Abstract
Transferrin (Tf)-based recombinant fusion protein approach was investigated to achieve oral delivery for human growth hormone (hGH). Plasmid constructs expressing the fusion proteins were established by fusing coding sequences of both hGH and Tf in frame. Fusion proteins were produced in serum free media by transient transfection of human embryonic kidney HEK293 cells. The SDS-PAGE analysis of conditioned media showed that fusion proteins expressed at high purity with a 100 kDa molecular weight; the Western blot analysis with anti-hGH and anti-Tf antibodies verified the identity of fusion proteins. The Nb2 cell proliferation and Caco-2 cell Tf receptor (TfR) binding assays demonstrated that fusion proteins retained bioactivity of both hGH and Tf, respectively. A helical linker was inserted as spacer between hGH- and Tf-domain to enhance the bioactivity and the yield of the fusion protein. Two fusion proteins, hGH-Tf (GT) and hGH-(H4)2-Tf (GHT) were obtained and assessed in hGH-deficient hypophysectomized rats for in vivo biological activity. Results from seven-day subcutaneous dosing (1.25 mg/kg/day) demonstrated that both GT and GHT fusion proteins were bioactive in vivo, comparable to native hGH. However, only the GHT, but not GT, fusion protein promoted a modest but statistically significant weight gain after oral dosing with 12.5 mg/kg/day.
Keywords: fusion protein, human growth hormone, transferrin, oral delivery, therapeutic protein
1. Introduction
Protein drug delivery is mainly limited to invasive needle injection, which is associated with pain, inconvenience, noncompliance and reduced-accessibility to children and elderly patients. As an alternative to needle injection, the pulmonary, nasal and trans-dermal routes have been explored for protein drug delivery with limited success including poor acceptance by patients and the medical community [1–3]. Although protein drugs with oral administration are highly desirable [4], development of oral dosage forms faces significant challenges including fragile structure with large molecular size, poor lipid solubility and reduced stability, leading to poor uptake across epithelial cells lining the gastrointestinal tract (GIT). To overcome poor oral absorption of protein drugs, different strategies including enteric coating, penetration enhancers and protease inhibitors have been attempted as part of formulations [5–7]. However, the safety and efficacy of such strategies are not well characterized for long-term applications [8].
Therefore, we sought to use a natural, physiological process to investigate the feasibility of developing a protein drug for oral delivery. This approach exploits the transferrin receptor (TfR) mediated transcytosis mechanism coupled with a transferrin-based recombinant fusion protein to achieve the oral delivery of a protein drug.
Transferrin (Tf) is an endogenous serum protein that transports iron to cells expressing the TfR on the plasma membrane through TfR-mediated endocytosis [9–11]. Tf has been applied as a vehicle to deliver small molecule drugs, peptides, proteins, and genes to the target tissues including blood brain barriers that abundantly express TfRs [12–16]. Tf is resistant to enzymatic digestion resulting from both trypsin and chymotrypsin enzymes [17], and TfRs express at high numbers in the human GI epithelial cells [18], a major factor in Tf-TfR mediated transcytosis. For example, the chemical conjugates of Tf and protein drugs such as granulocyte colony stimulating factor (G-CSF) and insulin show that both Tf and its conjugates are not only transported through epithelial cells, but also demonstrated in vitro as well as in vivo bioactivities in cell cultures and animal models, respectively [19, 20]. However, the high manufacturing cost, product heterogeneity and low reproducibility associated with the chemical approach generally limit its application as a viable choice as oral therapeutics [4].
Human growth hormone (hGH) is an important regulator of metabolism and stimulates growth and differentiation of target tissues such as muscle, bone, cartilage and liver [21–23]. hGH deficiency is associated with several clinical indications including short stature, Turner syndrome, chronic kidney disease, HIV-associated wasting and abnormal metabolism [24, 25]. If left untreated, these indications pose significant health risks to the society. The current treatment regimen for patients with growth hormone deficiency is limited to needle injection of recombinant hGH several times a week [26]. This type of treatment is not favorable to patients including children and senior patients who need hGH treatment for an extended period of time, thereby suggesting that there is a pressing need for oral hGH, preferably with a prolonged plasma half-life.
Recombinant fusion protein approach has been used in the biopharmaceutical industry as a means to improve both plasma half-life and targeting of a protein drug with poor pharmacokinetics and efficacy [27–29]. Albumin-Interferon-2αb and Fc-TNFα fusion proteins are examples demonstrating the acceptance of this approach [30]. Recently, our lab produced GCSF-Tf recombinant fusion protein in mammalian cells, which demonstrated bioactivity when given orally in BDF1 mice [31]. To extend and further validate the application of Tf-based recombinant fusion protein approach for oral delivery, we investigated the feasibility of producing bioactive human growth hormone-transferrin fusion protein in mammalian embryonic kidney cells (HEK293T), focusing on oral absorption.
2. Materials and Methods
2.1. Cloning in pCR-Blunt II-TOPO vector
The DNA sequence coding for hGH [32] was amplified in a PCR reaction containing cDNA from human pituitary gland (Clontech, Mountain View, CA), platinum pfx DNA polymerase (Invitrogen, Carlsbad, CA), and hGH-specific primers with EcoRV and XhoI sites (USC Norris Cancer Center). In addition, the amplification primers incorporated both the Kozak and signal sequences upstream to coding sequences. The PCR amplified hGH fragment (668 bp) was ligated to pCR-Blunt II-TOPO vector (Invitrogen) and subsequently transformed to chemically competent TOP10 cells (Invitrogen). Plasmids prepared from overnight E. coli cultures were analyzed by restriction enzymes, and selected plasmids were further confirmed by DNA sequencing (USC Norris Cancer Center).
Likewise, the DNA sequence [33] coding for Tf was amplified from TFR27 plasmid (ATCC, Manassas, VA) using platinum pfx DNA polymerase with proofreading and Tf-specific primers incorporating the XhoI and XbaI restriction enzyme sites and stop codon. The resulting Tf-fragment (2052 bp) was then cloned into pCR-Blunt II-TOPO vector, and subsequently transformed to chemically competent TOP10 cells. The plasmids prepared from overnight E. coli culture were analyzed with restriction enzymes and DNA sequencing.
2.2. Fusion constructs in mammalian expression vector
2.2.1. hGH-Tf expression construct
The pCR-Blunt II-TOPO plasmid harboring the Tf fragment was double digested using XhoI and XbaI restriction enzymes to release the Tf coding sequences with cohesive ends, followed by gel extraction and purification (Invitrogen). Similarly, the pcDNA3.1(+) expression vector was digested using the same restriction enzymes (XhoI and XbaI), dephosphorylated with calf intestinal phosphatase (Invitrogen), and prepared by gel extraction and purification. Subsequently, the gel-purified Tf fragment was ligated to gel purified pcDNA3.1(+), and transformed into chemically competent TOP10 cells. The plasmid mini-preps from the overnight culture of E. coli were analyzed with restriction enzymes to identify positive colonies with the correct insert, and the selected plasmids were analyzed by DNA sequencing.
Next, the hGH coding region was released from the pCR-Blunt II-TOPO plasmid by EcoRV and XhoI digestion, gel purified, and fused upstream to Tf coding region in pcDNA3.1(+) plasmid with EcoRV and XhoI cohesive ends. The resulting plasmid construct, hGH-Tf, was transformed into chemically competent TOP10 cells, and the selected plasmids isolated from overnight cultures were analyzed for positive colonies with the correct sequences.
2.2.2. Inserting a helical linker between hGH- and Tf-moiety
Two copies of a helical linker, (H4)2, LEA(EAAAK)4ALEA(EAAAK)4ALE, were inserted according to a previous study [34]. The orientation, sequences and copy numbers of the helical linker were confirmed by DNA sequence analysis.
2.2.3. hGH expression construct
hGH coding region containing both the signal sequences and stop codon was prepared from cloning vector pCR-Blunt II-TOPO by EcoRV and XhoI digestion. The resulting fragment was ligated to the expression vector pcDNA3.1(+) with same cohesive ends and subsequently transformed into competent TOP10 cells. Selected plasmids were analyzed using both restriction enzymes and DNA sequencing to confirm its sequences and orientation.
2.3. Transient transfection
The human embryonic kidney 293 cells (HEK293T) (ATCC) were cultured in DMEM media (Mediatech, Manassas, VA) containing 10% FBS, 50 units penicillin/50 µg streptomycin in a humidified incubator at 37 °C with 5% CO2. HEK293T cells growing in log phase were seeded in 6-well plates (Corning Costar, Wilkes Barre, PA) with ~ 0.8 million cells per well, one day before transfection. Briefly, 2 µg expression construct and 5.5 µl Lipofectamine 2000 (Invitrogen) were diluted in 250 µl Opti-Mem media (Invitrogen), mixed and incubated for 15 minutes at room temperature to allow DNA-lipid complexes to form. The DNA-lipid complexes were carefully added to cells in 6-well plates, which were incubated for 5 hrs to internalize the plasmid, and then the Opti-Mem media was replaced with serum free CD293 media (Invitrogen). The transfected cells were allowed to express the fusion protein during a 5-day incubation in CD293 media.
2.4. SDS-PAGE
The conditioned media from 5-day post-transfection was harvested, clarified by centrifugation for 15 minutes at 4 °C, and concentrated using Amicon Ultra-4 or Ultra-15 filtering devices (Millipore, Billerica, MA) by centrifugation at 4 °C. Small aliquots of fusion protein were boiled for 5 minutes in sample loading buffer, fractionated on a 10% SDS-PAGE or precast gel (Thermo Scientific, Waltham, MA), and stained with 0.1% Coommassie blue in 10% acidic acid and 40% methanol for 1 hour. Protein bands were captured and analyzed using ChemiDoc XBR (Bio-Rad, Hercules, CA) and Quantity One software (Bio-Rad), and the quantity of fusion protein was estimated using SDS-PAGE or Western blot with known transferrin-standards.
2.5. Western blot
After separation using SDS-PAGE, samples were transferred to a PVDF membrane (GE Healthcare, Piscataway, NJ) using wet transfer, and the membrane was blocked with 5% non-fat milk for 1 hr at room temperature and probed with goat anti-hGH monoclonal antibody (1:1000) (R&D Systems, Minneapolis, MN) or goat anti-human Tf antibody (1:10000) (Sigma, St. Louis, MO). The signal was detected by using rabbit anti-goat secondary antibody conjugated to HRP (Sigma) and ECL plus reagents (GE Healthcare), captured using ChemiDoc XBR and quantified with Quantity One software.
2.6. Characterizing fusion proteins for in vitro bioactivity
2.6.1. TfR binding
Tf was labeled with radioactive iodine (ICN, Irvine, CA) using the chloramine-T catalyzed iodination reaction to obtain iodine-labeled Tf (125I-Tf), which was purified using Sephadex-G50 column chromatography (Amersham Biosciences, Piscataway, NJ). CaCo-2 cells (ATCC) cultured in DMEM supplemented with 20% FBS, 50 units penicillin/50 µg streptomycin in a humidified incubator at 37 °C with 5% CO2, were seeded in 12-well plates, allowed to grow as a monolayer and used for TfR binding assay as described previously [31].
2.6.2. Nb2 cell proliferation
Nb2 cells (Sigma) derived from rat T lymphoma cells were cultured in suspension in RPMI 1640 (Mediatech) media supplemented with 2 mM glutamine, 10% FBS, 10% horse serum (HS) (Invitrogen), 50 units of penicillin/50 µg streptomycin, and 50 µM 2-mercaptoethanol [35] in a humidified incubator at 37 °C with 5% CO2. For proliferation assays, Nb2 cells growing at log-phase were washed extensively in serum free RPMI 1640 media, re-suspended in assay media, which contained 10% HS (without FBS), and counted using Z1 Coulter particle counter (Beckman Coulter, Fullerton, CA). 5000–7000 Nb2 cells were seeded per well in 96-well plates in 200 µl assay media and starved for 24 h, followed by adding varying doses of hGH and fusion protein in 10 µl and 50 µl assay media, respectively. The dose used for the fusion protein was normalized to that of hGH, due to the differences in molecular weight of the fusion protein (100 kDa) and hGH (22 kDa). After four-day incubation, cells were incubated overnight with 20 µl of Alamar Blue dye (Biosource, Hopkinton, MA); the absorbance was measured at 570 nm using a Genios microplate reader (Tecan, San Jose, CA) and normalized against the non-treatment control.
2.7. Subcutaneous and oral administration in hypophysectomized rats
Hypophysectomized female Sprague Dawley rats were obtained from Taconic (Germantown, NY). Animal experiments were compliant with the Principles of Laboratory Animal Care (NIH Publication No. 85-23) and approved by the IACUC at USC. Hypophysectomized rats, housed two per cage in a temperature and light controlled room with free access to food and water, were allowed to adapt to the new condition. The weight of the rats was monitored and the base-line weight for each rat was established over 7-day period of pre-dose screening. Subsequently, the rats without capacity of gaining weight were selected for the in vivo experiments and randomly assigned to different groups, each with five rats. Rats were fasted for 4 hrs (10 a.m. – 2 p.m.), weighed, and then administered orally or subcutaneously with the following doses: 12.5 mg/kg fusion protein or 2.5 mg/kg hGH in a 0.5 ml volume for oral administration using a gavage needle, and 1.25 mg/kg fusion protein or 0.25 mg/kg hGH in a 0.2 ml volume for subcutaneous administration. The treatment was repeated for 7 consecutive days. The dose used for fusion protein was normalized to that of hGH to ensure that the hGH moiety in fusion protein is equal in molar to the native hGH. The oral dose was given in a formulation consisting of Bowman-Birk protease inhibitor (1.7 mg/ml), sodium bicarbonate (10 mg/ml), mannitol (88 mM) and phosphate (5 mM). The subcutaneous dose was administered in phosphate buffered saline to maintain isotonicity, while the mannitol-phosphate buffer (pH 7.3) was used as a vehicle control for both subcutaneous and oral administration. The 7-day post-dose weight was established by subtracting the weight of 1st day of dosing from that of one day after 7th day dosing; similarly, the base-line (pre-dose) weight was established by subtracting the 1st day of pre-screening weight from that of final day of pre-dosing. The mean of pre-and post-dose weight gain in each group was plotted as bar graph.
2.8. Statistical analysis
The unpaired two-tailed t-test (GraphPad Prism, GraphPad Software, Inc., San Diego, CA) was used to evaluate the difference between base-line weight gain against the 7-day oral dosing for hGH and its fusion proteins. The difference in the effect on weight gain was considered significant, if p <0.01.
3. Results
3.1. Expression constructs and fusion protein expression
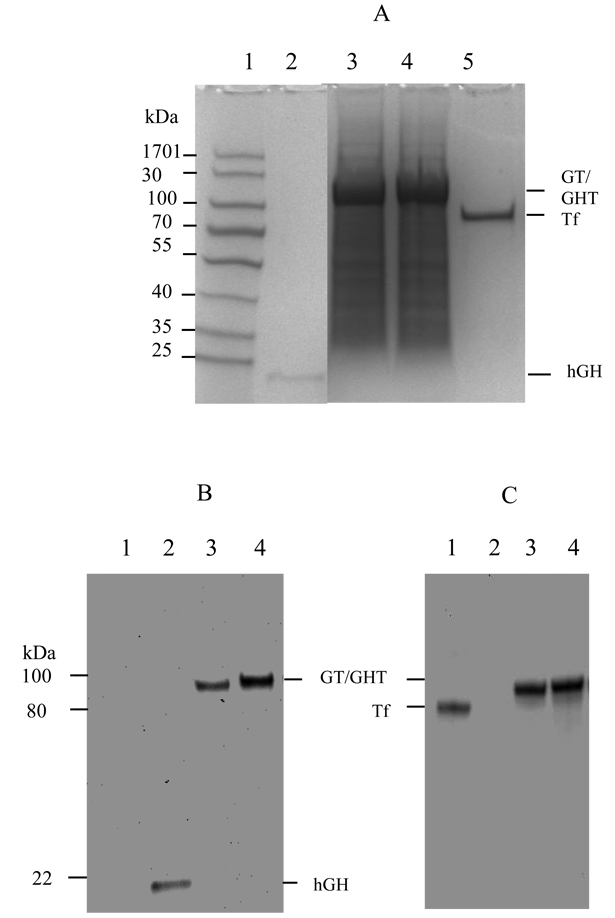
The mammalian vector pcDNA3.1(+) was used to engineer the constructs for the expression of fusion protein and hGH, respectively. The data from restriction enzyme and DNA sequence analysis showed that both hGH and Tf were fused in frame without mutations, and connected by the helical linker. HEK293 cells, known to express high quality recombinant proteins, are selected as a host for the expression of fusion protein, including hGH-Tf (GT) and hGH-Tf with (H4)2 helical linker(GHT), and hGH. The SDS-PAGE analysis of serum free, conditioned media demonstrated that GT and GHT expressed as a single, dominant band with about 90% abundance and 100-kDa molecular weight (Fig.1A lanes 3 and 4, respectively), which is the sum of hGH (22 kDa) and Tf (79 kDa). SDS-PAGE also showed that the hGH expressed at a predicted molecular weight of 22 kDa (Fig.1A lane 2). As previously reported [36], the production of GHT is significantly higher than that of GT (Fig.1).
Fig. 1.
Expression and confirmation of GT and GHT fusion protein by SDS-PAGE (A) and Western blot (B and C) analysis. A. Samples from conditioned media were analyzed for fusion protein expression on a SDS-PAGE gel, stained with Coomassie blue. Lane 1: molecular weight marker; lane 2: hGH; lane 3: GT fusion protein; lane 4: GHT fusion protein; lane 5:Tf. B. Samples from conditioned media were analyzed by Western blot using anti-hGH (B) and anti-Tf antibody (C). Lane 1: Tf; lane 2: hGH; lane 3: GT fusion protein; lane 4: GHT fusion protein.
3.2. Identifying the fusion protein
To confirm identity of the fusion protein, the conditioned media was analyzed using Western blot and probed with both anti-Tf and anti-hGH antibodies, respectively. The Western blot data revealed that both antibodies including the anti hGH and anti-Tf were positive and detected the fusion protein with 100 kDa molecular weight (Fig.1B and 1C, lanes 3 and 4).
3.3. In vitro biological activity
3.3.1. TfR binding
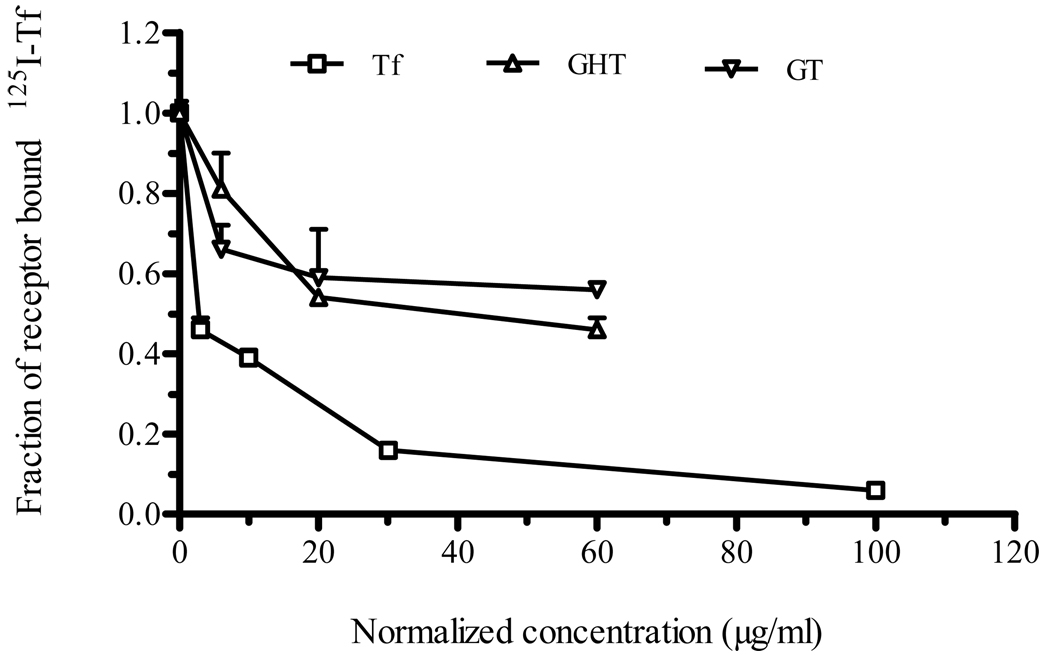
To evaluate the biological function of the Tf-domain, the fusion protein was assessed for its ability to bind TfR in CaCo-2 cells. To this end, TfR binding competition assay was performed in the presence of 5 µg/ml radioactively labeled-Tf (125I-Tf). The addition of a 12-fold excess of GT and GHT fusion protein (60 µg/ml) resulted in approximately 50% and 60% reduction of surface bound 125I-Tf, respectively (Fig. 2). When added at relatively low dose (6 µg/ml), the fusion protein still decreased the surface bound 125I-Tf (Fig. 2). The reduction of surface bound 125I-Tf as a result of competition binding by the fusion protein suggested that the fusion protein retained the receptor-binding activity of Tf.
Fig. 2.
Fusion protein demonstrated TfR binding activity. Both GT and GHT fusion proteins were assessed for Tf bioactivity using a TfR competition binding assay in which different doses of GT or GHT fusion protein was added to CaCo-2 cells in the presence of radioactively labeled-Tf (125I-Tf). Data were collected as triplicates and represent one of several independent experiments; each data point is mean ± SEM (n = 3).
3.3.2. Nb2 cell proliferation
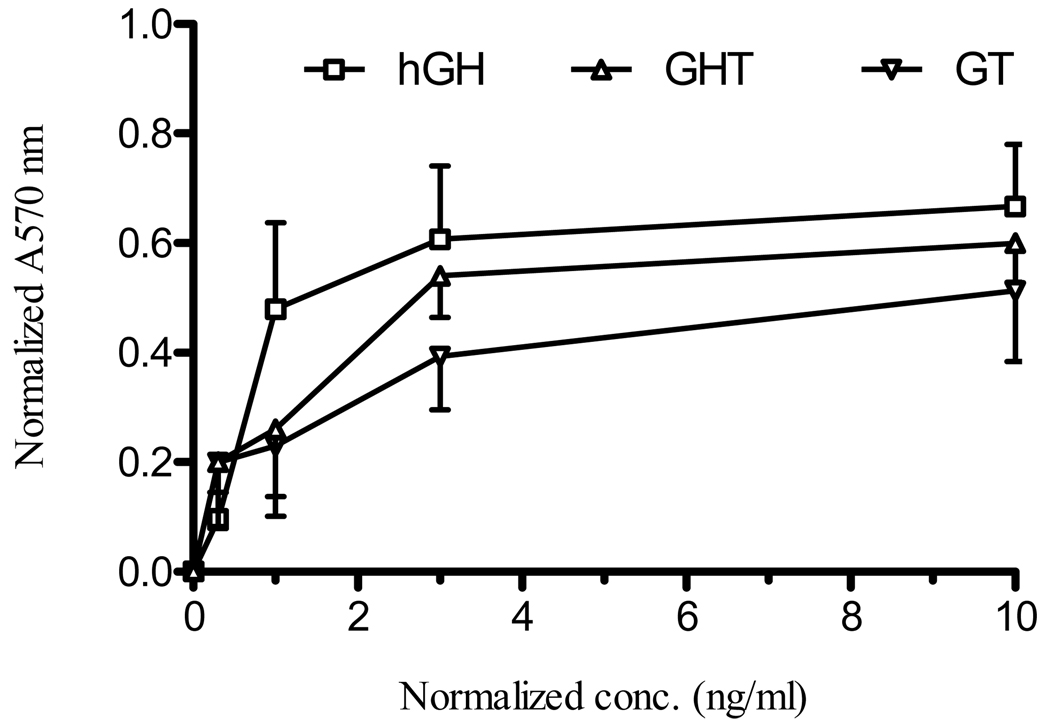
Nb2 cells are known to respond to the exogenous hGH stimulation by proliferation in a dose-dependent manner when cultured in assay media containing 10% horse serum without FBS [35]. The Nb2 cells starved in assay media were treated with different doses of fusion protein to evaluate whether the fusion protein triggers hGH-dependent Nb2 cell proliferation. The normalized absorbance values from the proliferation assay showed that both fusion proteins resulted in a dose-dependent proliferation of Nb2 cells with an ED50 value of approximately 2 ng/ml, which is higher than the ED50 of native hGH (~ 1 ng/ml) (Fig. 3). In contrast, the untreated cells did not proliferate under the same condition. Therefore, the dose-dependent proliferation of Nb2 cells indicated that the fusion proteins preserved the biological activity of the hGH-domain.
Fig. 3.
Stimulation with fusion protein triggered Nb2 cell proliferation. To further assess GT and GHT fusion proteins for hGH bioactivity, Nb2 cells were starved for 24 hr in assay media and treated with different doses of hGH, GT or GHT fusion protein. After four day incubation, Nb2 cells were stained with alamar-blue dye overnight and UV absorbance was measured at 570 nm. The absorbance values were then normalized to that of un-treated control. Data represent mean ± SEM (n = 3).
3.4. Subcutaneous administration of hGH, GT and GHT fusion proteins
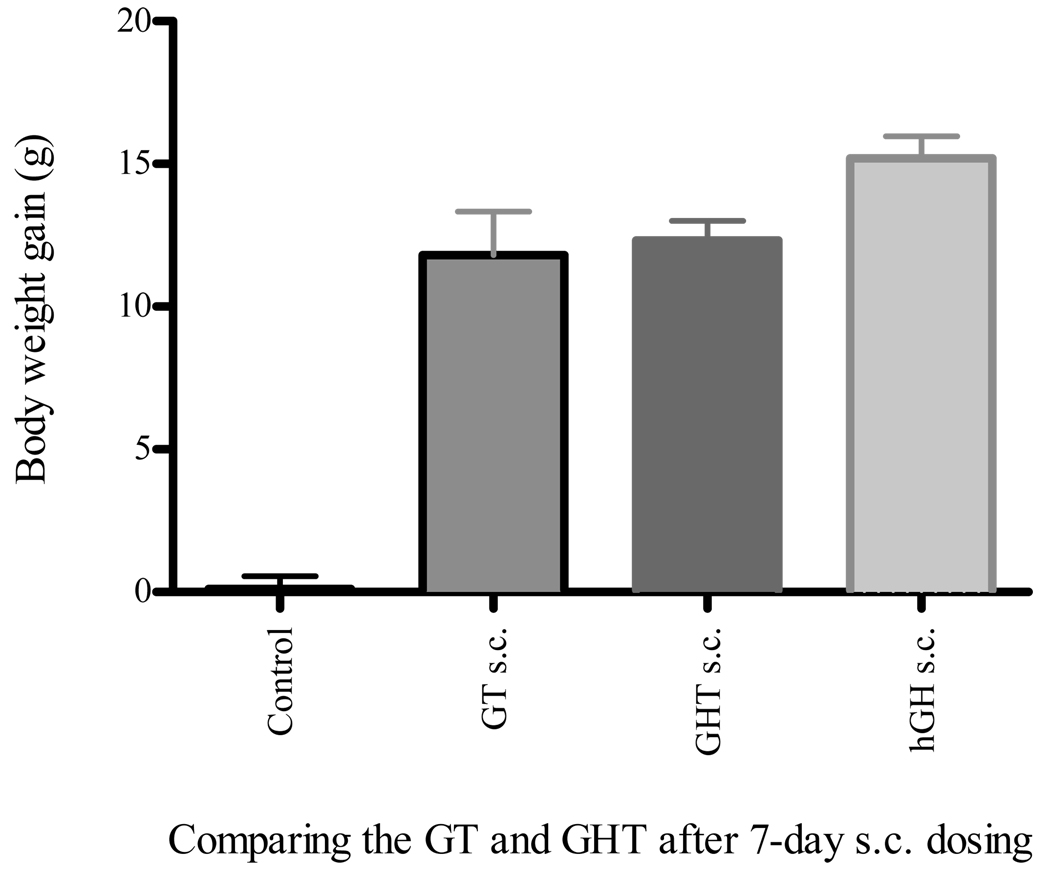
To evaluate intrinsic bioactivity of the GT and GHT fusion proteins, the rats were administered with the above fusion proteins, along with the vehicle control and hGH. Results from the 7-daily subcutaneous injections with 1.25 mg/kg dose showed that both the GT and GHT were biologically active in vivo and promoted 11.8 g and 12.3 g of body weight, respectively (Fig. 4). This effect on body weight gain was smaller than the hGH, which led to a 15.2 g of body weight gain with the same dose (Fig. 4). Conversely, the hypophysectomized rats injected with the vehicle control were unable to gain weight (Fig. 4). The subcutaneous injection of these fusion proteins therefore resulted in a strong growth response by promoting weight gain in hypophysectomized rats.
Fig. 4.
Both GT and GHT fusion proteins showed intrinsic in vivo bioactivity after subcutaneous dosing. The hypophysectomized rats that did not gain body weight during the pre-screening period were randomized to four groups and evaluated for intrinsic bioactivity. The fasted rats were then subcutaneously administered (s.c.) with vehicle control, hGH (0.25 mg/kg), GT (1.25 mg/kg), or GHT (1.25 mg/kg), for 7-consecutive days, respectively. The weight gain was determined by subtracting the weight on the day of dosing (day 1) from that of last day of dosing (day 8). Data represent mean of body weight gain ± SEM (n = 5, except GHT s.c. where n = 4).
3.5. Oral administration of GHT fusion protein
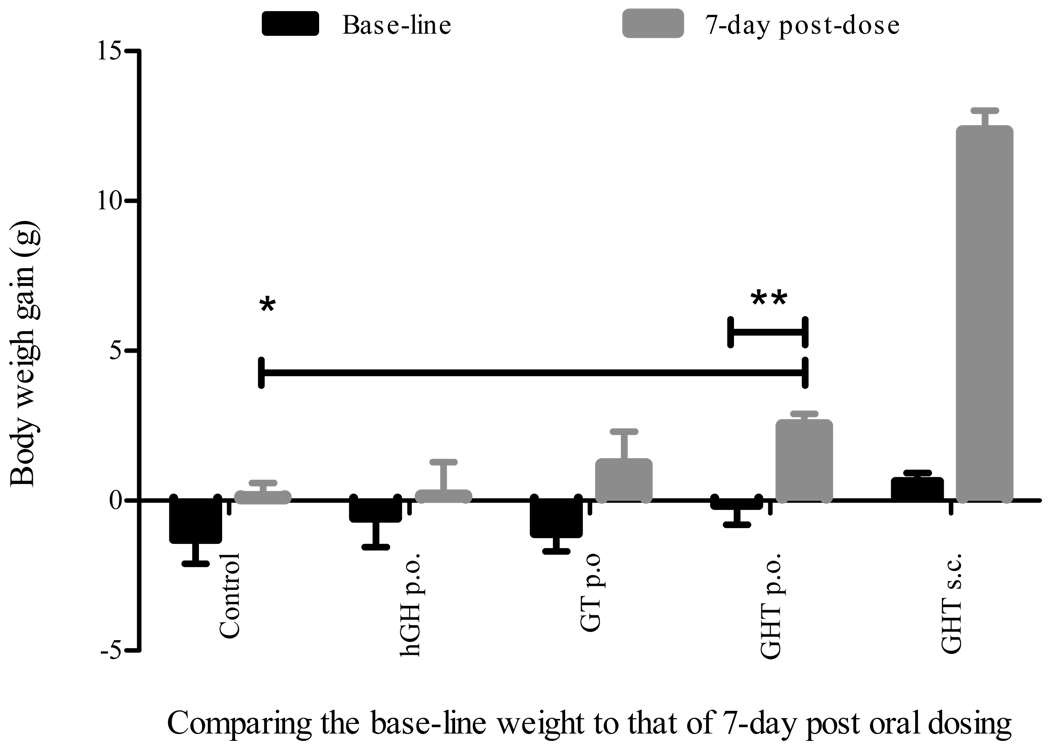
When subcutaneously administered, both the GT and GHT fusion protein exhibited intrinsic bioactivity by increasing the body weight of hypophysectomized rats, and subsequently, these fusion proteins were evaluated for oral bioactivity. To demonstrate oral hGH activity of the GT and GHT fusion protein, hypophysectomized rats were orally dosed with 12.5 mg/kg of GT, GHT fusion protein, or 2.5 mg/kg hGH for 7-consecutive days, along with the vehicle and subcutaneously administered GHT controls, respectively. The change in body weight after 7 day dosing was analyzed as compared to that of 7-day base-line weight. Results from 7-daily treatments demonstrated that the oral administration of GHT fusion protein led to a statistically significant 2.5 g of body weight gain (p < 0.01) in hypophysectomized rats that showed no change of base-line weight during the 7-day pre-dose monitoring (Fig. 5). Furthermore, the body weight in the group that received oral administration of GHT fusion protein was significantly higher than that in the control group (p < 0.01) (Fig. 5). Conversely, the hypophysectomized rats dosed with either hGH or GT fusion protein orally did not gain weight compared to the base-line weight (p > 0.1) (Fig. 5). Taken together, the GHT fusion protein showed modest but statistically significant weight gain with oral administration and strong growth promotion with subcutaneous administration.
Fig. 5.
Oral administration of the GHT fusion protein led to body weight gain. Body weight of the hypophysectomized rats were monitored for 7-days and the base-line weight were established for five experimental groups. The fasted rats were orally administered (p.o.) with GHT fusion protein (12.5 mg/kg), GT fusion protein (12.5 mg/kg), hGH (2.5 mg/kg), and vehicle control for 7-consecutive days, respectively, and one group of rats were given GHT fusion protein s.c. as a positive control. The body weight gain was determined by subtracting the weight on the day of dosing (day 1) from that of one day after last dosing (day 8), and then compared to that of base-line weight. Data represent mean of body weight gain ± SEM (n = 5, except GHT s.c. where n = 4 and vehicle control where n = 3). The two-tailed t-test showed that the differences of base line weight gain versus oral GHT weight gain and of the vehicle control versus oral GHT weight gain were both statistically significant (**p < 0.01, *p < 0.01). However, the difference between base line weight versus 7-day oral dosing with GT, hGH, or control was not significant (p > 0.1).
4. Discussion
The Tf activity of the fusion protein was determined using a TfR binding assay, because it can determine if the fusion protein has the capacity to bind to TfR. TfR binding of fusion protein is a crucial step in oral delivery that enables GI absorption via Tf-TfR mediated transcytosis. The fusion protein-dependent displacement of radioactively labeled-Tf from TfR on CaCo-2 cells indicated that fusion proteins recognize and bind to TfR (Fig. 2). This result was in line with the previous study in which G-CSF-Tf fusion protein maintained TfR binding capacity in a similar study [31]. The IC50 value for fusion protein is about 20-fold higher than the native Tf, suggesting that fusion protein binds to TfR with reduced affinity. One possible reason for this reduced binding is that the hGH located at the N-terminus or upstream of Tf could influence optimal receptor binding in negative fashion by introducing steric hindrance [9, 37]. However, this lower affinity binding to TfR by the fusion protein could be enough to elicit a biological effect in vivo.
After characterizing the fusion proteins for Tf bioactivity, we sought to determine whether the fusion protein possesses hGH bioactivity using hGH-dependent proliferation of rat Nb2 cells. The results from the Nb2 cell proliferation assay indicated both fusion proteins still maintain the activity of hGH (Fig. 3). These results were consistent with the findings of hGH and its fusion protein-dependent phosphorylation of JAK2 and STAT5 (unpublished result) in IM-9 cells that express hGH-receptor. The dose of fusion protein required to stimulate the activation of signaling molecules JAK2 and STAT5 was similar to the dose needed for effective proliferation of Nb2 cells. Thus these two assays were consistent and correctly assessed the fusion protein for hGH bioactivity.
Subcutaneous injection of GHT fusion protein in hypophysectomized rats demonstrated substantial growth promoting activity (Fig. 4 and 5). Even though the fusion protein had a higher in vitro EC50 compared to the native hGH (Fig. 3), the GHT fusion protein had similar cell proliferation effect at a higher dose (10 ng/ml). It is conceivable that Tf-moiety of the fusion protein could trap the hGH-moiety by binding TfRs expressed on the surface of Nb2 cells. However, high dose of fusion protein could enhance hGH receptor binding and subsequent cell proliferation activity by overcoming TfR binding. This slow but robust response profile of the GHT fusion protein in Nb2 cell proliferation could suggest that the selection of a suitable dose is critical factor in achieving in vivo bioactivity. The dose of the fusion protein we used in subcutaneous injection was 1.25 mg/kg, which appears to result in good bioavailability leading to a substantial growth promotion in hypophysectomized rats. The GT fusion protein with subcutaneous injection produced a similar growth promotion as compared to the GHT fusion protein with the same dose. These in vivo results seem to be supported well by the in vitro Nb2 cell proliferation that showed the GT fusion protein had slightly lower cell proliferation activity than the GHT fusion protein (Fig. 3). Although both fusion proteins displayed a good in vivo bioactivity with subcutaneous delivery, only the GHT fusion protein promoted weight gain with oral administration. This effect could be due to the enhanced intrinsic bioactivity of the GHT fusion protein by the insertion of a helical linker, a similar observation has been reported in Tf fusion proteins of G-CSF [34]. In deed, both Nb2 cell proliferation and oral administration indicated that the GHT fusion protein with helical linker showed better intrinsic activity than that of GT fusion protein without the helical linker (Fig. 3 and 4).
It was observed that some rats in the same experimental group gained weight differently. This variation following the oral dosing could be attributed to many factors including TfR expression, transcytosis efficiency, proteolytic enzyme concentration, fasting and eating habits, and gastric empting behavior of individual rats [6, 7, 38–40]. Prolonged stability and effective transcytosis of the fusion protein after oral dosing is essential for increased absorption and subsequent oral bioactivity. Our unpublished data regarding the effect of trypsin and chymotrypsin on fusion protein stability suggested that even though the Tf domain in fusion protein is resistant to protease digestion, the hGH domain in both GT and GHT fusion proteins is susceptible to enzymatic degradation. Studies that address in vivo stability and pharmacokinetics of the fusion protein are necessary for the design of future in vivo experiments to achieve better oral bioavailability. In addition, formulations including enteric coating, protease inhibitors, microspheres and nanoparticles and hydrogels [2, 3, 8, 41–43] with controlled release at the absorption site should prove helpful in the effort of oral protein delivery with the acceptable bioavailability.
5. Conclusion
Two fusion proteins, i.e. GT and GHT, have been engineered, produced in mammalian cells, characterized, and validated for in vitro/in vivo bioactivities. Results from this study demonstrated the oral growth activity of the GHT fusion protein in an animal model of hGH response. Thus this project provides further evidence for the feasibility of using Tf-based fusion protein approaches to develop a potential oral dosage form for therapeutic proteins.
Acknowledgements
This work was supported in part by NIH Grant GM063647 and a contract from Pfizer, Inc. The authors would like to thank Daisy Shen for her excellent assistance in animal experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, Liu S, Zhang M, Wen LP. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 2.Bosquillon C, Préat V, Vanbever R. Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. J Control Release. 2004;96(2):233–244. doi: 10.1016/j.jconrel.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Hussain A, Arnold JJ, Khan MA, Ahsan F. Absorption enhancers in pulmonary protein delivery. J Control Release. 2004;94(1):15–24. doi: 10.1016/j.jconrel.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Shen WC. Oral peptide and protein delivery: unfulfilled promises? Drug Discov Today. 2003;8(14):607–608. doi: 10.1016/s1359-6446(03)02692-8. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NM. Oral delivery and recombinant production of peptide hormones. Part I: Making oral delivery possible. Biopharm Int. 2004;17(6):38–43. [Google Scholar]
- 6.Hamman JH, Enslin GM, Kotzé AF. Oral Delivery of Peptide Drugs: Barriers and Developments. BioDrugs. 2005;19(3):165. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kompella UB, Lee VHL. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 2001;46(1–3):211–245. doi: 10.1016/s0169-409x(00)00137-x. [DOI] [PubMed] [Google Scholar]
- 8.Bernkop-Schnürch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release. 1998;52(1–2):1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the Human Transferrin Receptor-Transferrin Complex. Cell. 2004;116(4):565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 10.Gomme PT, McCann KB, Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov Today. 2005;10(4):267–273. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 11.Richardson DR. Mysteries of the Transferrin-Transferrin Receptor 1 Interaction Uncovered. Cell. 2004;116(4):483–485. doi: 10.1016/s0092-8674(04)00165-5. [DOI] [PubMed] [Google Scholar]
- 12.Bickel U, Yoshikawa T, Landaw EM, Faull KF, Pardridge WM. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc Natl Acad Sci USA. 1993;90(7):2618–2622. doi: 10.1073/pnas.90.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22(3):225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 14.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1(2):131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge WM. Molecular Trojan horses for blood–brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Shin SU, Friden P, Moran M, Olson T, Kang YS, Pardridge WM, Morrison SL. Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci USA. 1995;92(7):2820. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azari PR, Feeney RE. Resistance of metal complexes of conalbumin and transferrin to proteolysis and to thermal denaturation. J Biol Chem. 1958;232(1):293–302. [PubMed] [Google Scholar]
- 18.Banerjee D, Flanagan PR, Cluett J, Valberg LS. Transferrin receptors in the human gastrointestinal tract. Relationship to body iron stores. Gastroenterol. 1986;91(4):861–869. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- 19.Widera A, Kim KJJ, Crandall ED, Shen WC. Transcytosis of GCSF-Transferrin Across Rat Alveolar Epithelial Cell Monolayers. Pharm Res. 2003;20(8):1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- 20.Xia CQ, Wang J, Shen WC. Hypoglycemic Effect of Insulin-Transferrin Conjugate in Streptozotocin-Induced Diabetic Rats. J Pharmacol Exp Ther. 2000;295(2):594. [PubMed] [Google Scholar]
- 21.Ankersen M, Hansen TK, Ahnfelt-Rønne I, Kappelgaard AM. Growth hormone secretagogues: recent advances and applications. Drug Discov Today. 1999;4(11):497–506. doi: 10.1016/s1359-6446(99)01415-4. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MJ. The molecular basis of growth hormone action. Growth Horm IGF Res. 1998;8(1):3–11. doi: 10.1016/s1096-6374(98)80316-x. [DOI] [PubMed] [Google Scholar]
- 23.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol. 2006;36(1):1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- 24.Staub JM, Garcia B, Graves J, Hajdukiewicz PTJ, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L, Ward D, Ye GN, Russell DA. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol. 2000;18(3):333–338. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, Kaji H, Okimura Y, Goji K, Abe H, Chihara K. Short Stature Caused by a Mutant Growth Hormone. N Engl J Med. 1996;334(7):432. doi: 10.1056/NEJM199602153340704. [DOI] [PubMed] [Google Scholar]
- 26.Walsh G. Biopharmaceutical benchmarks 2006. Nat Biotechnol. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 27.Duttaroy A, Kanakaraj P, Osborn BL, Schneider H, Pickeral OK, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54(1):251–258. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 28.Halpern W, Riccobene TA, Agostini H, Baker K, Stolow D, Gu ML, Hirsch J, Mahoney A, Carrell J, Boyd E, Albugranin TM. a Recombinant Human Granulocyte Colony Stimulating Factor (G-CSF) Genetically Fused to Recombinant Human Albumin Induces Prolonged Myelopoietic Effects in Mice and Monkeys. Pharm Res. 2002;19(11):1720–1729. doi: 10.1023/a:1020917732218. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Ou Y, Shi Y. Albubnp, a Recombinant B-type Natriuretic Peptide and Human Serum Albumin Fusion Hormone, as a Long-Term Therapy of Congestive Heart Failure. Pharm Res. 2004;21(11):2105–2111. doi: 10.1023/b:pham.0000048203.30568.81. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian GM, Fiscella M, Lamousé-Smith A, Zeuzem S, McHutchison JG. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat Biotechnol. 2007;25:1411–1419. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 31.Bai Y, Ann DK, Shen W-C. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci USA. 2005;102(20):7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roskam WG, Rougeon F. Molecular cloning and nucleotide sequence of the human growth hormone structural gene. Nucleic Acids Res. 1979;7(2):305. doi: 10.1093/nar/7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Lum JB, McGill JR, Moore CM, Naylor SL, van Bragt PH, Baldwin WD, Bowman BH. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci USA. 1984;81(9):2752. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm Res. 2006;23(9):2116–2121. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa M, Nimura A, Horikawa R, Katsumata N, Arisaka O, Wada M, Honjo M, Tanaka T. A Novel Specific Bioassay for Serum Human Growth Hormone. J Clin Endocrinol Metab. 2000;85(11):4274–4279. doi: 10.1210/jcem.85.11.6983. [DOI] [PubMed] [Google Scholar]
- 36.Amet N, Lee HF, Shen WC. Insertion of the Designed Helical Linker Led to Increased Expression of Tf-Based Fusion Proteins. Pharm Res. 2009;26(3):523–528. doi: 10.1007/s11095-008-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence CM, Ray S, Babyonyshev M, Galluser R, Borhani DW, Harrison SC. Crystal Structure of the Ectodomain of Human Transferrin Receptor. Science. 1999;286(5440):779. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 38.Blanchette J, Kavimandan N, Peppas NA. Principles of transmucosal delivery of therapeutic agents. Biomed Pharmacother. 2004;58(3):142–151. doi: 10.1016/j.biopha.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Chan LMS, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci. 2004;21(1):25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Lee VH, Yamamoto A, Kompella UB. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Crit Rev Ther Drug Carrier Syst. 1991;8(2):91–192. [PubMed] [Google Scholar]
- 41.Carino GP, Jacob JS, Mathiowitz E. Nanosphere based oral insulin delivery. J Control Release. 2000;65(1–2):261–269. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 42.Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin–transferrin conjugates. Biomater. 2006;27(20):3846–3854. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Rekha MR, Sharma CP. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J Control Release. 2009;135(2):144–151. doi: 10.1016/j.jconrel.2009.01.011. [DOI] [PubMed] [Google Scholar]