
| Fusion inhibition |
| Virus inactivation assays (virucidal activity) |
| RT inhibition assays |
| CCR5 and CXCR4-tropism for attachment inhibitors |
| DC-SIGN inhibition assay for attachment inhibitors |
| gp120/CD4 ELISA |
| Stage 4: Range of action assays |
| Efficacy against range of HIV-1 subtypes (clades) |
| Efficacy against CCR5- and CXCR4-tropic viruses |
| Efficacy against resistant viruses |
| Efficacy against HIV-2, SIV, and SHIV |
| Efficacy versus other STIs |
| Step 5: Vaginal/Rectal Environmental Toxicity |
| Toxicity to Lactobacillus sp. |
| MatTek epivaginal assays |
| Vaginal cell toxicity |
| Cervical cell toxicity |
| Rectal cell toxicity |
| Step 6: Combination assays with other potential microbicides |
| In attachment assay |
| In standard PBMC assays |
| In transmission/sterilization assay |
| Step 7: Resistance |
| Transmission of resistant strains in attachment assay |
| Selection of resistant strains in microbicide-like conditions |
| Step 8: Cervical explant or other ex vivo model |
| Step 9: Activity in candidate gel formulations |
| Step 10: Non-human primate models/Mouse models |
4.1 Regulatory Guidance Relevant to the Development of Topical Microbicides
At present the Food and Drug Administration (FDA) has not issued a specific guidance document for the development of anti-HIV topical microbicides; however, there is some consensus that the key points to consider are similar to those used for the development of systemic HIV inhibitors. The International Working Group for Microbicides (IWGM) has published a detailed and extensive list of development milestones that are highly applicable (Lard-Whiteford et al. 2004). The requirements for systemic Investigational New Drug (IND)-directed development include efficacy and toxicity evaluations in relevant cell cultures systems, range-of-action evaluations against clinically relevant wild type and resistant organisms, definition of the product’s mechanism of action, a detailed evaluation of the combination microbicide interactions and combination prevention strategies, and drug resistance evaluations. In the microbicide environment, these points would necessarily be modified to specifically include efficacy and toxicity testing in cells or systems appropriate for vaginal or rectal transmission, range-of-action testing with subtypes of virus expected to be encountered in clinical trials in sub-Saharan Africa or Southeast Asia, definition of mechanisms of action that occur prior to the integration of HIV into target cell DNA, combination studies with other microbicidal compounds in appropriate formulations using appropriate target cells, and evaluation of the ability of the agents to prevent the transmission of resistant virus strains (in addition to actually selecting for resistant strains under prolonged culturing or treatment durations).
4.2 Primary Topical Microbicide Screening
The primary evaluation of candidate topical microbicides is routinely performed by evaluation in both cell-based assays that serve to define both the antiviral efficacy and toxicity of the compound (PBMC assays with low passage clinical strains) as well as the selection of compounds with optimal microbicide mechanism of action (attachment, entry, fusion, or RT inhibitors). In addition, toxicity is usually evaluated immediately with regard to the effects of the candidate on normal vaginal flora (Lactobacillus sp.) and cells that are specific to the vaginal, cervical, and/or rectal environments. Compounds developed and identified through mechanism-based screening for direct virucidal activity or the ability to inhibit attachment, entry, fusion, or reverse transcription are also immediately evaluated for activity in these cell-based efficacy and toxicity defining assays. Toxicity evaluations should also include evaluation of the toxicity of the candidate microbicide at high concentrations with limited exposure times (1, 2, 4, 8, and 24 hour exposures) to more closely mimic the interaction of cells with the microbicide and the product’s length of residence time in the vagina must be understood in the performance of these assays to assure that appropriate toxicity endpoints are evaluated. In our laboratory, primary screening is performed in both PBMC assays using low passage clinical virus strains (Morner et al. 1999) (currently focusing on subtype C or subtype E viruses based on their prevalence in areas expected to be utilized in initial human clinical trials) and with entry inhibition assays utilizing MAGI, GHOST or TZM-bl cells with CCCR5, CXCR4, and dual tropic receptor expression (Kimpton et al. 1992; Morner et al. 1999). It has become widely accepted that primary virus infections routinely involve the transmission of CCR5-tropic virus and thus most primary screening algorithms are performed using these viruses. Toxicity testing is performed using three strains of Lactobacilli and with cervical and vaginal epithelial and endothelial cells (Fichorova et al. 1999; Fichorova et al. 2001; Fichorova et al. 2001).
4.3 Range of Anti-HIV Action
The most important considerations when defining the range of action of a potential microbicide is the ability of the product to prevent cell-free and cell-associated virus transmission to target cells and to prevent transmission to cells that do or do not express cell surface CD4. These assays are routinely performed in the presence and absence of mucopolysaccharides, such as porcine mucin, or vaginal or seminal fluids (Owen et al. 1999; Owen et al. 2005), to more closely mimic the environment in which the compounds must be active. These virus transmission assays have been developed to evaluate all possible means of virus transmission in the vaginal vault and are performed using appropriate epithelial target cells [MAGI, GHOST, or TZM-bl cells) expressing appropriate receptors and coreceptors or the cervical epithelial cell line ME180 (that does not express CD4)] with the infectious virus in a cell-free or cell-associated form. It has become commonly accepted that virus transmission primarily involves CCR5-tropic virus strains and thus testing should be performed with these viruses, but confirmed with CXCR4-tropic viruses as a component of the range of action evaluations. Since the primary mechanism of virus transmission in the vagina and rectum remains unclear, it is important to evaluate the ability of a microbicide candidate to inhibit all potential means of virus transmission. Culture conditions can be modified to evaluate compound pretreatment or delayed addition as well as variability in the multiplicity of infection (MOI) of the viral inoculum. For microbicides, knowledge of the pretreatment effects and variable MOIs are critical since the product will be applied prior to introduction of the viral inoculum.
In addition, range-of-action assays should be employed to determine the efficacy profile of the microbicide compound against geographically distinct clinical virus subtypes, or clades, found throughout the world and to drug resistant and multi-drug resistant (MDR) virus strains that might be encountered during sexual transmission. For a microbicide that essentially forms a barrier between the virus and its target cell, it is very important to determine the ability of pre-existing drug-resistant virus strains to bypass the chemical barrier. Since infectious replicating virus will not be repeatedly exposed to drugs being used in the microbicide product, the issues of resistance (described in more detail below) must be focused on preventing the transmission of drug-resistant virus strains rather than the selection of resistant strains. Thus, transmission and PBMC-based assays utilizing drug resistant virus strains are important to the development of the microbicide product.
In the microbicide environment it would be especially useful to have compounds that are able to suppress the replication or cellular infection of multiple STIs in addition to HIV. Thus, as a component of the range-of-action testing, it is suggested that the ability of the microbicide to inhibit replication of other viral, bacterial, and fungal pathogens that are known to be sexually transmitted should be evaluated. Screening is routinely performed against the STIs listed in Table 3.
Table 3.
Sexually Transmitted Infection (STI)-Causing Organisms to be Evaluated during Topical Microbicide Development
| PATHOGEN | CLASSIFICATION | INDICATION |
|---|---|---|
| Herpes Simplex Virus-Type 2 | Virus | Herpes |
| Hepatitis C (BVDV) | Virus | Hepatitis |
| Hepatitis B Virus | Virus | Hepatitis |
| Gardnerella vaginalis | Bacteria | Bacterial vaginosis |
| Prevotella corporis | Bacteria | Bacterial vaginosis |
| Neisseria gonorrhoeae | Bacteria | Gonorrhea |
| Bacterioides fragilis | Bacteria | Bacterial vaginosis |
| Mobiluncus curtisii | Bacteria | Bacterial vaginosis |
| Candida albicans | Fungus | Vulvovaginal candidiasis |
| Trichomonas vaginalis | Protist | Trichomoniasis |
| Lactobacillus crispatus | Bacteria | Normal Vaginal Flora |
| Lactobacillus jensenii | Bacteria | Normal Vaginal Flora |
| Lactobacillus acidophilus | Bacteria | Normal Vaginal Flora |
| Chlamydia trachomatis | Bacteria | Chlamydia |
4.4 Specialized Microbicidal Transmission Inhibition Assays
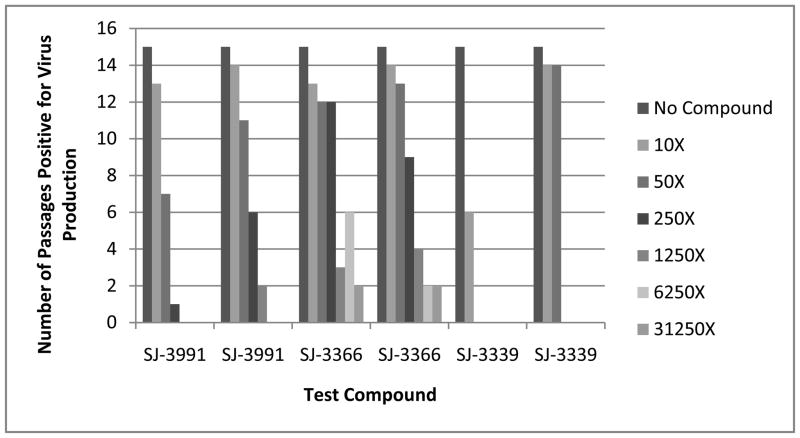
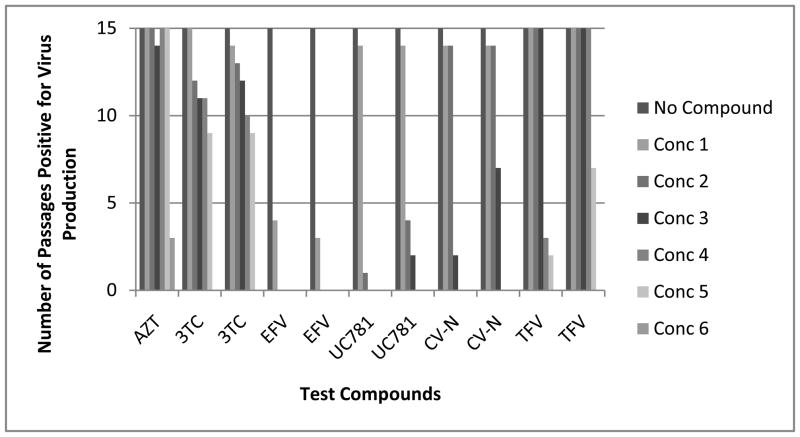
We have recently described a highly sensitive transmission assay based on methodology originally described by Balzarini et al. (Balzarini et al. 1993; Watson et al. 2008) that serves to define the concentrations of a microbicide that can completely suppress the transmission and subsequent replication of viruses in culture. Though the assays defined above that quantify the cell-free and cell-associated transmission of HIV are sensitive, they lack the robustness of the new microbicide transmission and sterilization assay (MTSA) because of the timing of the endpoint analysis. In the MTSA, virus is added to the culture in a cell-free or cell-associated form and the infection is allowed to proceed over the course of 30 days in the presence of various high, fixed concentrations of the microbicide test compound. The concentrations chosen are based on the selectivity index of the compound but the initial culture concentration is generally ten times the 50% effective concentration (EC50) of the compound. The cells are subcultured every three days by adding 20% of the infected culture (cells and supernatant) to the same original volume of uninfected cells in fresh medium with the same fixed concentration of test agent. These assays serve to define the concentration of the microbicide compound required to completely suppress or sterilize a culture and the sterilizing concentration of a given compound is totally unique. A representative example of the data obtained in the specialized microbicide transmission assay is shown in Figure 1. Compounds from the same highly related structure activity relationship (SAR) series that possess only extremely minor differences in chemical structure have been found to have equivalent activity in PBMC cultures and standard transmission assays, but to be widely different in their ability to suppress virus production in the MTSA (see Figure 1). Surprisingly we have found that most approved HIV drugs require significantly higher (1000 × EC50 concentration defined in the CPE assay) concentrations to completely suppress virus transmission and sterilize a culture of replicating virus than required for effective inhibition in the CPE assay. These transmission assays can be performed in simulated vaginal or seminal fluids or other mucopolysaccharides and drug treatment can be modified to more accurately reflect microbicidal use. In addition, a variety of virus strains, including drug resistant strains, may be used as the infectious inoculum.
Figure 1. Evaluation of Pyrimidinediones in a Specialized Transmission Inhibition Assay.
Figure 1A: Sterilizing Concentration Determinations for Pyrimidinedione Inhibitors: SJ-3991, SJ-3366 and SJ-3339 were evaluated in the MTSA and the results are presented as the number of passages which were positive for virus replication at each compound concentration. The results of two replicate assays for each compound are presented. Each pyrimidinedione was evaluated at concentrations that were 10-, 50-, 250-, 1,250-, 6,250- and 31,250-times the EC50 concentration that was defined in the CPE inhibition assay. All tested concentrations were significantly below the defined toxic concentration to CEM-SS cells. Passages which were positive for virus production were defined by detection of virus in the cell-free supernatant by RT assay. Cells were passaged for 10 passages and in the absence of compound for an additional 5 passages.
Figure 1B: Sterilizing Concentration Determinations for Control Compounds: The entry inhibitor cynovirin –N, the nucleotide RT inhibitors AZT and 3TC, the nucleotide RT inhibitor Tenofovir and the nonnucleoside RT inhibitors UC781 and efavirenz were evaluated in the MTSA and the results are presented as the number of passages which were positive for virus replication at each compound concentration. The results of two replicate assays for each compound are presented. The concentrations utilized for each compound in the series are as follows: AZT: 10 to 31,250 nM (10- through 31,250-times the EC50 concentration); 3TC: 100 to 62,500 nM (10- through 6,250-times the EC50 concentration); Efavirenz: 10 to 6,250 nM (10- through 6,250-times the EC50 concentration); UC781: 15 to 46,875 nM (10- through 31,250-times the EC50 concentration); cyanovirin N: 0.1 to 62.5 μg/mL (10- through 6,250-times the EC50 concentration); Tenofovir: 2.5 to 97.7 μM (2.5- through 97.7-times the EC50 concentration). All concentrations were in 5-fold increments with the exception of Tenofovir which was in 2.5-fold increments. Passages which were positive for virus production were defined by detection of virus in the cell-free supernatant by RT assay. Cells were passaged for 10 passages and in the absence of compound for an additional 5 passages.
4.5 Mechanism of Anti-HIV Action
Mechanistic studies to define how a potential microbicide prevents HIV infection are an important step in microbicide development since the optimal action of the microbicide is to prevent HIV infection prior to the establishment of the proviral state (pre-integration inhibitors). Both cell-based and biochemical/enzymatic assays are available to precisely define the mechanism of action of a test compound. In the case of microbicides, these assays are prioritized to include those that monitor virus attachment and entry, cell-cell fusion, and reverse transcription in light of the prevailing hypothesis that a microbicide product should prevent de novo infection. Entry assays can be performed to evaluate chemokine receptor specificity as well as the conformational epitopes that are formed upon co-culture of virus with target cells (post-attachment inhibition). Specialized testing for microbicides should include virucidal assays to determine whether compounds inhibit virus replication by directly inactivating HIV.
4.6 Combination Therapy Strategies
Combination therapy strategies are just beginning to be developed for microbicide inhibitors. At present two microbicide trials include the evaluation of a combination of a microbicide with an oral anti-retroviral agent: tenofovir (1% gel) plus oral tenofovir disoproxil fumarate (TDF) and tenofovir gel plus oral Truvada (TDF + emtricitabine). Recent NIH funding initiatives have specifically included support for the development of combination prevention strategies. The challenge of developing a combination microbicide strategy is a highly interesting opportunity, especially since the creative development of formulation strategies can be used to place each of the microbicides in the right place within the vaginal or rectal environment at the appropriate time to prevent infection. For example, viral inactivating agents would be expected to have activity upon contact with HIV-infected semen in the vaginal/rectal vault, viral entry inhibitors would be expected to work at the vaginal/rectal epithelium, and reverse transcriptase inhibitors would need to be present inside the target cells lining the epithelium. Combination assays have routinely been performed in either established or fresh human cell systems for the development of systemic inhibitors. In light of the specialized requirements for infection in the microbicide environment which include hematopoietic cells (T-cells, macrophages), dendritic cells, and epithelial cells, combination assays utilized should evaluate the activity of the microbicides alone and in combination in each of these cell types. For example, in addition to the standardized combination assays in PBMCs and other cell established hematopoietic cell lines, we have adapted the MAGI or GHOST cell based entry inhibition assays as a microbicide combination assay, as this assay might more closely resemble the environment in which the test agents will need to function. In this assay, MAGI or GHOST cells are pretreated with the combination of microbicides. Mucopolysaccharides and/or simulated/authentic vaginal and/or seminal fluid may also be added. Infectious virus is added and the cells are cultured for approximately two hours before the unattached and unabsorbed virus are removed by washing. The washing step may be modified to allow a more gradual disappearance of virus and compound to more accurately mimic the vaginal microbicide treatment and infection kinetics. The infection of the target cells and the ability of the compounds to prevent infection are then evaluated using the MacSynergy II analysis program at 48 hours post-infection.
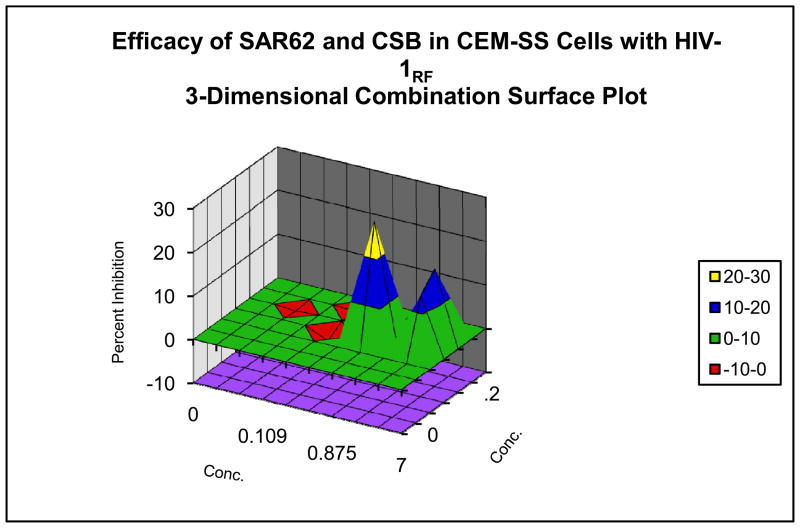
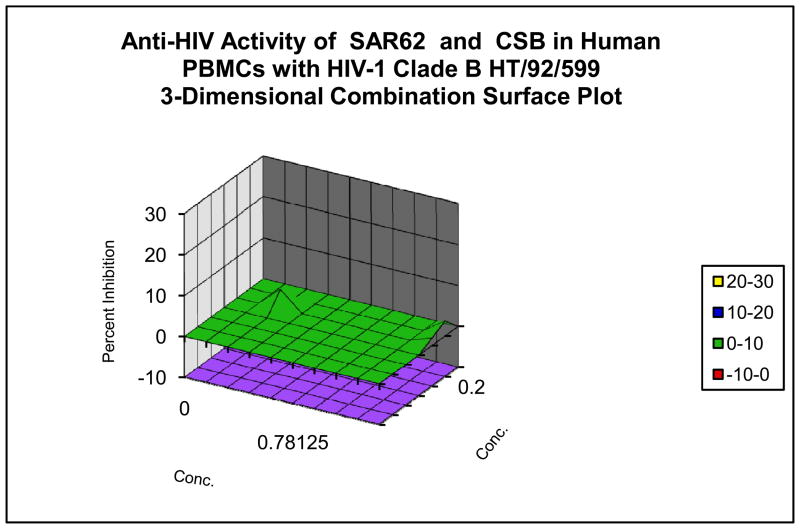
These microbicide-specific assays sometimes provide significantly different results than those performed with established or fresh cells as can be seen in data provided in Figure 2. In the systemic-type inhibitory assays, the compound and virus are allowed to remain in contact with the cells for 6 to7 days post-infection. In cases where significant synergy has been observed with the systemic assays, the microbicide assay has in some cases defined additive to only slightly synergistic results, and vice versa. Thus far, the data would suggest that evaluation in the microbicide specific assays is warranted since different answers are obtained with regard to the interaction of test compounds.
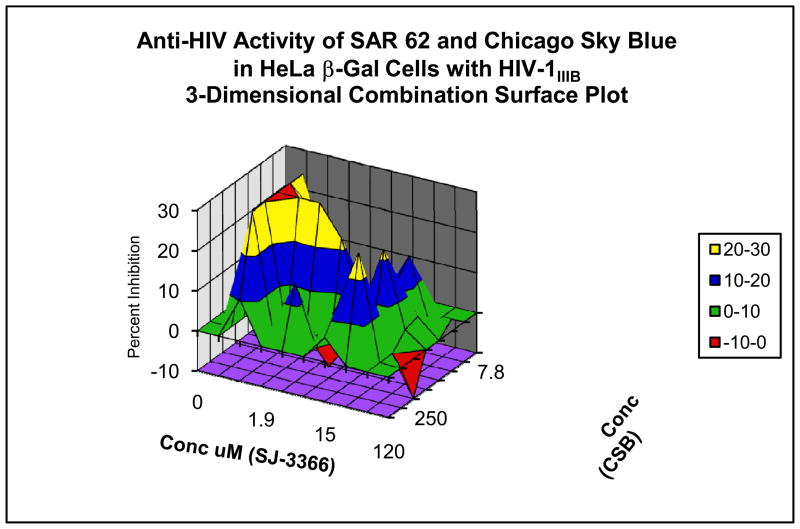
Figure 2. Evaluation of Combination Drug Interactions in Three Cell System.
Combination anti-HIV activity interactions of pyrimidinedione IQP-0410 (SAR 62) and Chicago Sky Blue in CEM-SS cells infected with a laboratory strain of HIV-1 (Panel A), in PBMCs infected with a low passage clinical strain of HIV-1 (Panel B) and in the microbicide-like attachment inhibition assay (Panel C). Slightly synergistic and additive interactions were observed with the CEM-SS and PBMC cultures, while significant synergy was observed in the microbicide-like attachment assay in MAGI cells.
4.7 Resistance Issues and Considerations For Development of Microbicides
As described above, infectious virus in the inoculum will be exposed to a microbicide product in the vaginal or rectal vaults and most likely will not have the opportunity to actually replicate in the presence of the compound (unless the active product is systemically absorbed). Thus, resistance selection in the context of microbicide development implies the rapid selection of a virus that likely pre-exists in the infectious inoculum and that is able to penetrate the microbicide barrier immediately because of the presence of the resistance-engendering mutation. For example it would be expected that the use of a compound such as the non-nucleoside RT inhibitor (NNRTI) nevirapine would provide an effective barrier to wild type viruses in a population, but would allow transmission of a virus possessing the nevirapine-resistant Y181C or Y181C+K103N mutations in the RT. In the case of these resistant viruses, the microbicide barrier would be immediately abrogated and the mutant viruses would transmit to the target cells as if the microbicide compound were not present. Thus, it is important to consider the impact of the presence of rare pre-existing drug resistant viruses whenever a microbicide product is evaluated. Compounds in which multiple amino acid changes are required for high-level resistance as a monotherapy would be the better choices for mono or combination microbicide strategies. As combination microbicide products are developed, in vitro antiviral evaluations should be performed to assure that virus strains resistant to one of the microbicide compounds remain completely susceptible to the other microbicide compound in the combination product. The quantification of resistance selection in the microbicide environment essentially involves the use of the short-term and specialized microbicide transmission assays (MTSA) in which the ability of the resistant virus to transmit is compared side by side with wild type virus. These assays effectively demonstrate the ease by which resistant viruses can bypass a microbicide barrier. The resistant viruses that should be employed in these evaluations should include any virus(es) that possess a known resistance profile to the microbicide being evaluated, viruses resistant to other members of the same inhibitor class, as well as resistant viruses known to be transmitted with some frequency as primary infections, including MDRs. In our laboratory, we define the sterilizing concentration of each microbicide compound using both wild type and various drug-resistant virus strains, including MDRs, and calculate a ratio that would demonstrate the fold-increase in drug concentration required to suppress the transmission of a drug resistant virus versus a wild type virus.
Another consideration for the development of microbicides is that the microbicide compound could be absorbed through the vaginal or rectal mucosa and enter the bloodstream of an individual that is HIV infected. Additionally, absorption may occur through the exposure of the unprotected penis to the microbicide compound in the vaginal or rectal vaults, or through exposure of mucous membranes in the mouth during oral sex. These secondary means of absorption and the possibility of selecting for resistant viruses should be carefully considered. At present most absorption studies are performed by evaluation of compound entering the blood stream from vaginal or rectal administration and studies are not performed to evaluate penile or oral absorption. Microbicide use is predicated on the ability of the compound to prevent transmission to uninfected sexual partners (females or males) from infected males through exposure to infectious virus in semen. However, the infected male partner must be carefully considered with regard to resistance selection if the opportunity exists for this infected male to receive small suboptimal doses of a microbicide through penile or oral absorption during sexual relations with his partner. In this scenario, the secondary absorption may drive resistance selection in the infected male, allowing subsequent infection of his sexual partner with resistant virus that is insensitive to the barrier effects of the microbicide that this partner has been using. Animal studies should be performed in the advanced preclinical development stage to quantify penile and oral absorption.
4.8 Ex Vivo Evaluations using Cervical Explants
Ex vivo organotypic cultures offer an indispensable bridge between in vitro culture systems and clinical studies by providing a controlled format where microbicides can be comparatively evaluated for (1) toxicity against the mucosal epithelium and (2) anti-HIV-1 activity in target cells present within the submucosa of human tissue. Three ex vivo ectocervical explant systems have been described (Collins et al. 2000; Greenhead et al. 2000). In one method, tissues are completely embedded/submerged in tissue culture fluids and HIV-1 is applied apically to the explant tissue with or without added microbicide (Collins et al. 2000). In the second method, the tissue is exposed to HIV-1 with or without an added microbicide in a nonpolarized manner and the explants are cultured submerged in tissue culture medium (Greenhead et al. 2000). Recently, a third method which more closely mimics vaginal infection has been described (Cummins et al. 2007). In this explant culture system, tissue is maintained in a polarized state with the epithelial surface positioned at the air/tissue interface and the submucosa (stroma) submerged in tissue culture medium. Unlike the other explant culture systems (Collins et al. 2000; Greenhead et al. 2000), this positioning of the cervical tissue allows application of virus and candidate topical microbicides directly to the epithelium and allows access to the cells in the submucosa. This explant culture along with the other published explant systems (Collins et al. 2000; Greenhead et al. 2000), have several limitations, including the lack of hormone modulation, the lack of recruitment of immune cells, the loss of epithelium, and the inability of the epithelial tissue to regenerate or repair damage. However, the last two attributes make this system sensitive to any potential toxic effect by the topical microbicide. Further, this explant culture demonstrates the capacity to be infected with HIV-1 with the subsequent evaluation of efficacy of several topical microbicide compounds. Additionally, this explant model may be performed with the addition of semen and vaginal fluids to more closely mimic the actual environment in which the microbicide must function.
4.9 Animal Models of HIV Sexual Transmission
A nonhuman primate model involving the vaginal and rectal inoculation of SIV or SHIV using rhesus macaques that has become the method of choice for the evaluation of HIV microbicides (Miller et al. 2005; Patton et al. 2009). In this model, the virus utilized must be SIV, but depending on the mechanism of action of the microbicide, SHIV strains with HIV envelope or HIV reverse transcriptase have also been utilized. Nearly all microbicides that are in clinical development have been evaluated in this particular animal model and the model can be used to monitor the ability of potential microbicides to inhibit both vaginal and rectal transmission of virus. Unlike the scenarios for development of systemic inhibitors of HIV infection, the use of the nonhuman primate model for the evaluation of microbicide products is much more frequent, however significant debate over the relevance of the nonhuman primate model versus actual human infection exists in the field and it is commonly accepted that better and more predictive models are needed to prioritize microbicide development. The animal models not only provide needed efficacy information on the product, but also allow for the evaluation of potential toxic effects of the microbicide product through direct observation of lesions in the vaginal or rectal lining and the quantification of inflammatory cytokine production. A topical microbicide development model using mice has also been developed (Sun et al. 2007; Denton et al. 2008). This model employs humanized bone marrow-liver-thymus (BLT) mice reconstituted with human CD4+ T and other relevant human cells and the mice are susceptible to intravaginal infection by HIV-1. The advantages of the BLT model include the use of humanized tissue and HIV-1, as well as the benefits of significantly reduced quantity requirement for compounds for screening and the ease of working with small animals as opposed to nonhuman primates.
4.10 Advanced Preclinical Testing Leading to the IND
Specialized testing for HIV microbicide development that differs significantly from studies proposed for systemic HIV inhibitors includes Rabbit Vaginal Irritation (RVI) (Draize 1944; Eckstein et al. 1969) and Rabbit Penile Irritation (RPI) evaluations (Zaneveld et al. 2002), contact hypersensitivity testing such as the Local Lymph Node Assay (LLNA) (Buehler 1994; Dearman et al. 1999; ICCVAM 1999) and absorption studies to evaluate the potential for systemic absorption of the microbicide (Lard-Whiteford et al. 2004; Balzarini et al. 2005) The remainder of the preclinical pharmacology and toxicology is identical to that described for the systemic inhibitors (Buckheit 2006) though some safety pharmacology studies may be avoided if a microbicide candidate can be shown conclusively not to be absorbed into the systemic circulation. Evaluation of inflammatory responses in the vaginal environment as measured by cytokine and chemokine profiles remains in the experimental stage (Belec et al. 1995; Doncel et al. 2004), but may soon become a relevant component of development algorithms. Evaluation of inflammatory responses would allow for greater understanding of potential immunomodulatory activities upon microbicide administration.
5. Microbicide Formulation and Delivery
The importance of the role of product formulation in the success of the development of a safe and effective microbicide product has been acknowledged. Successful formulation development requires systematic combination of vaginal microbicide candidates with excipients in a scientifically rational way to produce a stable, safe, and effective product. In addition to product safety and efficacy, patient acceptability is essential for the success of a microbicide product. The drug delivery method appropriate for each microbicide will depend upon factors such as chemical characteristics of the compound to be delivered and the mechanism by which the respective microbicide agent inhibits HIV infection. If the microbicide binds to the receptor or co-receptor of the target host cells to prevent HIV infection (macrophages, T-cells, and dendritic cells), then the drug delivery method must be able to deliver the drug to the site of action within the tissue. It is of critical importance to consider the target and eventual formulations even in the early phases of microbicide development. Therefore a “one size fits all” approach to microbicide drug delivery may not be possible, necessitating the consideration of “alternative” or other novel formulations such as polymeric films and nanoparticles. Several dosage forms have been identified and are under investigation for use as vaginal drug delivery systems for microbicide products (Rohan et al. 2009).
5.1 Semisolids – Gels and Creams
In microbicide formulation development, semi-solid dosage forms, or gels, are the most common for vaginal delivery. For these water-based dosage forms, the stability, retention, and distribution are most dependent upon the viscoelastic properties of the gel. In clinical trials, a “universal placebo” gel, a hydroxyethylcellulose (HEC) based gel, has been developed as a baseline comparison in microbicide development (Tien et al. 2005). Currently, there are several gel based formulations undergoing development. BufferGel is a carbopol-based gel that displayed spermicidal, anti-HIV, and HSV-2 activity, while safe in female trials (Mayer et al. 2001). PRO2000, a naphthalene sulfonate polymer, is also currently under development. PRO2000 has been shown effective in inhibiting HIV-1 infection in mice models (Pearce-Pratt et al. 1996; Bourne et al. 1999). Additionally, in Phase I trials, PRO2000 has been shown safe and acceptable in sexually inactive women and HIV-1 infected women (Van Damme 2000; Mayer et al. 2003; Morrow et al. 2003). Currently, PRO2000 has moved onto Phase II and Phase III clinical trials. In addition to formulating gels as microbicide agents, various microbicide candidate molecules are currently under development as a gel dosage form. UC-781 and Dapivirine, other NNRTI s, and Tenofovir, have shown significant promise in being formulated into a safe, effective, and acceptable product (Mayer et al. 2006; Patton et al. 2007; 2008; Schwartz et al. 2008; Fletcher et al. 2009; Romano et al. 2009).
Despite wide-spread use and numerous studies into gel formulations, the semi-solid nature of the products results in leakage or general “messiness” being a common problem encountered. In product acceptability studies for cellulose sulfate (CS) vaginal gel and KY gel, 20% of users reported product leakage where such leakage would prevent future gel use (Malonza et al. 2005; Holt et al. 2006; Schwartz et al. 2006). However, in an acceptability study involving sex, while 40% of women reported that the CS-containing gel leaked out during sex, 100% reported that the gel made the sexual activity feel “more wet” and 96% said they would probably or definitely use the gel (El-Sadr et al. 2006). In studies addressing the application of gel microbicides, a significant, but not a majority of participants reported messy applications, leakage, and an overall negative experience (Gross et al. 1999; Bentley et al. 2000; Coetzee et al. 2001).
5.2 Vaginal Rings
Intravaginal rings (IVRs) offer a unique delivery method over other solid and semi-solid formations. For the most part, topical gel microbicides are applied as a single dose before intercourse. This coital dependency may not be the most optimal dosage form for women in all parts of the world. Therefore, solid dosage forms, such as IVRs, which offer long term coitus-independent release of active pharmaceutical ingredient (API) into the vagina are being investigated. IVRs are torus-shaped polymeric devices either loaded with API within the polymer matrix of the ring or within a reservoir core at the center of the ring. Depending upon the composition of the polymeric ring, based upon excipient concentration, the release rate of drug from the ring can be controlled. The largest controlling factor of the IVR is the pliability of the polymer ring backbone. The rings need to allow for compression as they are inserted into the vagina, and placed in the upper third of the vagina to prevent involuntary expulsion (Novak et al. 2003; Sarkar 2005). Currently three IVR devices have been approved for commercial use by the FDA. Two rings are silicon based hormone replacement rings (Estring and Femring), and the third is a thermoplastic urethane ring (Nuvaring). As a microbicide dosage form delivery system, the NNRTI dapivirine is currently being evaluated in clinical trials as a silicon-based microbicide IVR (Woolfson et al. 2006; Gupta et al. 2008). Results from the Phase I studies indicated that the ring was safe for use, and provided controlled long term in vitro drug release.
5.3 Vaginal Films
While semi-solid gel applications are widely used today in clinical studies for vaginal drug delivery systems in the prevention of HIV, there still exist acceptability issues with gels that can be improved upon. Additionally, economic and social conditions, as well as consumer/patient preference, may interfere with acceptability of the traditional dosage forms of IVRs and semi-solids. Such issues would result in products being unacceptable to consumers, resulting in an overall reduced effectiveness of microbicide products. Ultimately, since patient acceptability and their ability and willingness to use a product directly impacts efficacy, it may be necessary to develop multiple dosage platforms for a single active agent to provide users with options they can use within the constraints of their social environment, personal choice, and environmental conditions. Therefore, rapid-dissolving polymeric films as a novel solid dosage formulation to delivery microbicides to the vagina are currently under investigation as an additional alternative.
In a survey study, it was reported that the ideal microbicide product would be odorless, offer protection from pregnancy and STI s, allow insertion up to 8 hours prior to sexual activity, and have minimal to no leakage (Holt et al. 2006). Others also indicate a desire for products that are unobtrusive to the sexual experience (or that enhance it) and offer flexibility in the timing of use. Advances in the field of polymer sciences have increased interest in the development of drug delivery systems which utilize these newly available polymers. Polymeric films are increasingly being used as a means of drug delivery. For vaginal drug delivery, a vaginal contraceptive film (VCF) has been developed that contains the spermicide Nonoxynol-9. Such film-formulated products, through ease of storage and application, have been shown to be very convenient for the user (Elias et al. 2001).
Therefore, polymeric vaginal films could address the acceptability issues observed in gel microbicides. They have been investigated for use as a drug delivery system for mucosal delivery and transdermal delivery. There are several attributes which make vaginal films attractive as a microbicide product dosage form. The films are a solid dry drug delivery system, which may eliminate any product odor and avoid any “messy” application. Polymeric films provide rapid drug release and bioadhesive properties that may increase retention time at the target tissue. The rapid dissolving nature of the films once in contact with the fluids present in the vagina may also reduce microbicide leakage. Recently, vaginal films have been investigated for use as contraceptives and more recently as contraceptive and microbicide formulations (Mauck et al. 1997; Roddy et al. 1998; Garg et al. 2005). A polystyrene sulfonate (PSS) microbicide film has been developed as an antimicrobial contraceptive agent that is transparent, pliable, and quick dissolving in solution. PSS has been demonstrated to be safe for vaginal use in phase I clinical trials in its gel formulation. The polymeric film based formulated resulted in similar safety and contraceptive activity (Garg et al. 2005).
5.4 Nanoparticles
More advanced forms of formulation that aim to enhance and improve API characteristics are being investigated in tandem to the aforementioned delivery systems. Through biotechnology, the use of nanoparticle encapsulation is being investigated as a drug delivery formulation that could provide a microbicide with long term coitus-independent efficacy. There has been significant effort in developing nanotechnology for the purpose of drug delivery since it offers a means of delivering both small molecules and macromolecules, such as peptides and proteins, by localized or targeted delivery to the tissue of interest (Moghimi et al. 2001). Biodegradable nanoparticles are commonly used due to their ability to be reabsorbed by the body, presenting lower toxicity than non-degradable polymers (Feng 2004; Gao et al. 2006). Nanoparticles can be loaded with a variety of bioactive agents, using various methods of encapsulation including emulsification-solvent evaporation, solvent displacement, and spray drying techniques (Konstantinos 2004; Xie et al. 2007).
Poly(lactic-co-glycolic acid) (PLGA) is one of the most widely accepted biodegradable polymers used in encapsulation drug delivery (Bala et al. 2004). PLGA has been extensively investigated to readily encapsulate hydrophobic drugs and provide them with properties reducing immunogenic response, increasing blood circulation lifetime, protecting against degradation, enhancing tissue penetration, and providing sustained drug release (Park 1995; Penco et al. 1996; Pettit et al. 1997; Shive et al. 1997; Lamprecht et al. 1999; Oh et al. 1999; Panyam et al. 2003; Castellanos et al. 2005; Wischke et al. 2008). The physical properties of the nanoparticles can be modified to alter the characteristics of the nanoparticles: surface charge to alter solubility (Musyanovych et al. 2008), particle size to alter intracellular trafficking and localization (Gaumet et al. 2009), and surface functionalization to increase in vivo residence time and active specific targeting (Chan et al. 2009).
Nanoparticle encapsulation as a drug delivery technology is currently being extensively investigated for various therapeutic applications in oral, nasal, transdermal, brain, infectious diseases, and cardio-vascular drug delivery systems (Dinauer et al. 2005; Heffernan et al. 2005; Roney et al. 2005; Coester et al. 2006; Dailey et al. 2006; Peng et al. 2006; Westedt et al. 2007; Ham et al. 2009). Many of these current studies focus on the encapsulation of larger molecules, e.g. proteins, peptides, and DNA/RNA. Their sub-micron size allows the nanoparticles to penetrate into the tissues and be readily available for cell uptake (Vinogradov et al. 2002). However, the vast majority of new drug therapies being developed today are not larger molecules like proteins and peptides, but low molecular weight molecules. Additionally, many of these molecules display low aqueous solubility which can be overcome through nanoparticle encapsulation (Straub et al. 2005).
For microbicide development, a nanoparticle drug delivery system for the delivery of PSC-RANTES, a CCR5 chemokine inhibitor, has recently been investigated (Ham et al. 2009). In formulation development, in vitro release studies demonstrated a release profile of PSC-RANTES that maintains anti-HIV bioactivity and protein stability over 30 days with increased ex vivo tissue permeability. Nanoparticle encapsulation has proven to be quite effective in research environments; however, commercially, it has met with little success. Marketing on Nutropin CDEpot, the first and only protein-loaded PLGA microparticle formulation on the market, was stopped due to the difficulties and high cost associated with protein microparticle development and commercial preparation (Wischke et al. 2008). This brings up the concern as to the feasibility of drug loaded nanoparticles as a viable and profitable product. However, such decisions need to be considered on a case by case basis. Additionally, with the advent of small molecule drugs, which are generally cheaper to synthesize, characterize, and encapsulate, nanoparticle encapsulation as a drug delivery system may be a significant vector in enhancing the efficacy of hydrophobic microbicides.
6. Microbicide Acceptability
A successful microbicide product must have biological effectiveness as well as be acceptable to the end user of the product. The biological effectiveness of a microbicide is dependent on both an effective anti-HIV product which is optimally formulated, and which can be adequately delivered to the location where it can interrupt the transmission of infectious virus to target cells in the vagina or rectum. An acceptable microbicide is one that users trust to be efficacious and whose formulation and/or delivery mechanism does not interfere with (or preferably will actually enhance) the experience of sex.
Studies have found that women are interested in using microbicides (Darroch et al. 1999; Hammett et al. 2000; Bentley et al. 2004; Weeks et al. 2004); however, there are certain aspects of topical gel use that women find unacceptable or bothersome. To date, microbicide gel acceptability studies have been limited to formative studies of user preferences using hypothetical or available over-the-counter “surrogate” products (Steiner et al. 1995; Hardy et al. 1998; Darroch et al. 1999; Hammett et al. 2000; Hammett et al. 2000; Weeks et al. 2004), and preferences based on the limited experiences of women participating in clinical trials (Bentley et al. 2000; Coggins et al. 2000; Coggins et al. 2000; Mauck et al. 2001; Mason et al. 2003; Morrow et al. 2003; Bentley et al. 2004; Mauck et al. 2004; Mauck et al. 2004; El-Sadr et al. 2006; Rosen et al. 2008). While these studies have been highly valuable, and a majority of users in most studies reported a willingness to use study products under at least some circumstances, acceptability issues have indeed emerged, including issues of leakage, how consistency and leakage affect sexual pleasure, how the feel of the product impacts the product’s ability to be used covertly, and how coitus-dependent gels may not offer the most useful strategy for application of microbicidal products.
Vaginal ring acceptability has been specifically assessed in several studies (Roumen et al. 2001; Novak et al. 2003; Speroff 2003; Ballagh 2004; Ahrendt et al. 2006). In one study, 84% of participants were either satisfied or very satisfied with the ring and 87% said they would (absolutely/most probably) recommend it to others (Ahrendt et al. 2006). Another study of 1950 women concluded that there was an overall high level of both user and partner acceptability for the contraceptive ring (Novak et al. 2003). Vaginal ring acceptability research often considers effectiveness, and duration and systemic effects of the drugs delivered in comparison with other existing drugs regimens, such as combined oral contraception, in the case of NuvaRing (Veres et al. 2004; Oddsson 2006), and oral hormones for menopause in the case of Estring. In addition to these more clinical aspects of ring use, Novak and colleagues (Novak et al. 2003) reported on acceptability dimensions of instruction clarity, ease of use (including insertion and removal), sexual comfort, overall satisfaction, and cycle-related characteristics. While these data offer promise regarding IVR acceptability in general, unique issues related to this delivery system as a microbicide drug delivery system (e.g., whether delivering an antiretroviral drug via this system is acceptable) now need to be explored.
Conducting acceptability research earlier in the developmental process, and creating behavioral tools for developers to ascertain acceptability of candidate formulations and devices, may save valuable resources by providing critical data which might allow earlier definition of which microbicide candidates meet preclinical standards for acceptability. Many behavioral dimensions have been shown to be important to microbicide gel acceptability, in particular: leakage, application, sexual pleasure, covert use, access, packaging/portability/disposability, side effects, perceived product efficacy, hygiene, contraceptive properties, consistency, color, odor, taste, and other contextual factors. In addition, intravaginal ring acceptability studies include such dimensions as ring removal (Novak et al. 2003) and disposal, perceived moisture (Veres et al. 2004), and sexual comfort (Novak et al. 2003).
7. Preclinical Development of Novel Microbicide Strategies
As discussed above, microbicide strategies are primarily targeted at developing compounds that will act early in the virus replication cycle; microbicides generally directly inactivate virions or target one of the early stages in the replication cycle including attachment, fusion, entry, reverse transcription and/or integration. Recent advances have suggested that novel therapies that directly or indirectly inhibit virus replication may also be possible and the microbicide testing algorithm may need to accommodate evaluation of the strategies described below. In addition, the future development of combination strategies will obviously benefit from targeting of multiple viral replication pathways and utilizing compounds which inactivate virus, prevent virus entry, and/or suppress virus replication following infection.
7.1. Pre-Exposure Prophylaxis
Pre-exposure prophylaxis (PrEP) is a promising experimental prevention method that would utilize anti-retrovirals (ARVs) to protect uninfected individuals from their infected sexual partners (Youle et al. 2003). PrEP follows in the footsteps of post-exposure prophylaxis (PEP) which has proven to be effective in the prevention of mother-to-child transmission of HIV (Lallemant et al. 2004). HIV challenge studies in animals have provided preliminary evidence that PrEP could partially prevent the transmission of the virus (Subbarao et al. 2006; Denton et al. 2008). Tenofovir and Truvada (tenofovir plus emtricitabine) are currently being evaluated in clinical PrEP trials. Although these FDA approved drugs have been determined to be safe and effective as therapeutic agents, additional safety and efficacy studies are in progress to evaluate their use as prevention technologies.
PrEP candidate selection has focused on identifying candidate molecules with several important characteristics. To assure effectiveness these microbicides should have a high genetic barrier to resistance as well as a unique resistance profile that would include minimal to no cross-resistance. Additionally, since there is a risk of selection of resistant viruses, the selected drug-resistant viruses should meet criteria that include being less transmissible compared to wild type virus and having a reduced replication fitness compared to wild type virus. Selected compounds should also have a favorable safety profile and be easy to use, have a mode of action that occurs prior to the integration of the virus into the host cell, be able to achieve effective concentrations of the active drug at the site of transmission, maintain its antiviral profile against wild-type and resistant viruses, and be cost-effective (Derdelinckx et al. 2006). PrEP offers a potentially convenient strategy for prevention in light of the ease of administration of selected compounds, as well as the advantages of acceptability and potentially enhanced adherence. Obvious disadvantages involve the potential toxic effects of the compounds, relative pharmacokinetics in the vaginal and rectal compartments, and drug-drug interactions which would need to be closely monitored.
7.2. Lactobacillus Vectors to Produce Microbicides in Vivo
Lactobacilli normally flourish in the vagina and offer some protection from STI transmission through production of hydrogen peroxide and by maintenance of the vaginal pH at levels low enough to inhibit pathogen replication. Molecular engineering approaches have been utilized to attempt to generate Lactobacillus species that constitutively produce a microbicidal protein (Martin et al. 1999; Turpin 2002; D’Cruz et al. 2004). Colonization of the vagina by the engineered Lactobacilli would provide a combination approach to STI inhibition: the Lactobacilli would inhibit STI transmission through its normal functions and the antimicrobial protein, such as cyanovirin-N (Boyd et al. 1997; Colleluori et al. 2005) or griffithsin (Mori et al. 2005), would offer an additional barrier to STI transmission.
7.3. Broadly Active Anti-Infective Agents
Perhaps the most sought after microbicides are broadly active anti-infective agents. These compounds would be expected to have activity against multiple viral and/or bacterial agents. The detailed testing algorithm proposed above is especially well suited for testing broadly active therapeutic agents and is designed to provide needed experimental support for an IND. Currently microbicides with multi-potent activity include sulfated, high molecular weight proteins and engineered proteins such as cyanovirin-N (Boyd et al. 1997; Colleluori et al. 2005) or griffithsin (Mori et al. 2005). In vitro evaluations with PRO2000 have suggested that this compound also possesses a broad range of anti-viral action with efficacy against both HIV-1 and herpesviruses (Bourne et al. 1999). ISIS 5320, an 8-mer oligonucleotide being developed by ImQuest BioSciences also has been documented to possess activity against HIV and HSV-2 (RWB, KMW, unpublished data). It is anticipated that the definition of compounds with activity against both viral infections and bacterial vaginosis causing organisms will be highly difficult. Combination microbicide approaches, especially those involving antimicrobial peptides plus specific small molecule microbicides might yield the desired result.
7.4. Antimicrobial peptides
Natural antimicrobial peptides are universally occurring, ancient and highly potent defense molecules against bacteria, viruses, fungi and parasites, representing an important component of the initial innate human response to microbial infection. However, only a few of these peptides have been subjected to evaluation in antiviral assays (Cole 2005; Jenssen et al. 2006). Known examples are brevinin-1 (Yasin et al. 2000), caerin 1.1, caerin 1.9, maculatin 1.1 (VanCompernolle et al. 2005), dermaseptin S4 (Lorin et al. 2005), esculentin 2P, ranatuerin 2P (Chinchar et al. 2001), and magainins (Albiol Matanic et al. 2004) from amphibians and cecropin A and melittin from insects (Wachinger et al. 1998). In mammals, defensins and cathelicidins are the two major classes of antimicrobial peptides. All human α-defensins and human β-defensin 3 inhibit HIV infection (Hazrati et al. 2006), but θ-defensins are more effective (Cole et al. 2002; Cordes et al. 2002; Munk et al. 2003; Wang et al. 2003; Wang et al. 2004) inhibitors of HIV. Distinct from defensins, cathelicidins vary in both sequence and three-dimensional structure. As the only cathelicidin in humans, LL-37 is also active against HIV. Currently, more than 1459 such peptides are catalogued in the Antimicrobial Peptide Database (APD) which was originally constructed by investigators at the University of Nebraska Medical Center in 2004 and was substantially updated during 2007–2008 (http://aps.unmc.edu/AP/main.html). Wang et al. have recently reported on the anti-HIV activity of 20 antimicrobial peptides derived from human and bovine cathelicidins (LL-37 and BMAP-27) (Wang et al. 2008) and have shown that peptides with HIV inhibitory activity can be defined by screening peptides from the APD. In addition, they have demonstrated that the biological activity of lead peptides can be improved through peptide engineering to define essential regions for antimicrobial activity as well as more specific motifs that might be conserved among the peptides that impact peptide efficacy or toxicity. A blood derived inhibitory agent designated virus-inhibitory peptide (VIRIP) has also been described which targets gp41 and blocks virus entry (Munch et al. 2007). VIRIP is a 20-residue peptide corresponding to the C-proximal region of the most abundant serine protease inhibitor. Naturally occurring blood products may not all be beneficial to preventing HIV infection, as semen derived amyloid fibrils have been reported to enhance HIV transmission and reduce the efficacy of polyanionic microbicides (Patel et al. 2007).
7.5. Cellular Targets
Microbicidal strategies that are directed at cellular targets may result in modulation of expression or blockade of function of cellular proteins required for virus entry, such as CD4 or the chemokine receptor molecules. For example, it has been suggested that siRNA strategies might be utilized to degrade mRNAs for receptor proteins, causing these proteins to be removed from the cell surface for extended periods of time and rendering the target cells resistant to infection during that transient period (Novina et al. 2002; Song et al. 2003; Song et al. 2003; Palliser et al. 2006). Additional studies will need to be performed to determine if HIV can evade these types of therapeutic strategies by utilizing novel entry strategies, especially since it is not known what receptors are critical for infection in the vaginal and rectal environments. A similar siRNA-based microbicide development program targeting herpes simplex virus has demonstrated inhibition of HSV-2 in a mouse model (Palliser et al. 2006).(Palliser et al. 2006)
7.6. Immune Modulation and Neutralizing Antibodies
A great deal of discussion has recently centered on the potential for stimulating innate or natural immune defense mechanisms to inhibit the transmission of HIV and other STIs in the mucosal environment. These strategies may target the NK cell response or may activate small immunomodulatory proteins that can directly inactivate infectious organisms. A special case of immune modulation would be appropriate stimulation of the mucosal immunity to generate neutralizing antibodies in the mucosa. We have shown that neutralizing antibodies can prevent infection in the in vitro microbicide assays described above and most interestingly we have demonstrated high levels of synergistic anti-HIV activity with neutralizing antibodies used in combination with some small molecule microbicidal compounds (RWB, unpublished data). The potential synergism between adaptive or natural immunity in the mucosal environment together with a combination microbicide therapeutic might be an excellent approach to suppressing virus transmission.
8. Summary
The development of an effective anti-HIV microbicidal compound will likely have a much greater impact on the spread of HIV than systemic therapy, especially in the developing world. By one estimate, a single microbicide with 60% efficacy could prevent over one million new infections per year (Watts et al. 2002). In the absence of an effective prophylactic vaccine, it is critical that the world’s pharmaceutical capability be directed toward the development of an effective topical microbicide. The development of a microbicide from discovery through the IND currently follows a path similar to that of systemic anti-HIV compounds with modifications that are reflective of the preventative strategy and environment. Along with microbicide development, formulation issues must be accounted for. As such, several formulation strategies, including semi-solids, IVR, films, and nanoparticles, are being developed to accommodate the growing number of microbicides as well as their acceptability by users. Microbicide testing must evaluate (1) toxicity to normal vaginal flora and cells and tissues found in the vaginal or rectal environments, (2) efficacy in assays that represent the biological mode of infection during sexual transmission, and (3) the acceptability of anticipated dosage forms. Although the bulk of microbicide development has been directed at vaginal microbicides, significant attention must be directed towards the equally important development of rectal microbicides. The present approach of utilizing vaginally formulated microbicides for testing in rectal environments must be modified to consider appropriate formulation of the rectal microbicide using microbicide compounds that are similar to or identical to those developed for vaginal use. With an initial clinical success with PRO2000 and several failures in clinical trials, the development of topical microbicides continues with a wide variety of microbicides entering or in Phase 3 human clinical trials. The adaptation of preclinical testing algorithms will continue to naturally evolve to reflect the clinical trial experiences and maturation of the microbicide field.
Acknowledgments
The authors wish to express their sincere appreciation to the many dedicated scientists around the globe that have diligently labored for the humanitarian purpose of developing topical microbicides for use throughout the world. No greater reward is possible than the prevention of HIV transmission to millions of individuals, especially women, that will result from the successful development and deployment of an effective microbicide. Similarly, the authors acknowledge and express their appreciation to the thousands of women that have participated in microbicide trials and thank them for their contributions to the development of a microbicide product. Closer to home, the authors acknowledge the efforts of Christa Buckheit Sturdevant, Robert W. Buckheit III, Tracy Hartman, Nick Kaludov, Joe Kurczewski, Todd Parsley, Kathleen Powers, and Lu Yang in the development of microbicides at ImQuest BioSciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Food and Drug Administration. from www.fda.gov/oashi/aids/virals.html.
- Guidance for Industry - Antiviral Drug Development Conducting Virology Studies and Submitting the Data to the Agency.
- From the Centers of Disease Control and Prevention. CDC statement on study results of product containing nonoxynol-9. Jama. 284(11):1376. doi: 10.1001/jama.284.11.1376-jwr0920-4-1. [DOI] [PubMed] [Google Scholar]
- Test of new vaginal microbicide gel show promise for women. AIDS Read. 18(4):165–6. [PubMed] [Google Scholar]
- Anti-HIV Gel Shows Promise in Large Scale Study in Women. NIH News.
- Ahrendt HJ, Nisand I, Bastianelli C, Gomez MA, Gemzell-Danielsson K, Urdl W, Karskov B, Oeyen L, Bitzer J, Page G, Milsom I. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 microg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74(6):451–7. doi: 10.1016/j.contraception.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Albiol Matanic VC, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents. 2004;23(4):382–9. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21(12):757–66. doi: 10.2165/00002512-200421120-00001. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Karlsson A, Perez-Perez MJ, Camarasa MJ, De Clercq E. Knocking-out concentrations of HIV-1-specific inhibitors completely suppress HIV-1 infection and prevent the emergence of drug-resistant virus. Virology. 1993;196(2):576–85. doi: 10.1006/viro.1993.1513. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Damme L. Intravaginal and intrarectal microbicides to prevent HIV infection. Cmaj. 2005;172(4):461–4. doi: 10.1503/cmaj.1041462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer BE, Doncel GF, Krebs FC, Shattock RJ, Fletcher PS, Buckheit RW, Jr, Watson K, Dezzutti CS, Cummins JE, Bromley E, Richardson-Harman N, Pallansch LA, Lackman-Smith C, Osterling C, Mankowski M, Miller SR, Catalone BJ, Welsh PA, Howett MK, Wigdahl B, Turpin JA, Reichelderfer P. In vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob Agents Chemother. 2006;50(2):713–23. doi: 10.1128/AAC.50.2.713-723.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belec L, Gherardi R, Payan C, Prazuck T, Malkin JE, Tevi-Benissan C, Pillot J. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine. 1995;7(6):568–74. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- Bentley ME, Fullem AM, Tolley EE, Kelly CW, Jogelkar N, Srirak N, Mwafulirwa L, Khumalo-Sakutukwa G, Celentano DD. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94(7):1159–64. doi: 10.2105/ajph.94.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley ME, Morrow KM, Fullem A, Chesney MA, Horton SD, Rosenberg Z, Mayer KH. Acceptability of a novel vaginal microbicide during a safety trial among low-risk women. Fam Plann Perspect. 2000;32(4):184–8. [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3(1):42–7. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Capetti A, Rizzardini G. HIV disease treatment in the era of HAART. Biomed Pharmacother. 1999;53(2):93–105. doi: 10.1016/s0753-3322(99)80066-3. [DOI] [PubMed] [Google Scholar]
- Bourne N, Bernstein DI, Ireland J, Sonderfan AJ, Profy AT, Stanberry LR. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J Infect Dis. 1999;180(1):203–5. doi: 10.1086/314853. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, 2nd, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, 2nd, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–30. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit R. Expedited PreClinical Development of Inhibition of the Human Immunodeficiency Virus: Evaluation and Char. Expert Opin Investig Drugs To Be Published 2006 [Google Scholar]
- Buehler EV. Occlusive patch method for skin sensitization in guinea pigs: the Buehler method. Food Chem Toxicol. 1994;32(2):97–101. doi: 10.1016/0278-6915(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. Jama. 2000;283(3):381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- Carreno MP, Chomont N, Kazatchkine MD, Irinopoulou T, Krief C, Mohamed AS, Andreoletti L, Matta M, Belec L. Binding of LFA-1 (CD11a) to intercellular adhesion molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) triggers transmigration of human immunodeficiency virus type 1-infected monocytes through mucosal epithelial cells. J Virol. 2002;76(1):32–40. doi: 10.1128/JVI.76.1.32-40.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos IJ, Flores G, Griebenow K. Effect of the molecular weight of poly(ethylene glycol) used as emulsifier on alpha-chymotrypsin stability upon encapsulation in PLGA microspheres. J Pharm Pharmacol. 2005;57(10):1261–69. doi: 10.1211/jpp.57.10.0004. [DOI] [PubMed] [Google Scholar]
- Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, Farokhzad OC. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–34. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Wang J, Murti G, Carey C, Rollins-Smith L. Inactivation of frog virus 3 and channel catfish virus by esculentin-2P and ranatuerin-2P, two antimicrobial peptides isolated from frog skin. Virology. 2001;288(2):351–7. doi: 10.1006/viro.2001.1080. [DOI] [PubMed] [Google Scholar]
- Coester C, Nayyar P, Samuel J. In vitro uptake of gelatin nanoparticles by murine dendritic cells and their intracellular localisation. Eur J Pharm Biopharm. 2006;62(3):306–14. doi: 10.1016/j.ejpb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Coetzee N, Blanchard K, Ellertson C, Hoosen AA, Friedland B. Acceptability and feasibility of Micralax applicators and of methyl cellulose gel placebo for large-scale clinical trials of vaginal microbicides. Aids. 2001;15(14):1837–42. doi: 10.1097/00002030-200109280-00013. [DOI] [PubMed] [Google Scholar]
- Coggins C, Blanchard K, Alvarez F, Brache V, Weisberg E, Kilmarx PH, Lacarra M, Massai R, Mishell D, Jr, Salvatierra A, Witwatwongwana P, Elias C, Ellertson C. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Infect. 2000;76(6):480–3. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins C, Blanchard K, Friedland B. Men’s attitudes towards a potential vaginal microbicide in Zimbabwe, Mexico and the USA. Reprod Health Matters. 2000;8(15):132–41. doi: 10.1016/s0968-8080(00)90015-6. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391–1393. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- Cole AM. Antimicrobial peptide microbicides targeting HIV. Protein Pept Lett. 2005;12(1):41–7. doi: 10.2174/0929866053406101. [DOI] [PubMed] [Google Scholar]
- Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci U S A. 2002;99(4):1813–8. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleluori DM, Tien D, Kang F, Pagliei T, Kuss R, McCormick T, Watson K, McFadden K, Chaiken I, Buckheit RW, Jr, Romano JW. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr Purif. 2005;39(2):229–36. doi: 10.1016/j.pep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6(4):475–9. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, Dragavon J, Peterson G, Hooton TM, Collier AC, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- Cordes FS, Bright JN, Sansom MS. Proline-induced distortions of transmembrane helices. J Mol Biol. 2002;323(5):951–60. doi: 10.1016/s0022-2836(02)01006-9. [DOI] [PubMed] [Google Scholar]
- Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51(5):1770–9. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz OJ, Uckun FM. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10(3):315–36. doi: 10.2174/1381612043386374. [DOI] [PubMed] [Google Scholar]
- Dailey LA, Jekel N, Fink L, Gessler T, Schmehl T, Wittmar M, Kissel T, Seeger W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol. 2006;215(1):100–8. doi: 10.1016/j.taap.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Darroch JE, Frost JJ. Women’s interest in vaginal microbicides. Fam Plann Perspect. 1999;31(1):16–23. [PubMed] [Google Scholar]
- Dearman RJ, Basketter DA, Kimber I. Local lymph node assay: use in hazard and risk assessment. J Appl Toxicol. 1999;19(5):299–306. doi: 10.1002/(sici)1099-1263(199909/10)19:5<299::aid-jat591>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, Garcia JV. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5(1):e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdelinckx I, Wainberg MA, Lange JM, Hill A, Halima Y, Boucher CA. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med. 2006;3(11):e454. doi: 10.1371/journal.pmed.0030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio S, Giannini G, Lapenta C, Spada M, Binelli A, Germinario E, Sestili P, Belardelli F, Proietti E, Vella S. Vaginal transmission of HIV-1 in hu-SCID mice: a new model for the evaluation of vaginal microbicides. Aids. 2001;15(17):2231–8. doi: 10.1097/00002030-200111230-00003. [DOI] [PubMed] [Google Scholar]
- Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26(29):5898–906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Doncel GF, Chandra N, Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004;37(Suppl 3):S174–80. [PubMed] [Google Scholar]
- Draize ea. J Pharmacol Exp Ther. 1944;82:377–390. [Google Scholar]
- Eckstein P, Jackson MC, Millman N, Sobrero AJ. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20(1):85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- El-Sadr WM, Mayer KH, Maslankowski L, Hoesley C, Justman J, Gai F, Mauck C, Absalon J, Morrow K, Masse B, Soto-Torres L, Kwiecien A. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. Aids. 2006;20(8):1109–16. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going? J Womens Health Gend Based Med. 2001;10(2):163–73. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- Feng SS. Nanoparticles of biodegradable polymers for new-concept chemotherapy. Expert Rev Med Devices. 2004;1(1):115–25. doi: 10.1586/17434440.1.1.115. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60(2):508–14. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69(9):5840–8. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184(4):418–28. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317(4):185–91. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Harman S, Azijn H, Armanasco N, Manlow P, Perumal D, de Bethune MP, Nuttall J, Romano J, Shattock R. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2009;53(2):487–95. doi: 10.1128/AAC.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PS, Wallace GS, Mesquita PM, Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Pope M. The enigma of dendritic cell-immunodeficiency virus interplay. Curr Mol Med. 2002;2:229–248. doi: 10.2174/1566524024605716. [DOI] [PubMed] [Google Scholar]
- Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, Fu S. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–90. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Garg S, Kandarapu R, Vermani K, Tambwekar KR, Garg A, Waller DP, Zaneveld LJ. Development pharmaceutics of microbicide formulations. Part I: preformulation considerations and challenges. AIDS Patient Care STDS. 2003;17(1):17–32. doi: 10.1089/108729103321042881. [DOI] [PubMed] [Google Scholar]
- Garg S, Tambwekar KR, Vermani K, Kandarapu R, Garg A, Waller DP, Zaneveld LJ. Development pharmaceutics of microbicide formulations. Part II: formulation, evaluation, and challenges. AIDS Patient Care STDS. 2003;17(8):377–99. doi: 10.1089/108729103322277402. [DOI] [PubMed] [Google Scholar]
- Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJ. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm Res. 2005;22(4):584–95. doi: 10.1007/s11095-005-2490-1. [DOI] [PubMed] [Google Scholar]
- Gaumet M, Gurny R, Delie F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur J Pharm Sci. 2009;36(4–5):465–73. doi: 10.1016/j.ejps.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74(12):5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999;26(10):572–8. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Gupta KM, Pearce SM, Poursaid AE, Aliyar HA, Tresco PA, Mitchnik MA, Kiser PF. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008;97(10):4228–39. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- Gupta P, Mellors J, Kingsley L, Riddler S, Mandaleshwar KS, Schreiber S, Cronin M, Rinaldo CR. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26(3):502–11. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett TM, Mason TH, Joanis CL, Foster SE, Harmon P, Robles RR, Finlinson HA, Feudo R, Vining-Bethea S, Jeter G, Mayer KH, Doherty-Iddings P, Seage GR., 3rd Acceptability of formulations and application methods for vaginal microbicides among drug-involved women: results of product trials in three cities. Sex Transm Dis. 2000;27(2):119–26. doi: 10.1097/00007435-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Hammett TM, Norton GD, Mason TH, Langenbahn S, Mayer KH, Robles RR, Feudo R, Seage GR., 3rd Drug-involved women as potential users of vaginal microbicides for HIV and STD prevention: a three-city survey. J Womens Health Gend Based Med. 2000;9(10):1071–80. doi: 10.1089/152460900445983. [DOI] [PubMed] [Google Scholar]
- Hardy E, Jimenez AL, de Padua KS, Zaneveld LJ. Women’s preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packaging. Contraception. 1998;58(4):245–9. doi: 10.1016/s0010-7824(98)00104-8. [DOI] [PubMed] [Google Scholar]
- Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, Lehrer RI, Herold BC. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol. 2006;177(12):8658–66. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16(6):1340–2. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S17–21. [PubMed] [Google Scholar]
- Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- Holt BY, V, Morwitz G, Ngo L, Harrison PF, Whaley KJ, Pettifor A, Nguyen AH. Microbicide preference among young women in California. J Womens Health (Larchmt) 2006;15(3):281–94. doi: 10.1089/jwh.2006.15.281. [DOI] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74(13):6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Pope M, Brown C, O’Doherty U, Miller CJ. Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys. Lab Invest. 1998;78(4):435–51. [PubMed] [Google Scholar]
- Ibata B, Parr EL, King NJ, Parr MB. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol Reprod. 1997;56(2):537–43. doi: 10.1095/biolreprod56.2.537. [DOI] [PubMed] [Google Scholar]
- ICCVAM. NIH Publication No. 99–4494. Research Triangle Park: National Institute of Environmental Health Sciences; 1999. [Google Scholar]
- Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66(4):2232–9. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinos A. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1(4):321–33. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- Lackman-Smith C, Osterling C, Luckenbaugh K, Mankowski M, Snyder B, Lewis G, Paull J, Profy A, Ptak RG, Buckheit RW, Jr, Watson KM, Cummins JE, Jr, Sanders-Beer BE. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52(5):1768–81. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, Kanshana S, McIntosh K, Thaineua V. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351(3):217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- Lamprecht A, Ubrich N, Hombreiro Perez M, Lehr C, Hoffman M, Maincent P. Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm. 1999;184(1):97–105. doi: 10.1016/s0378-5173(99)00107-6. [DOI] [PubMed] [Google Scholar]
- Lard-Whiteford SL, Matecka D, O’Rear JJ, Yuen IS, Litterst C, Reichelderfer P. Recommendations for the nonclinical development of topical microbicides for prevention of HIV transmission: an update. J Acquir Immune Defic Syndr. 2004;36(1):541–52. doi: 10.1097/00126334-200405010-00001. [DOI] [PubMed] [Google Scholar]
- Letvin N. Annu Rev Med. 2004. [DOI] [PubMed] [Google Scholar]
- Lorin C, Saidi H, Belaid A, Zairi A, Baleux F, Hocini H, Belec L, Hani K, Tangy F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology. 2005;334(2):264–75. doi: 10.1016/j.virol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Malonza IM, Mirembe F, Nakabiito C, Odusoga LO, Osinupebi OA, Hazari K, Chitlange S, Ali MM, Callahan M, Van Damme L. Expanded Phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. Aids. 2005;19(18):2157–63. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- Mason TH, Foster SE, Finlinson HA, Morrow KM, Rosen R, Vining S, Joanis CL, Hammett TM, Seage GR., 3rd Perspectives related to the potential use of vaginal microbicides among drug-involved women: focus groups in three cities in the United States and Puerto Rico. AIDS Behav. 2003;7(4):339–51. doi: 10.1023/b:aibe.0000004726.61630.96. [DOI] [PubMed] [Google Scholar]
- Mathijs JM, Hing M, Grierson J, Dwyer DE, Goldschmidt C, Cooper DA, Cunningham AL. HIV infection of rectal mucosa. Lancet. 1988;1(8594):1111. doi: 10.1016/s0140-6736(88)91932-0. [DOI] [PubMed] [Google Scholar]
- Mauck C, Weiner DH, Ballagh S, Creinin M, Archer DF, Schwartz J, Pymar H, Lai JJ, Callahan M. Single and multiple exposure tolerance study of cellulose sulfate gel: a Phase I safety and colposcopy study. Contraception. 2001;64(6):383–91. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]
- Mauck CK, Baker JM, Barr SP, Abercrombie TJ, Archer DF. A phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9. Postcoital testing and colposcopy. Contraception. 1997;56(2):89–96. doi: 10.1016/s0010-7824(97)00097-8. [DOI] [PubMed] [Google Scholar]
- Mauck CK, Weiner DH, Ballagh SA, Creinin MD, Archer DF, Schwartz JL, Pymar HC, Lai JJ, Rencher WF, Callahan MM. Single and multiple exposure tolerance study of polystyrene sulfonate gel: a phase I safety and colposcopy study. Contraception. 2004;70(1):77–83. doi: 10.1016/j.contraception.2004.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck CK, Weiner DH, Creinin MD, Barnhart KT, Callahan MM, Bax R. A randomized Phase I vaginal safety study of three concentrations of C31G vs. Extra Strength Gynol II. Contraception. 2004;70(3):233–40. doi: 10.1016/j.contraception.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Karim SA, Kelly C, Maslankowski L, Rees H, Profy AT, Day J, Welch J, Rosenberg Z. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. Aids. 2003;17(3):321–9. doi: 10.1097/00002030-200302140-00005. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, Masse B, Eshleman SH, Hendrix C, Morrow K, Rooney JF, Soto-Torres L. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. Aids. 2006;20(4):543–51. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Peipert J, Fleming T, Fullem A, Moench T, Cu-Uvin S, Bentley M, Chesney M, Rosenberg Z. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin Infect Dis. 2001;32(3):476–82. doi: 10.1086/318496. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d’Arminio Monforte A, Yust I, Bruun JN, Phillips AN, Lundgren JD. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352(9142):1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- Mori T, O’Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–53. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Morner A, Bjorndal A, Albert J, Kewalramani VN, Littman DR, Inoue R, Thorstensson R, Fenyo EM, Bjorling E. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol. 1999;73(3):2343–9. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow K, Rosen R, Richter L, Emans A, Forbes A, Day J, Morar N, Maslankowski L, Profy AT, Kelly C, Abdool Karim SS, Mayer KH. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J Womens Health (Larchmt) 2003;12(7):655–66. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- Munch J, Standker L, Adermann K, Schulz A, Schindler M, Chinnadurai R, Pohlmann S, Chaipan C, Biet T, Peters T, Meyer B, Wilhelm D, Lu H, Jing W, Jiang S, Forssmann WG, Kirchhoff F. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell. 2007;129(2):263–75. doi: 10.1016/j.cell.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Munk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses. 2003;19(10):875–81. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- Musyanovych A, Schmitz-Wienke J, Mailander V, Walther P, Landfester K. Preparation of biodegradable polymer nanoparticles by miniemulsion technique and their cell interactions. Macromol Biosci. 2008;8(2):127–39. doi: 10.1002/mabi.200700241. [DOI] [PubMed] [Google Scholar]
- Novak A, de la Loge C, Abetz L, van der Meulen EA. The combined contraceptive vaginal ring, NuvaRing: an international study of user acceptability. Contraception. 2003;67(3):187–94. doi: 10.1016/s0010-7824(02)00514-0. [DOI] [PubMed] [Google Scholar]
- Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P, Sharp PA. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8(7):681–6. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- Oddsson K. NuvaRing contraception and combined oral contraception were equally efficacious and tolerable. Evidence-based Ostetrics and Gynocology. 2006;8:26–27. [Google Scholar]
- Oh JE, Nam YS, Lee KH, Park TG. Conjugation of drug to poly(D,L-lactic-co-glycolic acid) for controlled release from biodegradable microspheres. J Control Release. 1999;57(3):269–80. doi: 10.1016/s0168-3659(98)00123-0. [DOI] [PubMed] [Google Scholar]
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. Journal of Andrology. 2005;26(4):459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439(7072):89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- Panyam J, Sahoo SK, Prabha S, Bargar T, Labhasetwar V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly(D,L-lactide-co-glycolide) nanoparticles. Int J Pharm. 2003;262(1–2):1–11. doi: 10.1016/s0378-5173(03)00295-3. [DOI] [PubMed] [Google Scholar]
- Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16(15):1123–30. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196(9):1394–402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski MA, Garcia P. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153(2):481–90. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Sweeney YT, Balkus JE, Rohan LC, Moncla BJ, Parniak MA, Hillier SL. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51(5):1608–15. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Sweeney YT, Paul KJ. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009;36(6):350–6. doi: 10.1097/OLQ.0b013e318195c31a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce-Pratt R, Phillips DM. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol Reprod. 1996;54(1):173–82. doi: 10.1095/biolreprod54.1.173. [DOI] [PubMed] [Google Scholar]
- Penco M, Marcioni S, Ferruti P, DAS, Deghenghi R. Degradation behaviour of block copolymers containing poly(lactic-glycolic acid) and poly(ethylene glycol) segments. Biomaterials. 1996;17(16):1583–90. doi: 10.1016/0142-9612(95)00323-1. [DOI] [PubMed] [Google Scholar]
- Peng T, Cheng SX, Zhuo RX. Synthesis and characterization of poly-alpha, beta-[N-(2-hydroxyethyl)-L-aspartamide]-g-poly(L-lactide) biodegradable copolymers as drug carriers. J Biomed Mater Res A. 2006;76(1):163–73. doi: 10.1002/jbm.a.30550. [DOI] [PubMed] [Google Scholar]
- Pettit DK, Lawter JR, Huang WJ, Pankey SC, Nightlinger NS, Lynch DH, Schuh JA, Morrissey PJ, Gombotz WR. Characterization of poly(glycolide-co-D,L-lactide)/poly(D,L-lactide) microspheres for controlled release of GM-CSF. Pharm Res. 1997;14(10):1422–30. doi: 10.1023/a:1012176823155. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, THC, Eron JJ, Jr, Vernazza PL, LSY, Stewart PW, Goh LE, Cohen MS Q. Study and D.-U.-E. A. H. Consortium. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- Poynten IM, I, Millwood Y, Falster MO, Law MG, Andresen DN, Van Damme L, Kaldor JM. The safety of candidate vaginal microbicides since nonoxynol-9: a systematic review of published studies. Aids. 2009;23(10):1245–54. doi: 10.1097/QAD.0b013e32832b4271. [DOI] [PubMed] [Google Scholar]
- Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- Richman DD. HIV therapeutics. Science. 1996;272(5270):1886–8. doi: 10.1126/science.272.5270.1886. [DOI] [PubMed] [Google Scholar]
- Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339(8):504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. Aaps J. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, Temmerman M, Verstraelen H, Van Bortel L, Weyers S, Mitchnick M. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25(5):483–8. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Mallikarjuana NN, Manohar S, Liang HF, Kulkarni AR, Sung HW, Sairam M, Aminabhavi TM. Targeted nanoparticles for drug delivery through the blood-brain barrier for Alzheimer’s disease. J Control Release. 2005;108(2–3):193–214. doi: 10.1016/j.jconrel.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE, Hoffman S, Gai F, Maslankowski L, El-Sadr WM, Mayer KH. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008;17(3):383–92. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- Roumen FJ, Apter D, Mulders TM, Dieben TO. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod. 2001;16(3):469–75. doi: 10.1093/humrep/16.3.469. [DOI] [PubMed] [Google Scholar]
- Sarkar NN. The Combined Contraception Vaginal Device (NuvaRing): A Comprehensive Review. Contraception and Reproductive Health Care. 2005;10(2):73–78. doi: 10.1080/13625180500131683. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Kovalevsky G, Lai JJ, Ballagh SA, McCormick T, Douville K, Mauck CK, Callahan MM. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35(4):414–9. doi: 10.1097/OLQ.0b013e318162c4d8. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Mauck C, Lai JJ, Creinin MD, Brache V, Ballagh SA, Weiner DH, Hillier SL, Fichorova RN, Callahan M. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind Phase I safety study. Contraception. 2006;74(2):133–40. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9654):1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, Shankar P. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77(13):7174–81. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9(3):347–51. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102(4):823–34. doi: 10.1016/s0029-7844(03)00764-6. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285(5431):1261–5. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- Steiner M, Windchy A, Gould AR, Kushner GM, Weber R. Effects of chemotherapy in patients with dental implants. J Oral Implantol. 1995;21(2):142–7. [PubMed] [Google Scholar]
- Straub JA, Chickering DE, Lovely JC, Zhang H, Shah B, Waud WR, Bernstein H. Intravenous hydrophobic drug delivery: a porous particle formulation of paclitaxel (AI-850) Pharm Res. 2005;22(3):347–55. doi: 10.1007/s11095-004-1871-1. [DOI] [PubMed] [Google Scholar]
- Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, Adams DR, Bashirian S, Johnson J, Soriano V, Rendon A, Hudgens MG, Butera S, Janssen R, Paxton L, Greenberg AE, Folks TM. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194(7):904–11. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, Garcia JV. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204(4):705–14. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Pearce-Pratt R, Phillips DM. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67(11):6447–52. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Phillips DM. Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch Virol. 1996;141(7):1177–89. doi: 10.1007/BF01718823. [DOI] [PubMed] [Google Scholar]
- Tao W, Richards C, Hamer D. Enhancement of HIV infection by cellulose sulfate. AIDS Res Hum Retroviruses. 2008;24(7):925–9. doi: 10.1089/aid.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845–53. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- Trabattoni D, Lo Caputo S, Biasin M, Seminari E, Di Pietro M, Ravasi G, Mazzotta F, Maserati R, Clerici M. Modulation of human immunodeficiency virus (HIV)-specific immune response by using efavirenz, nelfinavir, and stavudine in a rescue therapy regimen for HIV-infected, drug-experienced patients. Clin Diagn Lab Immunol. 2002;9(5):1114–8. doi: 10.1128/CDLI.9.5.1114-1118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin JA. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin Investig Drugs. 2002;11(8):1077–97. doi: 10.1517/13543784.11.8.1077. [DOI] [PubMed] [Google Scholar]
- UNAIDS. UNAIDS Executive Summary 2004 Report on the Global AIDS Epidemic 2004 [Google Scholar]
- UNAIDS/WHO. AIDS Epidemic. 2007 December; 2007. [Google Scholar]
- Van Damme L. Clinical research with topical microbicides as a potential HIV prevention method. AIDS Read. 2000;10(9):552–4. [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- VanCompernolle SE, Taylor RJ, Oswald-Richter K, Jiang J, Youree BE, Bowie JH, Tyler MJ, Conlon JM, Wade D, Aiken C, Dermody TS, KewalRamani VN, Rollins-Smith LA, Unutmaz D. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J Virol. 2005;79(18):11598–606. doi: 10.1128/JVI.79.18.11598-11606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol. 2004;104(3):555–63. doi: 10.1097/01.AOG.0000136082.59644.13. [DOI] [PubMed] [Google Scholar]
- Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54(1):135–47. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79(Pt 4):731–40. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- Wang G, Watson KM, Buckheit RW., Jr Anti-HIV-1 Activity of Antimicrobial Peptides Derived from Human and Bovine Cathelicidins. Antimicrob Agents Chemother. 2008 doi: 10.1128/AAC.00452-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170(9):4708–16. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- Wang W, Owen SM, Rudolph DL, Cole AM, Hong T, Waring AJ, Lal RB, Lehrer RI. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173(1):515–20. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- Watson KM, Buckheit CE, Buckheit RW., Jr Comparative evaluation of virus transmission inhibition by dual-acting pyrimidinedione microbicides using the microbicide transmission and sterilization assay. Antimicrob Agents Chemother. 2008;52(8):2787–96. doi: 10.1128/AAC.01657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C, Zimmerman C. Violence against women: global scope and magnitude. Lancet. 2002;359(9313):1232–7. doi: 10.1016/S0140-6736(02)08221-1. [DOI] [PubMed] [Google Scholar]
- Weeks MR, Mosack KE, Abbott M, Sylla LN, Valdes B, Prince M. Microbicide acceptability among high-risk urban U.S. women: experiences and perceptions of sexually transmitted HIV prevention. Sex Transm Dis. 2004;31(11):682–90. doi: 10.1097/01.olq.0000143113.04524.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westedt U, Kalinowski M, Wittmar M, Merdan T, Unger F, Fuchs J, Schaller S, Bakowsky U, Kissel T. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Control Release. 2007;119(1):41–51. doi: 10.1016/j.jconrel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Woodsong C. Covert use of topical microbicides: implications for acceptability and use. Int Fam Plan Perspect. 2004;30(2):94–8. doi: 10.1363/3009404. [DOI] [PubMed] [Google Scholar]
- Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325(1–2):82–9. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- www.microbicide.org Volume, DOI:
- Xie J, Wang CH. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor cone-jet mode. Biotechnol Bioeng. 2007;97(5):1278–90. doi: 10.1002/bit.21334. [DOI] [PubMed] [Google Scholar]
- Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19(3):187–94. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, Fischl MA, Gatell JM, Gazzard BG, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Schooley RT, Thompson MA, Vella S, Volberding PA. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. Jama. 2004;292(2):251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- Youle M, Wainberg MA. Pre-exposure chemoprophylaxis (PREP) as an HIV prevention strategy. J Int Assoc Physicians AIDS Care (Chic Ill) 2003;2(3):102–5. doi: 10.1177/154510970300200302. [DOI] [PubMed] [Google Scholar]
- Zaneveld LJ, Waller DP, Anderson RA, Chany C, 2nd, Rencher WF, Feathergill K, Diao XH, Doncel GF, Herold B, Cooper M. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate) Biol Reprod. 2002;66(4):886–94. doi: 10.1095/biolreprod66.4.886. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286(5443):1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]