Abstract
The recently discovered exchange protein directly activated by cAMP (Epac), a guanine exchange factor for the G-protein RAP-1, is directly activated by cAMP independently of PKA. While cAMP is known to be an important second messenger in the retina, the presence of Epac has not been investigated in this tissue. The goal of the present study was to determine if the Epac1 and Epac2 genes are present and to characterize their location within the retina. Western blot analysis revealed that Epac1 and Epac2 proteins are expressed within the retina, and the presence of mRNA was demonstrated with the aid of RT-PCR. Additionally, we used immunofluorescence and confocal microscopy to demonstrate that Epac1 and Epac2 have overlapping as well as unique distributions within the retina. Both are present within horizontal cells, rod and cone bipolar cells, cholinergic amacrine cells, retrograde labeled retinal ganglion cells, and Müller cells. Uniquely, Epac2 was expressed by cone photoreceptor inner and outer segments, cell bodies, and synaptic terminals. In contrast, Epac1 was expressed in VGlut1 and CtBP2 positive photoreceptor synaptic terminals. Together, these results provide evidence that Epac1 and Epac2 are differentially expressed within the retina and provide the framework for further functional studies of cAMP pathways within the retina.
Keywords: cAMP, PKA, Long-Evans Hooded, immunohistochemistry, PDE, CNG, Rap, H89
Introduction
Cyclic nucleotide second messengers play an important role in signaling pathways within the central nervous system. Similarly, these cyclic nucleotides are essential for development and functioning of the retina. The signaling pathway of cGMP is well established within the first stages of the visual transduction pathway, however less is know about cAMP signaling within the retina. Some studies have established that cAMP contributes to retinal synapse formation (Stellwagen et al., 1999), retinal cell death (Linden et al., 2005), and the post-synaptic signaling for various neurotransmitters (Martins and Pearson, 2008). There is however, an inherent problem associated with identifying the specific roles of cAMP, which is the ubiquitous nature of this second messenger. Anatomical studies of adenylate cyclase, the protein responsible for cAMP production, have revealed that 9 splice variants are present and differentially expressed throughout the retina (Abdel-Majid et al., 2002). Similarly, phosphodiesterases (PDEs), proteins responsible for hydrolyzing cyclic nucleotides, are expressed within the retina (Santone et al., 2006, Whitaker and Cooper, 2007). Thus, to better understand the function of cAMP within the retina, a focus on the distribution of functionally related proteins such as the exchange proteins directly-activated by cAMP is in order.
At present, 3 families of proteins are known to be directly-activated by cAMP: (1) protein kinase A (PKA) (Seino and Shibasaki, 2005), (2) cyclic nucleotide gated channels (CNGs) (Craven and Zagotta, 2006, Kaupp and Seifert, 2002), including closely related hyperpolarization-activated cyclic nucleotide modulated channels (HCN) (Craven and Zagotta, 2006), and (3) exchange proteins directly activated by cAMP (Epacs) (de Rooij et al., 1998, Kawasaki et al., 1998). Initially, studies focused on cAMP signaling through PKA, which was originally believed to be responsible for essentially all cAMP signaling within the CNS and retina. Originally discovered, photoreceptors (Fesenko et al., 1985), CNGs were additionally found to be targets of cAMP (Kramer and Tibbs, 1996). Recently these channels have been localized to various cells throughout the retina including specific bipolar cells (Ivanova and Muller, 2006, Muller et al., 2003) and retinal ganglion cells (Oi et al., 2008), where they purportedly contribute to signaling within the neural network of the retina.
Recently discovered Epac proteins also convey cAMP signaling, independently of PKA. Various studies have reported that PKA and Epac signaling can produce synergistic and / or antagonistic effects within the same cells (Cheng et al., 2008). A detailed review on Epac structure has been reported (Bos, 2006). In brief, Epacs are the products of two genes, Epac1 and Epac2. Both Epacs belong to a family of proteins called guanine exchange factors (GEFs). GEFs catalyze the activation of small monomeric G-proteins by facilitating dissociation of GDP and replacement with GTP (Vetter and Wittinghofer, 2001). In the case of Epac1 and Epac2, they catalyze the activation of two G-proteins, Rap-1 and Rap-2 (de Rooij et al., 2000). Structurally, both Epacs are similar although there is an additional cyclic nucleotide binding (CNB) site located on Epac2. Both are multi-domain proteins and contain an inhibitory regulatory domain which contains the CNB and a catalytic domain which contains the GEF domain.
Advancements in pharmacological agonists and interest in the roles of Epac signaling have led us to investigate the presence of Epac1 and Epac2 within the retinas of adult, Long Evans Hooded rats as a prelude to more functional studies. The present study uses western blotting techniques, RT-PCR, retrograde labeling, and immunofluorescence to establish for the first time that both Epac genes are present in the retina. We have identified their relative distributions with the aid of double-labeling immunofluorescence with known retinal cell markers. Portions of these results have been presented in abstract form (Whitaker and Cooper, 2007 Whitaker and Cooper, 2008).
Materials and Methods
Tissue Preparation
Tissues for western blot analysis and rt-PCR, were obtained from euthanized (sodium pentobarbital, 100mg/kg, i.p.), adult male (250–300g) Long Evan’s hooded rats (Harlan, Indianapolis, IN). Retinas were removed from each eye and flash frozen in liquid nitrogen. Dissected tissues which were not used immediately were stored at −80°C for subsequent use. For immunohistochemistry, rats were deeply anesthetized with sodium pentobarbital (100mg/kg, i.p.) and transcardially perfused with calcium-free Tyrodes solution, followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB), pH 7.4. Eyes were enucleated and post-fixed overnight in 4% paraformaldehyde at 4°C followed by several rinses in 0.1M PB. Whole eyes were cryoprotected in 30% sucrose in 0.1M PB for 3–5 days at 4°C before cryostat sectioning at 14μm. Sections were stored at −80°C for subsequent use. All animals were housed in 12:12 hour light:dark cycle with food and water, ad libitum. The protocols for treatment and care of all animals used in this study were approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC) and were carried out in accordance with the Association for Research in Vision and Ophthalmology (ARVO) guidelines.
Retrograde Labeling
Retrograde labeling from the superior colliculus was used to identify retinal ganglion cells. Rats were anesthetized with a cocktail of ketamine (37.5mg/kg, i.m.) and xylazine (5 mg/kg, i.m.) and mounted on a stereotaxic apparatus. The area of incision was cleansed with 70% ethanol followed by Betadine™ surgical scrub. Following incision, bilateral holes were drilled over the superior colliculus as measured from bregma using known brain coordinates (Paxinos and Watson, 2005). Approximately 4–5μl of 1% cholera toxin B subunit (CTB) (Sigma, St. Louis, MO) was injected bilaterally using a 10μl Hamilton syringe (Hamilton Co., Reno, NV) (Rivera and Lugo, 1998, Zhang and Diamond, 2006). Animals were given a 7 day recovery period before being perfused. Sections of superior colliculus were processed and visualized for CTB to confirm the correct location of the injection sites (data not shown).
Primary Antibodies
Several antibodies were used to differentiate various cells within the retina. Table 1 provides source, catalog, host, and dilution for all antibodies used in this study. Antibody to Epac1 was raised against amino acids 1–70 at the N-terminus of human Epac1 sequence. This antibody detected a ~120kDa band in imunolabeled western blots (manufacturer’s product sheet). Specificity of this H70 Epac1 antibody has been determined using small-inhibitory-RNA (siRNA) knockdown, where an absence of Epac1 was determined with the aid of western blotting (Huston et al., 2008). Antibody to Epac2 was raised against amino acids 1–220 at the N-terminus of human Epac2 sequence. This antibody detected a 126kDa band in immunolabeled western blots (manufacturer’s product sheet). Specificity was determined by the absence of bands in western blots following preadsorption with a recombinant protein consisting of amino acids 1–436 of the mouse Epac2 sequence (Aivatiadou et al., 2008).
Table 1.
List of primary antibodies, source, species, hosts, and dilutions used for immunofluorescence microscopy.
| Antibody | Source | Catalog /Clone No. | Host | Dilution |
|---|---|---|---|---|
| Epac 1 | Santa Cruz Biotechnologies, Santa Cruz, CA | SC-25632; H-70; Lot # E1905 | Rabbit | 1:100 |
| Epac 2 | Santa Cruz Biotechnologies | SC-25633; H-220; Lot #B1705 | Rabbit | 1:50 |
| VGLUT1 | Chemicon Temecula, CA | AB5905 | Guinea Pig | 1:1000 |
| CtBP2 | BD Biosciences, San Jose, CA | 612044; clone 16 | Mouse | 1:500 |
| Calbindin | Sigma, St. Louis, MO | C9848; clone CB-955 | Mouse | 1:500 |
| Chx10 | Exalpha Biologicals, Inc, Watertown, MA | X1180P | Sheep | 1:500 |
| PKCα | Upstate, Lake Placid, NY | 05–154; clone M4 | Mouse | 1:500 |
| Choline Acetyltransferase (ChAT) | Chemicon | AB144P | Goat | 1:200 |
| Glutamine Synthetase | Chemicon | MAB302; clone GS-6 | Mouse | 1:1000 |
| GLAST | Chemicon | AB1782 | Guinea Pig | 1:1000 |
| CTB | List Biological Laboratories, Inc, Campbell, CA | 703 | Goat | 1:1000 |
Antibody to vesicular glutamate transporter 1 (VGlut1) labeled ribbon synapses of the photoreceptor cells and bipolar cells within the outer plexiform layer (OPL) and the inner plexiform layer (IPL), respectively (Johnson et al., 2003, Mimura et al., 2002, Sherry et al., 2003). Raised against a synthetic peptide located at the C-terminal end of the rat VGlut1 protein sequence (GATHSTVQPPRPPPPVRDY), VGlut1 antibody detected a ~60kDa band in immunolabeled western blots (Melone et al., 2005). Specificity was determined with preadsorption where immunoreactivity was abolished (Johnson et al., 2003). Also, antibody to C-terminal binding protein (CtBP2) labeled ribbon synapses (Schmitz et al., 2000, tom Dieck et al., 2005). The antibody used was raised against the mouse CtBP2 protein at amino acids 361–445 and detected a 48kDa band in immunolabeled western blots (manufacture’s data sheet).
Antibody to calbindin labeled mammalian horizontal cells (Peichl and Gonzalez-Soriano, 1994, Rabie et al., 1985). The antibody used here was derived against purified bovine kidney calbindin-D-28K and recognized a ~28kDa band in western blots which was abolished after preadsorption (Gargini et al., 2007). Antibody to Chx10, a homeodomain protein, was restricted to bipolar cells in the adult retina (Elshatory et al., 2007, Liu et al., 1994). Raised against a recombinant protein to amino acids 1 to 131 of N-terminal of the human Chx10 protein, this antibody detected a ~46kDa protein in western blots of rat retina (manufacturer’s product sheet). Antibody labeling coincides with previous reports (Elshatory et al., 2007, Mojumder et al., 2007) and in situ hybridization results (Liu et al., 1994). Antibody to PKCα specifically labeled rod bipolar cells of the rodent retina (Greferath et al., 1990, Negishi et al., 1988). Monoclonal antibody to PKCα was raised against proteins derived from rabbit brain cytosol (Jaken and Kiley, 1987) and detected a ~82kDa band in western blots (Leach et al., 1988).
Antibody to choline acetyltransferase (ChAT) specifically labeled cholinergic amacrine cells (Haverkamp and Wassle, 2000, Voigt, 1986). This antibody was raised against a protein derived from a human placental enzyme which detected ~68kDa protein (Brunelli et al., 2005). Specificity was previously determined with preadsorption which abolished immunostaining (Motts et al., 2008). Two antibodies were used to differentiate Müller cells. Antibody to glutamine synthetase (GS) (Riepe and Norenburg, 1977) and antibody to L-glutamate / L-aspartate transporter (GLAST) (Lehre et al., 1997, Rauen et al., 1996) specifically labeled the cytosolic and membrane compartments of Müller cells, respectively (Derouiche and Rauen, 1995, Ding and Weinberg, 2007). Antibody to glutamine synthetase was raised against purified protein from sheep brain and detected ~45kDa band in western blots (manufacture’s data sheet). Antibody to GLAST was raised against a synthetic peptide from the C-terminus of the rat GLAST sequence (QLIAQDNEPEKPVADSETKM) (manufacturer’s data sheet) and detected ~65kDa band in western blots (Vermeiren et al., 2005). To label cone photoreceptors we used the fluorescein isothyocynate (FITC) conjugated peanut agglutinin (PNA) lectin from Arachis hypogaea, which specifically binds glycoproteins located on the inner and outer segments and synaptic pedicles of cones (Blanks and Johnson, 1984, Johnson et al., 1986).
Western Blot Analysis
Frozen retinas were sonicated in ice cold RIPA buffer (50mM Tris-HCl, 150mM NaCl, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, pH 8.0, Sigma) twice for 5 seconds each. A cocktail of protease and phosphatase inhibitors were added (1:100 FabGennix, Frisco, TX) before use. Following sonication, tissue was centrifuged at 4°C under 14,000 r.p.m. for 30 minutes. The supernatant was collected and protein concentration was assessed using a BCA kit (Pierce Biotechnology Inc., Rockford, IL). 30μg of protein was loaded onto a % SDS-PAGE gel and electrophoresed at 120V for ~1hr, then transferred to PVDF membranes (Milipore, Billerica, MA). Membranes were blocked in 5% fat free dry milk dissolved in tris buffered saline pH 8.0 (TBS) plus 0.1% tween-20 (TBST). Primary antibodies were incubated overnight at 4°C in 5% bovine serum albumin (BSA) dissolved in TBST (Epac1 1:1000; Epac2 1:1000). Following several rinses in TBST, a donkey anti-rabbit secondary antibody conjugated to horse radish peroxidase (1:10000, Jackson Immuno, West Grove, PA) was diluted in 5% milk TBST and placed on the membrane for 1 hr at room temperature. Application of the secondary antibody was followed by several washes in TBST. To visualize bands, an ECL visualization kit (Pierce) was applied to the membrane before exposure on radiographic film. Relative molecular weights of bands were determined by comparison with protein standards and compared to reported molecular weights of known Epac proteins.
RT-PCR
Retinal tissue was homogenized in 1 ml of QIAzol Lysis Reagent (Qiagen, Valencia, CA) using a PolyTron homogenizer. Total RNA was extracted from retinas using the RNeasy Lipid Tissue Mini Kit (Qiagen) according to manufacturer’s directions. RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanaDrop Tech., Wilmington, DE). A total of 10μg of RNA was reverse transcribed using the high capacity cDNA archiving kit (Applied Biosystgems, Foster, CA) with random primers in accordance with the manufacturer’s specifications. PCR reactions were completed in 50μl reactions containing 10ng of reverse transcribed cDNA, 10mM forward and reverse primers (Integrated DNA Technologies, Coralville, IA), and TITANIUM™ Taq PCR kit (Clontech, Mountain View, CA) as per manufacturer’s specifications. For detection of Epac1, the following primers were used (forward 5′-GTGTTGGTGAAGGTCAATCTG-3′; reverse 5′-CCACACCACGGCATC-3′) (Ulucan et al., 2007). To detect Epac2 mRNA, the following primers were used (forward 5′-GTGGGGACGTTTGAACTGATGAGC-3′; reverse 5′-AGCCTGTACGCCTTGTGATTTCTG-3′) (Kang et al., 2006). PCR conditions used were 95°C for 1 min, (Denaturing 95°C 30 sec ≫ Annealing 55°C 1 min ≫ Extending 72°C 1 min) for 40 cycles, and lastly 72°C for 7 min on a MJ Research 225 Tetrad Thermal Cycler (Global Medical Instrumentation Inc., Ramsey, MN). PCR products were detected with ethidium bromide gels where candidate bands were extracted and purified with QIAquick Gel Extraction Kit (Qiagen). Authenticity of each band was confirmed using direct sequencing on a CEQ™ 8000 Genetic Analysis System (Beckman Coulter Inc., Fullerton, CA) and queried against NCBI’s Blastn software (Altschul et al., 1997).
Immunohistochemistry
Frozen tissue sections were initially thawed, encircled with a pap pen smear, and rehydrated in tris-buffered saline (TBS) pH 7.2. Slides were then blocked in 10% normal donkey serum (NDS, Jackson Immuno) diluted with 0.05% triton X-100 in TBS (Tx-TBS) for 1 hr at room temperature. After the blocking step, sections were incubated with primary antibodies overnight at 4°C. Following several rinses in TBS, species specific secondary antibodies, which were raised in donkey and conjugated to Cy2, Fitc, Cy3, and / or Cy5 (1:200, Jackson Immuno), were added to the slides in 5% NDS Tx-TBS for 1 hr at room temperature. This was followed by several rinses in TBS then sections were coverslipped using freshly made Mowiol (Sigma).
Image Acquisition and Processing
Immunofluorescent images were obtained using a laser confocal microscope (Olympus) and digitized with Fluoview 500 software (Mellville, NY). Adobe photoshop v9.02 (Adobe Systems Inc., San Jose, CA) was used to sharpen images, adjust brightness and contrast, and compose the final images.
Results
Overall Expression and Distribution of Epac1 and Epac2
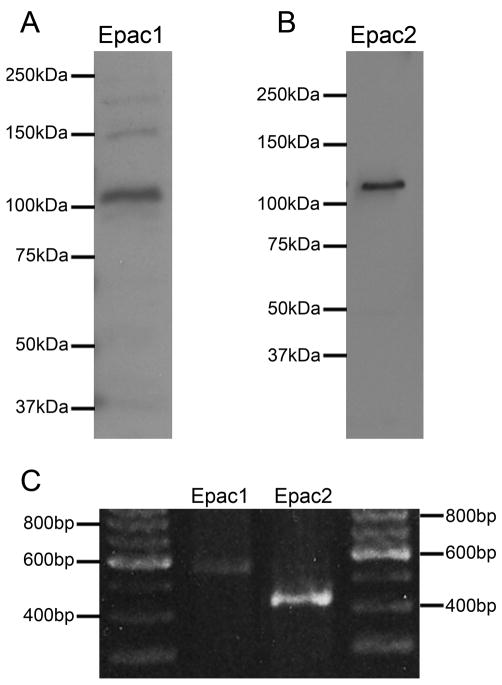
The presence of Epac proteins within the retina was determined with the aid of western blotting. Both Epac1 (Fig. 1A) and Epac2 (Fig. 1B) were found to be present within the retina at the expected sizes of ~120kDa and ~126kDa, respectively. The presence of Epac mRNAs within the retina was examined with the aid of RT-PCR. Candidate bands (Fig. 1C) were excised, sequenced, and confirmed as Epac1 and Epac2 using Blastn (NCBI).
Figure 1.
Epac protein and mRNA expression in the rodent retina. Epac1 (A) and Epac2 are represented within the retina at their predicted sizes 120kDA and 126kDA, respectively. RT-PCR of Epac1 and Epac2 within the retina (E) reveals bands of the predicted size for both Epacs.
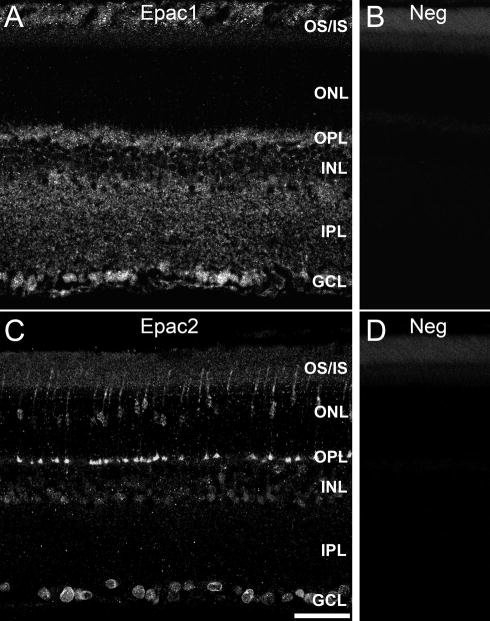
The relative distributions of Epac1 and Epac2 were determined using immunohistochemistry. Low magnification images of Epac1 and Epac2 reveal overlapping and also unique distributions within the retina (Fig. 2). Epac1 was expressed within both synaptic layers, the outer plexiform layer (OPL) and inner plexiform layer (IPL). Additional expression was located within cell bodies of the inner nuclear layer (INL) and ganglion cell layer (GCL). Epac2 expression was also found within the INL and GCL cell bodies. Uniquely, Epac2 immunoreactivity was specifically expressed within the inner and outer segments, as well as cell bodies of the outer nuclear layer (ONL), and processes with the appearance of photoreceptor synaptic terminals in the OPL.
Figure 2.
Epac expression in the rodent retina. Immunofluorescent images of Epac1 (A), Epac2 (C), and negative controls (B, D) demonstrate their differential localizations. Epac1 was expressed primarily within the inner retina where it localized to cell bodies and synaptic regions. Epac2 however, was expressed primarily within cell bodies of all layers and also within distinctive structures of the OPL. Scale bar = 50μm.
Epac expression within the photoreceptor terminals
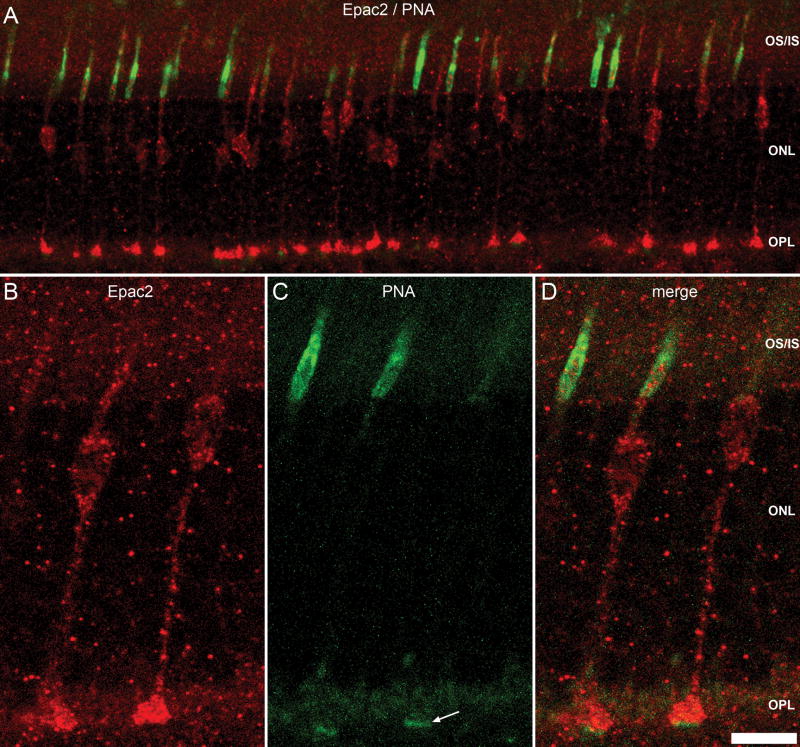
Overall distribution of Epac1 indicated a presence within the OPL. To determine whether Epac1 was expressed within photoreceptor terminals, we co-labeled with two commonly used markers for photoreceptor ribbon synapses, CtBP2 (Schmitz et al., 2000, tom Dieck et al., 2005) and VGlut1 (Johnson et al., 2003, Mimura et al., 2002, Sherry et al., 2003). Both CtBP2 (Fig. 3C) and VGlut1 (Fig. 3E) immunolabeling was restricted to the outer boundary of the OPL within photoreceptor terminals as previously reported (Johnson et al., 2003, Mimura et al., 2002, Schmitz et al., 2000, Sherry et al., 2003, tom Dieck et al., 2005). Epac1 was found to colocalize with both CtBP2 (Fig. 3B) and VGlut1 (Fig 3D). Epac2 was also found within the OPL, although the expression pattern was specific to large synaptic structures indicative of cone pedicles. To pursue this possibility we co-labeled with the PNA lectin, which is specific to cone photoreceptors and pedicles (Blanks and Johnson, 1984, Johnson et al., 1986). The results demonstrate colocalization of PNA labeling and Epac2 (Fig. 4A). Higher magnification revealed that Epac2 was completely expressed by the cone cells with expression in the inner / outer segments, cell body, and the PNA-labeled cone pedicles (Fig. 4B–D). Collectively these results indicate that both Epacs are expressed within photoreceptor terminals, where Epac2 was selectively expressed within cones (Fig. 4D).
Figure 3.
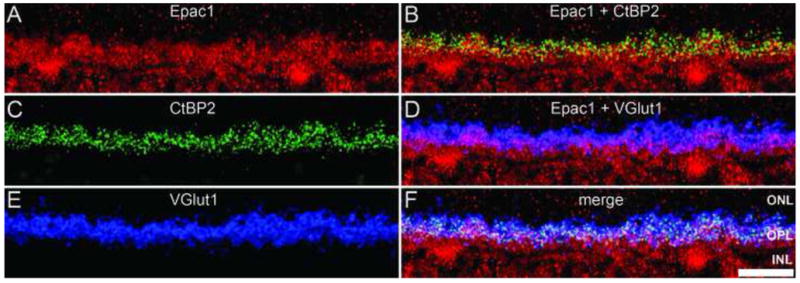
Epac1 expression in the photoreceptors. Co-labeling of Epac1 (A) with CtBP2 (C) and VGlut1 (E). CtBP2 and VGlut1 labeling was restricted to the outer margin of the OPL within photoreceptor synapses. Epac1 was highly expressed throughout the OPL and shared colocalization with both CtBP2 (B) and VGlut1 (C). Merging all three images reveal colocalizations between all three antibodies, this is denoted by the white color (F). Scale bar = 25μm.
Figure 4.
Epac2 expression in the photoreceptors. Double-labeling of Epac2 at low magnification (A) and high magnification (B) with PNA (A, C). PNA specifically binds glycoproteins on cone photoreceptor inner and outer segments and cone pedicles (arrow). At low magnification Epac2 and PNA double-labeling reveals colocalization with the majority of PNA labeled photoreceptors. Higher magnification (D) demonstrates the presence of Epac2 throughout the PNA labeled cone cell. Scale bar = 10μm (B–D) and 25μm (A).
Epac expression within the horizontal cells
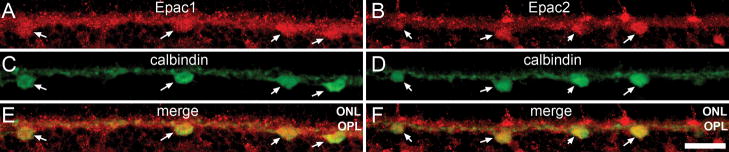
Antibody to Epac1 and Epac2 label cells located at the inner boundary of the OPL similar to the location of horizontal cell somata. To test for the presence of Epacs in these cells we double labeled with antibody to calbindin, previously demonstrated to specifically label all mammalian horizontal cells (Peichl and Gonzalez-Soriano, 1994, Rabie et al., 1985). Antibody to calbindin labeled horizontal cell somata (Fig. 5C, D). Both Epacs colocalized with calbindin containing horizontal cells (Fig. 5E, F). Some Epac containing cell bodies were not labeled with anti-calbindin. These may be the cell bodies of bipolar cells as described below.
Figure 5.
Epac expression within the horizontal cells. Double-labeling of Epac1 (A) and Epac2 (B) with calbindin (C, D). Calbindin specifically labels all horizontal cell bodies. Both Epacs (E, F) colocalized with calbindin positive horizontal cells (arrow). Scale bar = 25μm.
Epac expression within the bipolar cells
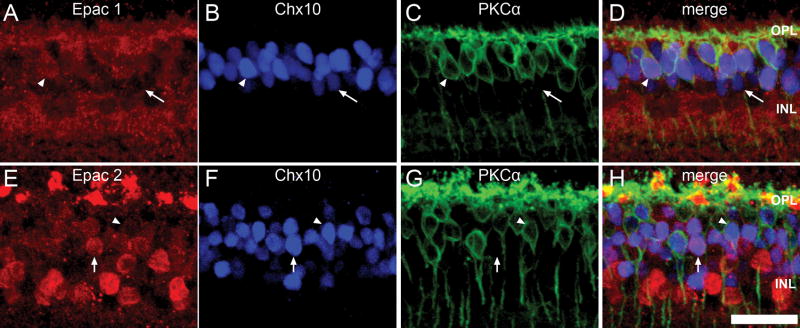
To date, 9 different cone bipolar cell types and one rod bipolar cell type have been identified in the mammalian retina with cell bodies typically located within the middle to outer INL (Ghosh et al., 2004, Pignatelli and Strettoi, 2004). Similarly, both Epac-antibodies labeled cell bodies located within the same area of the INL (Fig. 6A, E). To determine whether Epacs were expressed by bipolar cells, we used antibodies to PKCα, which label all rod bipolar cells (Greferath et al., 1990, Negishi et al., 1988) and Chx10 a purported pan-bipolar cell marker (Elshatory et al., 2007, Liu et al., 1994). We found that both Epacs were expressed in rod bipolar cells which we characterized as containing both PKCα and Chx10 labeling and in cone bipolar cells characterized by only Chx10 labeling (Fig 6D, H).
Figure 6.
Epac expression within the bipolar cells. Triple-labeling of Epac1 (A) and Epac2 (E) with Chx10 (B, F), and PKCα (C, G). PKCα immunoreactivity is present within bipolar cells, whereas PKCα expression is limited to rod bipolar cells. Both Epacs (D, H) colocalized with rod bipolar cells, which also contain Chx10 (arrowhead) and putative cone bipolar cells which are negative for PKCα labeling (arrow). Scale bar = 25μm.
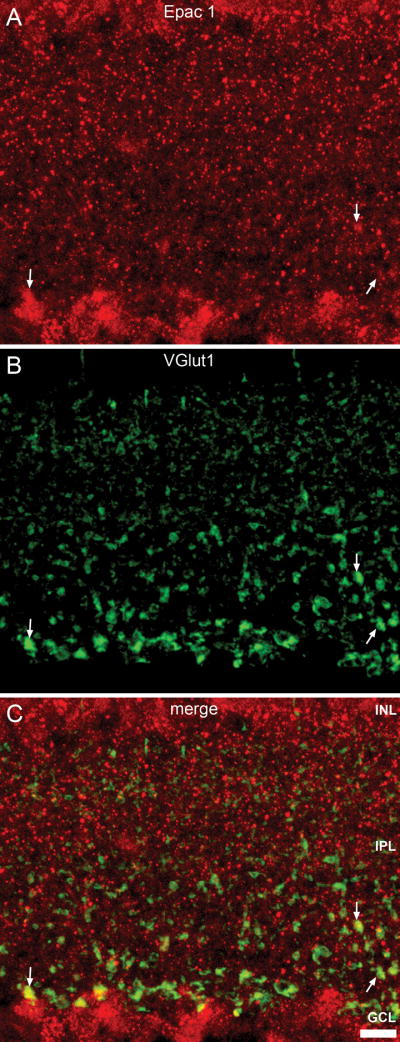
In addition, Epac1 antibody labeling was expressed within the IPL where bipolar cells terminate. VGlut1 antibody, in addition to labeling ribbon synapses of photoreceptor cells in the OPL, also labels ribbon synapses of bipolar cells within the IPL (Johnson et al., 2003, Mimura et al., 2002, Sherry et al., 2003). To determine whether bipolar cell terminals express Epac1 we co-labeled with an antibody to VGlut1. Figure 7 demonstrated colocalization of VGlut1 and Epac1 within some bipolar cell terminals. These colocalizations were most apparent within rod bipolar cell terminals defined by their large lobular synapses located at the inner-most boundary of the IPL, close to the retinal ganglion cell layer
Figure 7.
Epac1 expression within the bipolar cell terminals. Double-labeling of Epac1 (A) with VGlut1 (B). VGlut specifically labels bipolar cell ribbon synapses within the IPL. Epac1 (C) weakly colocalized with VGlut1, primarily within the larger terminals located at the inner boundary of the IPL, indicative of rod bipolar cell terminals (arrow). Scale bar = 10μm.
Epac expression within the amacrine cells
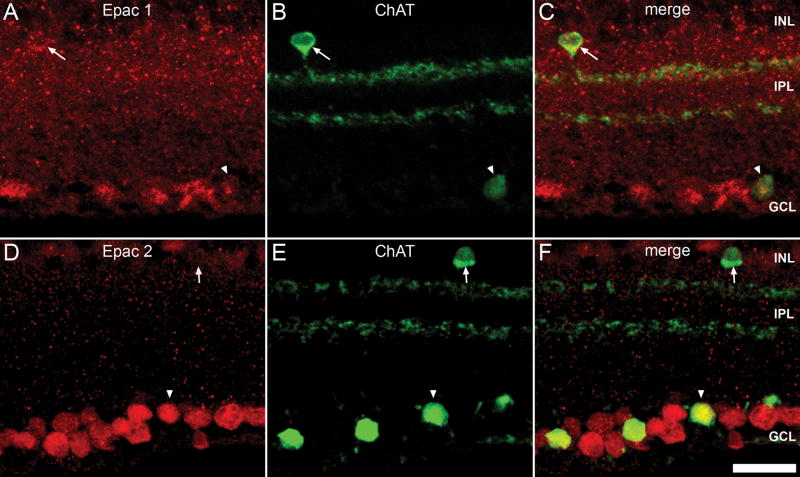
Epac1 and Epac2 are both expressed within cell bodies located within the inner boundary of the INL, perhaps indicative of amacrine cell bodies. Additionally both Epacs were present within numerous cells of the GCL, which contains over 50% displaced amacrine cells (Jeon et al., 1998). Cholinergic amacrine cells are a subset of amacrine cells with somata located within the INL and with displaced somata located within the GCL (Perry and Walker, 1980, Voigt, 1986). To determine the presence of Epac within these cells we double labeled with an antibody to ChAT. ChAT is expressed by cholinergic amacrine cells both within the INL and in the GCL and also labels their respective terminals corresponding to outer OFF cholinergic synapses and inner ON cholinergic synapses (Haverkamp and Wassle, 2000, Voigt, 1986). Co-labeling revealed that Epac1 colocalized with cell bodies of the INL and GCL, but were not expressed within their respective terminals (Fig. 8C). Epac2 also colocalized with cholinergic amacrine somata within the GCL, but demonstrated a weak expression within ChAT somata of the INL (Fig. 8F).
Figure 8.
Epac expression within the cholinergic amacrine cells. Double-labeling of Epac1 (A) and Epac2 (D) with ChAT (B, E). ChAT is expressed by cholinergic amacrine cells located at the INL and displaced cholinergic amacrine cells at the GCL and 2 strata within the IPL, an outer OFF cholinergic layer and an inner ON cholinergic layer. Epacs1 (C) colocalized with ChAT positive amacrine cells of both the INL (arrow) and GCL (arrowhead). Epac2 (F) was colocalized with ChAT positive cells of GCL (arrowhead), but demonstrated weak colocalization with ChAT cells of the INL (arrow). Scale bar = 25μm.
Epac expression within the retinal ganglion cells
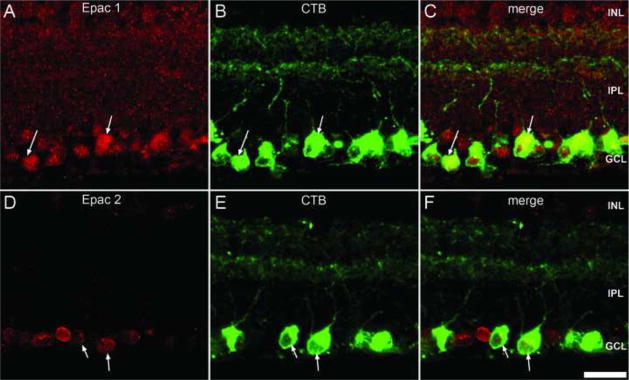
Approximately 90% of all rat retinal ganglion cell axons terminate within the superior colliculus and can be labeled by retrograde transport (Linden and Perry, 1983). In the current study we employed CTB as a retrograde label to positively identify retinal ganglion cells. Following 1 week of retrograde transport we observed that CTB was present within ganglion cell somata and their dendrites located within the middle to outer boarder of the IPL (Fig. 9B, E), as reported elsewhere (Zhang and Diamond, 2006). Double-labeling demonstrated that both Epac1 and Epac2 were expressed in retinal ganglion cells (Fig. 9C, F).
Figure 9.
Epac expression within the retinal ganglion cells. Double-labeling of Epac1 (A) and Epac2 (D) with CTB (B, E). Immunodetection of retrograde labeled retinal ganglion cells with CTB demonstrates expression within multiple retinal ganglion cell bodies and their dendrites within the IPL. Both Epacs (C, F) colocalized with retinal ganglion cell bodies (arrow). Scale bar = 25μm.
Epac expression within the Müller cells
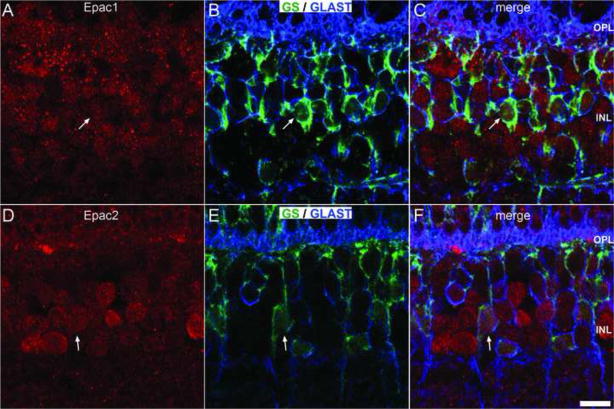
Müller cells are the major glial constituent of the retina (Jeon et al., 1998) and participate in multiple functions including recycling of neurotransmitters such as glutamate (Ehinger and Falck, 1971, White and Neal, 1976). Two antibodies raised against GS and GLAST were used to identify Müller cell cytosol and plasma membrane, respectively (Derouiche and Rauen, 1995, Ding and Weinberg, 2007). Double-labeling was used to demonstrate that both Epac1 and Epac2 colocalize with Müller cell bodies within the INL (Fig. 10).
Figure 10.
Epac expression within the Müller cells. Triple-labeling of Epac1 (A) and Epac (D) with GS and GLAST (B, E). GS and GLAST are expressed within Müller, specifically GS was expressed within the cytosol and GLAST was expressed at the plasma membrane. Classification of Müller cell bodies were determined by having both GS and GLAST immunoreactivity. Both Epacs (C, F) colocalized with Müller cell bodies. Scale bar = 10μm.
Discussion
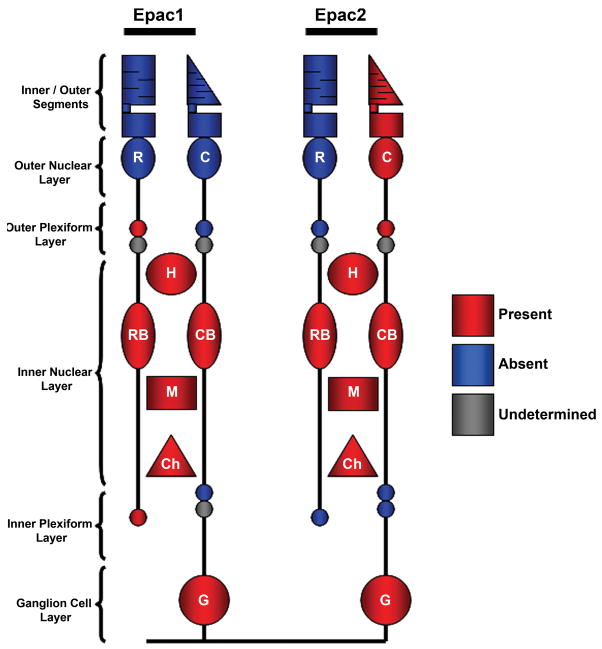
To better understand the roles of cAMP it is helpful to focus on the proteins that are downstream targets of the cAMP message. Previous studies have investigated the contributions of PKA and CNGs, but we are unaware of any study focusing on the presence or effects of Epac signaling within the retina. In the current study we demonstrate for the first time that 1) Epac1 and Epac2 are abundant within the retina and 2) they are differentially expressed. Figure 11 summarizes these findings.
Figure 11.
Summary of Epac expression within the rodent retina. Epac1 and Epac2 expression within various cell types where G – ganglion cells, Ch cholinergic amacrine cells, M – Müller cells, CB – cone bipolar cells, RB – rod bipolar cells, H – horizontal cells, C – cones, and R – rods.
Why study Epacs?
There is still much to be learned about the functioning of cAMP within the retina. Adenylate cyclases are found throughout the retina implying multiple, potential roles of cAMP (Abdel-Majid et al., 2002). Anatomical localization of PKA subtypes provides evidence that downstream cAMP components are specifically localized to specific retinal cell types (Mataruga et al., 2007). This is also true for different CNG and HCN genes (Mataruga et al., 2007, Muller et al., 2003, Oi et al., 2008). We demonstrate here that while the localization of Epac1 and Epac2 are differentially expressed, there are also instances of co-expression within the same cell types. Interestingly, within brain and spinal cord tissue Epac1 was expressed only during embryonic and neonatal time points, whereas Epac2 expression was highly expressed in adulthoot rather than embryonic or neonatal time points. (Murray and Shewan, 2008). Furthermore, PKA and Epac signaling can provide synergistic effects such as neurite extension (Christensen et al., 2003), regulation of neurotensin secretion (Dodge-Kafka et al., 2005), and regulation of phosphodiesterases (Li et al., 2007). In contrast, PKA and Epac signaling can have antagonistic effects including regulation of protein kinase B (PKB) phosphorylation (Mei et al., 2002), acetylcholine regulation (Brock et al., 2007), and plasticity (Ster et al., 2009). How then does cAMP differentiate between Epac and PKA? Differential distribution of PDE proteins lead to compartmentalization of cAMP signaling (Houslay et al., 2007, Huston et al., 2008). We have previously reported that PDE4 proteins are differentially expressed within the retina (Whitaker and Cooper, 2007). Variable PDE distributions may provide a potential mechanism to segregate Epac signaling from PKA (Huston et al., 2008).
Role of cAMP within cone photoreceptor inner and outer segments
In the current study, we demonstrated Epac2 expression within cone photoreceptor cell inner and outer segments. Proposed functions of cAMP within photoreceptors include disk shedding (Besharse et al., 1982) and inhibition of G-protein coupled receptor kinases (Horner et al., 2005). Decreases in cAMP following a light stimulus are attributed to dopamine D4 receptors located within photoreceptors which negatively regulates adenylate cyclases (Cohen and Blazynski, 1990, Cohen et al., 1992). Developmentally, anti-apoptotic effects of dopamine within the retina were only partially blocked with PKA inhibition indicating a potential role for Epacs in dopamine transmission (Varella et al., 1999). At this time however, the function of Epac2 within photoreceptors is unknown but could be related to these previously described pathways.
Role of cAMP in neurotransmitter release within the photoreceptor synapses
Both Epac1 and Epac2 are expressed within photoreceptor terminals. Epac1 expression colocalized with two known terminals markers, CtBP2 and VGlut1, where Epac2 was specifically expressed within cone pedicles. Evidence of the importance of cAMP in synapses is highlighted in studies of hippocampal (Nguyen and Woo, 2003) and cerebellar (Linden and Ahn, 1999) plasticity where it facilitates transmitter release (Fiumara et al., 2004). Hippocampal plasticity, both long term potentiation (LTP) and long term depression (LTD) were altered following application of cAMP agonists (Nguyen and Woo, 2003). Specifically, PKA facilitated LTP (Greengard et al., 1991, Wang et al., 1991) while inhibiting LTD (Kameyama et al., 1998, Santschi et al., 1999). Epac signaling has also been addressed using a hippocampal model of synaptic plasticity where it promoted a novel form of hippocampal LTD purportedly through a pre-synaptic mechanism (Ster et al., 2009), unlike the effect of PKA. Epac activation can regulate neurotransmitter release (Zhong and Zucker, 2005). A proposed mechanism for the neurotransmitter release is through the interaction between Epacs and the binding partner, piccolo (Fujimoto et al., 2002, Ozaki et al., 2000), a protein expressed in terminals, including photoreceptor synapses (Dick et al., 2001). The functional involvement of Epacs in photoreceptor terminals has yet to be determined. However both reportedly play significant roles in synaptic transmission throughout the CNS.
Role of cAMP within the traditional rod and cone pathway
There is evidence that cAMP signaling contributes to both ON and OFF cone bipolar cell signaling (Bragadottir and Jarkman, 1995, Maguire and Werblin, 1994). Immunohistochemical evidence demonstrated that PKA type II is selectively localized to a subtype of cone bipolar cells, type 3b, whereas HCN4 selectively labeled type 3a cone bipolar cells (Mataruga et al., 2007). This differential localization provides insight into the cellular specificity of cAMP signaling. While the current study did not attempt to localize Epacs to specific subtypes of bipolar cells, we do demonstrate the presence of both Epacs within rod bipolar cells and putative cone bipolar cells immuno-labeled with Chx-10, a pan-bipolar cell marker (Elshatory et al., 2007, Liu et al., 1994). These results collectively indicate that cAMP is expressed and most likely contribute to bipolar cell signaling through multiple effector proteins.
Retinal wave propagation guide synapse formation in the developing retina (Firth et al., 2005). These waves have been shown to be dependent upon PKA (Stellwagen et al., 1999). Possible upstream components include the neurotransmitters such as acetylcholine and GABA (Feller et al., 1996, Fischer et al., 1998). In the current study we examined colocalizations between Epacs and ChAT positive cholinergic amacrine cells. Our data demonstrate that both Epacs colocalize within cholinergic cells which begs the question of their functioning within developmental synapse formation. Inhibition of PKA attenuates synapse formation during development (Stellwagen et al., 1999), which would lead one to believe that Epac might not be involved. The particular function(s) of Epac within cholinergic amacrine cells is unknown at this time. However, it was recently reported that Epac activation produces opposite effects compared to PKA activation within a cholinergic cell culture paradigm (Brock et al., 2007). Further work will be necessary to examine Epac signaling in retinal development.
Role of cAMP in retinal ganglion regeneration
In the current study we demonstrate that both Epacs are expressed in retrograde labeled retinal ganglion cells. Elevation of cAMP has been demonstrated to successfully promote axonal regeneration within various in vitro and in vivo paradigms (Berry et al., 2008, Hannila and Filbin, 2008, Liu and Brady, 2004). Within the visual system increased cAMP significantly increases axonal regeneration following optic nerve crush (Chierzi et al., 2005, Choi et al., 2003, Monsul et al., 2004, Watanabe et al., 2003). Furthermore, Epac has been recently implicated in aiding neurite regeneration within spinal cord tissue (Murray and Shewan, 2008), providing a possible role for Epac within retinal axon regeneration.
Conclusion
This is the first study to establish the presence and expression of both Epac genes and their protein products within the retina. Our results indicate that these genes are expressed within the retina and have a differential distribution. Epac1 was specifically expressed at the synaptic layers and the ganglion cell layer. Epac2 expression was concentrated within cell bodies and the cone pedicles. These results contribute to an understanding of the involvement of the second messenger cAMP within the retina. This study provides the framework for numerous further functional studies which will begin to differentiate PKA vs Epac mediated signaling.
Acknowledgments
The authors would like to thank Drs. Martha E. Bickford and Robert F. Lundy for use of equipment and technical help. Support from grants R01EY017594, P20RR16481, and P30ES014443 are gratefully acknowledged.
Abbreviations
- BCA
bicinchoninic acid
- cAMP
cyclic adenosine monophosphate
- cDNA
complimentary deoxynucleic acid
- cGMP
cyclic guanosine monophosphate
- ChAT
choline acetalytransferase
- CNB
cyclic nucleotide binding site
- CNG
cyclic nucleotide gated channel
- CNS
central nervous system
- CTB
cholera toxin B subunit
- CtBP2
C-terminal binding protein 2
- Cy2
cyanine
- Cy3
indocarbocyanine
- Cy5
indodicarbocyanine
- dNTP
deoxyribonucleotide triphosphates
- ECL
enhanced chemiluminescence
- Epac
exchange protein directly activated by cAMP
- FITC
fluorescein isothyocynate
- GABA
γ amino butyric acid
- GCL
ganglion cell layer
- GDP
guanosine diphosphate
- GEF
guanine exchange factor
- GLAST
L-glutamate / L-aspartate transporter
- GS
glutamine synthetase
- GTP
guanosine triphosphate
- HCN
hyperpolarization-activated cyclic nucleotide modulated channel
- HRP
horseradish peroxidase
- INL
inner nuclear layer
- IPL
inner plexiform layer
- IS/OS
inner and outer segments
- kDa
kiloDaltons
- mRNA
messenger ribonucleic acid
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PDE
phosphodiesterase
- PFA
paraformaldehyde
- PKA
protein kinase A
- PKCα
protein kinase C α-subunit
- PNA
peanut agglutinin
- PVDF
polyvinylidene fluoride
- RT-PCR
reverse transcriptase polymerase chain reaction
- TBST
tris buffered saline and tween-20
- Tx-TBS
triton X-100 and tris buffered saline
- UCR1
upstream conserved region 1
- UCR2
upstream conserved region 2
- VGlut1
vesicular glutamate transporter 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Majid RM, Tremblay F, Baldridge WH. Localization of adenylyl cyclase proteins in the rodent retina. Brain Res Mol Brain Res. 2002;101:62–70. doi: 10.1016/s0169-328x(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Aivatiadou E, Ripolone M, Brunetti F, Berruti G. cAMP-Epac2-mediated activation of Rap1 in developing male germ cells: RA-RhoGAP as a possible direct down-stream effector. Mol Reprod Dev. 2008 doi: 10.1002/mrd.20963. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Ahmed Z, Lorber B, Douglas M, Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008;26:147–174. [PubMed] [Google Scholar]
- Besharse JC, Dunis DA, Burnside B. Effects of cyclic adenosine 3′,5′-monophosphate on photoreceptor disc shedding and retinomotor movement. Inhibition of rod shedding and stimulation of cone elongation. J Gen Physiol. 1982;79:775–790. doi: 10.1085/jgp.79.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest Ophthalmol Vis Sci. 1984;25:546–557. [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bragadottir R, Jarkman S. A cyclic adenosine monophosphate agonist elevates the b- and c-waves of the rabbit direct-current electroretinogram. Doc Ophthalmol. 1995;90:291–303. doi: 10.1007/BF01203864. [DOI] [PubMed] [Google Scholar]
- Brock M, Nickel AC, Madziar B, Blusztajn JK, Berse B. Differential regulation of the high affinity choline transporter and the cholinergic locus by cAMP signaling pathways. Brain Res. 2007;1145:1–10. doi: 10.1016/j.brainres.2007.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli G, Spano P, Barlati S, Guarneri B, Barbon A, Bresciani R, Pizzi M. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chierzi S, Ratto GM, Verma P, Fawcett JW. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci. 2005;21:2051–2062. doi: 10.1111/j.1460-9568.2005.04066.x. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim JA, Joo CK. Activation of MAPK and CREB by GM1 induces survival of RGCs in the retina with axotomized nerve. Invest Ophthalmol Vis Sci. 2003;44:1747–1752. doi: 10.1167/iovs.01-0886. [DOI] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res. 1995;42:131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstatter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: comparison with Bassoon. J Comp Neurol. 2001;439:224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- Ding JD, Weinberg RJ. Distribution of soluble guanylyl cyclase in rat retina. J Comp Neurol. 2007;500:734–745. doi: 10.1002/cne.21206. [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger B, Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971;33:157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007;503:182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Firth SI, Wang CT, Feller MB. Retinal waves: mechanisms and function in visual system development. Cell Calcium. 2005;37:425–432. doi: 10.1016/j.ceca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18:3767–3778. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumara F, Giovedi S, Menegon A, Milanese C, Merlo D, Montarolo PG, Valtorta F, Benfenati F, Ghirardi M. Phosphorylation by cAMP-dependent protein kinase is essential for synapsin-induced enhancement of neurotransmitter release in invertebrate neurons. J Cell Sci. 2004;117:5145–5154. doi: 10.1242/jcs.01388. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2.Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grunert U, Wassle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Horner TJ, Osawa S, Schaller MD, Weiss ER. Phosphorylation of GRK1 and GRK7 by cAMP-dependent protein kinase attenuates their enzymatic activities. J Biol Chem. 2005;280:28241–28250. doi: 10.1074/jbc.M505117200. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- Huston E, Lynch MJ, Mohamed A, Collins DM, Hill EV, MacLeod R, Krause E, Baillie GS, Houslay MD. EPAC and PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:12791–12796. doi: 10.1073/pnas.0805167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller F. Retinal bipolar cell types differ in their inventory of ion channels. Vis Neurosci. 2006;23:143–154. doi: 10.1017/S0952523806232048. [DOI] [PubMed] [Google Scholar]
- Jaken S, Kiley SC. Purification and characterization of three types of protein kinase C from rabbit brain cytosol. Proc Natl Acad Sci U S A. 1987;84:4418–4422. doi: 10.1073/pnas.84.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135. [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Tibbs GR. Antagonists of cyclic nucleotide-gated channels and molecular mapping of their site of action. J Neurosci. 1996;16:1285–1293. doi: 10.1523/JNEUROSCI.16-04-01285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach KL, Powers EA, McGuire JC, Dong L, Kiley SC, Jaken S. Monoclonal antibodies specific for type 3 protein kinase C recognize distinct domains of protein kinase C and inhibit in vitro functional activity. J Biol Chem. 1988;263:13223–13230. [PubMed] [Google Scholar]
- Lehre KP, Davanger S, Danbolt NC. Localization of the glutamate transporter protein GLAST in rat retina. Brain Res. 1997;744:129–137. doi: 10.1016/s0006-8993(96)01022-0. [DOI] [PubMed] [Google Scholar]
- Li J, O’Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM, Jr, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol. 2007;21:159–171. doi: 10.1210/me.2006-0340. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J Neurosci. 1999;19:10221–10227. doi: 10.1523/JNEUROSCI.19-23-10221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, Martins RA, Silveira MS. Control of programmed cell death by neurotransmitters and neuropeptides in the developing mammalian retina. Prog Retin Eye Res. 2005;24:457–491. doi: 10.1016/j.preteyeres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Linden R, Perry VH. Massive retinotectal projection in rats. Brain Res. 1983;272:145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- Liu HH, Brady ST. cAMP, tubulin, axonal transport, and regeneration. Exp Neurol. 2004;189:199–203. doi: 10.1016/j.expneurol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Maguire G, Werblin F. Dopamine enhances a glutamate-gated ionic current in OFF bipolar cells of the tiger salamander retina. J Neurosci. 1994;14:6094–6101. doi: 10.1523/JNEUROSCI.14-10-06094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Muller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502:1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492:495–509. doi: 10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Mogi K, Kawano M, Fukui Y, Takeda J, Nogami H, Hisano S. Differential expression of two distinct vesicular glutamate transporters in the rat retina. Neuroreport. 2002;13:1925–1928. doi: 10.1097/00001756-200210280-00019. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis. 2007;13:2163–2182. [PubMed] [Google Scholar]
- Monsul NT, Geisendorfer AR, Han PJ, Banik R, Pease ME, Skolasky RL, Jr, Hoffman PN. Intraocular injection of dibutyryl cyclic AMP promotes axon regeneration in rat optic nerve. Exp Neurol. 2004;186:124–133. doi: 10.1016/S0014-4886(03)00311-X. [DOI] [PubMed] [Google Scholar]
- Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci. 2003;17:2084–2096. doi: 10.1046/j.1460-9568.2003.02634.x. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Oi H, Partida GJ, Lee SC, Ishida AT. HCN4-like immunoreactivity in rat retinal ganglion cells. Vis Neurosci. 2008;25:95–102. doi: 10.1017/S095252380808005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Burlington, MA: Elsevier Academic; 2005. [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Walker M. Amacrine cells, displaced amacrine cells and interplexiform cells in the retina of the rat. Proc R Soc Lond B Biol Sci. 1980;208:415–431. doi: 10.1098/rspb.1980.0060. [DOI] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol. 2004;476:254–266. doi: 10.1002/cne.20207. [DOI] [PubMed] [Google Scholar]
- Rabie A, Thomasset M, Parkes CO, Clavel MC. Immunocytochemical detection of 28 000-MW calcium-binding protein in horizontal cells of the rat retina. Cell Tissue Res. 1985;240:493–496. doi: 10.1007/BF00222366. [DOI] [PubMed] [Google Scholar]
- Rauen T, Rothstein JD, Wassle H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res. 1996;286:325–336. doi: 10.1007/s004410050702. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenburg MD. Muller cell localisation of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Rivera N, Lugo N. Four retinal ganglion cell types that project to the superior colliculus in the thirteen-lined ground squirrel (Spermophilus tridecemlineatus) J Comp Neurol. 1998;396:105–120. doi: 10.1002/(sici)1096-9861(19980622)396:1<105::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Santone R, Giorgi M, Maccarone R, Basso M, Deplano S, Bisti S. Gene expression and protein localization of calmodulin-dependent phosphodiesterase in adult rat retina. J Neurosci Res. 2006;84:1020–1026. doi: 10.1002/jnr.21009. [DOI] [PubMed] [Google Scholar]
- Santschi L, Reyes-Harde M, Stanton PK. Chemically induced, activity-independent LTD elicited by simultaneous activation of PKG and inhibition of PKA. J Neurophysiol. 1999;82:1577–1589. doi: 10.1152/jn.1999.82.3.1577. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, Fagni L. Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol. 2009;587:101–113. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- Varella MH, de Mello FG, Linden R. Evidence for an antiapoptotic role of dopamine in developing retinal tissue. J Neurochem. 1999;73:485–492. doi: 10.1046/j.1471-4159.1999.0730485.x. [DOI] [PubMed] [Google Scholar]
- Vermeiren C, Najimi M, Maloteaux JM, Hermans E. Molecular and functional characterisation of glutamate transporters in rat cortical astrocytes exposed to a defined combination of growth factors during in vitro differentiation. Neurochem Int. 2005;46:137–147. doi: 10.1016/j.neuint.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986;248:19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- Wang LY, Salter MW, MacDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- Whitaker CM, Cooper NGF. Characterization of Phosphodiesterase 4 Within the Adult Rat Retina. Investigative Ophthalmology & Visual Science. 2007;48 E-Abstract 3047. [Google Scholar]
- White RD, Neal MJ. The uptake of L-glutamate by the retina. Brain Res. 1976;111:79–93. doi: 10.1016/0006-8993(76)91050-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]