Abstract
Anaplasma phagocytophilum is the causative agent of Tick-Borne Fever in small ruminants and has been identified as the zoonotic agent of human granulocytic anaplasmosis. The Norwegian strains of the rickettsia are naturally persistent in lambs and represent a suitable experimental system for analysing the mechanisms of persistence. Variation of the outer membrane protein MSP2(P44) by recombination of variable pseudogene segments into an expression site is believed to play a key role in persistence of the organism. The goal of the present study was to analyse the dynamics of the immune response towards A. phagocytophilum and MSP2(P44) during persistent infection of lambs. Responses to the hypervariable region of MSP2(P44) were detected shortly after appearance of the respective variants in cyclic rickettsemic peaks, consistent with a process of antigenic variation. In addition, there was a diminishing antibody response to MSP2(P44) and to other A. phagocytophilum antigens overall with time of infection, that was not associated with clearance of the infection.
Keywords: Anaplasma phagocytophilum, immune evasion, tick borne fever, sheep diseases, zoonosis, antigenic variation
Introduction
Anaplasma phagocytophilum (Ap) is the causative agent of Tick-Borne Fever in small ruminants and has been verified as the zoonotic agent of human granulocytic anaplasmosis (Bakken and Dumler, 2000; Dumler et al., 2007; Stuen, 2007; Woldehiwet, 2006). The disease has been described in sheep in Norway for at least 200 years, but human cases have been diagnosed within recent years (Bakken and Dumler, 2006; Bakken et al., 1996; Stuen and Bergstrom, 2001). Ap is an obligate intracellular rickettsia with primary tropism for neutrophil granulocytes (Granick et al., 2008; Woldehiwet et al., 2003). It has the ability to cause persistent infections in animals that can last for several years (Dumler et al., 2003; Dumler et al., 2001). The immunodominant MSP2(P44) outer membrane protein (OMP) of Ap is transcribed from a single expression site within the chromosome and this expression site is orthologous to that encoding the MSP2 OMP of A. marginale (Barbet et al., 2003). The msp2(p44) expression site encodes conserved N – and C termini of MSP2(P44) and a central hypervariable region (HVR) and is similar in structure among all Ap strains (Barbet et al., 2006). The genome of Ap consists of more than 100 msp2(p44) paralogs (Hotopp et al., 2006; Nelson et al., 2008), many of which are truncated, but most of which contain the HVR and some conserved flanking sequence. Segments of these functional pseudogenes are inserted into the msp2(p44) expression site by gene combinatorial conversion mechanisms (Barbet et al., 2003). Antigenic variation in the msp2(p44) gene is believed to have a major responsibility for the persistence of A. marginale and Ap (Brayton et al., 2001; Granquist et al., 2008). During the infection, modulation of antigenic properties would be expected to result in an altered immune response towards the constantly changing antigen. The mechanism of variation seems to be an intrinsic property of the bacterium and is controlled by RecF mediated gene conversion, most likely produced by insertion of partial or complete donor pseudogene sequences into the expression site as described for A. marginale (Brayton et al., 2002; Wang et al., 2004). However, in the absence of host immune responses as occurs in vitro with HL-60 cell cultures, homogenous expression profiles of the MSP2(P44) may be observed (Sarkar et al., 2008; Wang et al., 2004). A previous study revealed sequence variation of the expressed msp2(p44)gene in different peaks of rickettsemia during persistent infection in lambs (Granquist et al., 2008). The goal of the present study was to analyse the immune response towards Ap and MSP2(P44) during the persistent infection of lambs. We aimed to determine whether different MSP2(P44) variants were recognized at different times post-infection, as would be predicted by the prior genetic studies (Granquist et al., 2008). We also investigated how the rapidly changing OMPs might affect the overall dynamics of antibody responses to Ap during the different peaks of rickettsemia using different immunodiagnostic methods that have been proposed for detection of this organism in different animal species.
Materials and methods
Experimental infection of naive lambs and blood sampling
Four lambs were raised in an indoor environment, with barriers against tick entry and tick infestation. Two lambs (4203 and 4210) referred to as #1 and #2 were experimentally infected by intravenous injection of the Norwegian 16S rRNA variant 1 (GenBank no M73220) of Ap at an infection dose of approximately 2.0×106 infected neutrophils. The inoculum had been stored in 10% DMSO at −80°C. Two lambs were kept as negative controls throughout the sampling period of three months. All four lambs were examined and found negative for Mycoplasma ovis (formerly Eperythrozoon ovis) by blood smear analysis before inoculation. The rectal temperature and clinical status was recorded daily throughout the experimental period. EDTA blood samples were collected from the jugular vein. Samples were taken on day 0 of the infection and every second day for a three month period. One EDTA blood sample from each animal was used for blood smear analysis and differential blood cell counts (ADVIA, Bayer). The other was frozen at −75°C for later PCR analysis. The blood smears were stained with May Grünwald Giemsa for visual confirmation and quantitative assessment of infection using oil immersion microscopy (Stuen et al., 2003). Serum samples were collected on the day of infection and then weekly during the entire experimental period. The serum was separated from the cell fraction and frozen at −20°C for indirect fluorescent-antibody assay, ELISA and immunoblotting. The experiment was thoroughly planned and approved by the competent person at the animal experiment unit belonging to the Norwegian School of Veterinary Science, Section for Small Ruminant Research. The competent person and the facilities were approved by the National Animal Research Authority (Norway).
Real time PCR for relative quantification and identification of positive samples, targeting the msp2(p44) gene
DNA from EDTA blood was isolated using a QIAamp Mini kit™ (QIAGEN). Detection and selection of samples positive by PCR for further cloning and restriction fragment length polymorphism pattern (RFLP) analyzes was performed by Real Time PCR (Lightcycler 2.0, Roche), as described previously (Granquist et al., 2008).
Cloning and analysis of 2kb msp2/p44 expression site using the TOPO™ plasmid vector
DNA was isolated using a QIAamp DNA Blood Mini Kit ™ , as described by the manufacturer. A 2kb sequence of the msp2(p44) expression site of Ap was amplified and cloned that included the central hypervariable region flanked by semiconserved and conserved 5’ and 3’ ends. The primers were (AB1221) 5’-ATA GAA CAA GAG CAG GGA GAA GAC-3’ and (AB1227) 5’-TCT GTC TTG GAG AGT ATT GAG TC-3’ diluted to a concentration of 4µM as previously described (Granquist et al., 2008). The 2kb cloned PCR products were analyzed by single digestion with EcoRI to release inserted DNA and determine the presence and size of cloned fragments and by double digestions with EcoRI and RsaI to generate diagnostic RFLP patterns from each clone. Clones from each rickettsemic peak were selected for DNA sequencing based on the RFLP patterns obtained (Granquist et al., 2008).
Immunoblots
Gel electrophoresis used a 15% SDS-PAGE separating gel with a 4% stacking gel. The antigen was RF6A endothelial cells infected with the NY-18 strain of Ap and solubilized in SDS gel sample buffer by heating at 100 °C for 5 minutes. The electrophoretic separation was achieved with a Bio-Rad PowerPac 3000 at 300 Volts for 43 minutes. The transfer was done overnight (15 hours) at 30 Volts and increased to 70 Volts in the morning (two hours). The membranes were cut into 17 strips and 2.5 ml of serum (1:1000 dilution) was added to each strip. Pre-serum from dogs were applied to strip one as a negative control while dog postinfection serum was applied to strip two as a positive control. Pre-serum from lamb #1 was added to strip three. Strips four through 17 were incubated with post-peak sera collected at intervals after the peaks of rickettsemia from lamb #1. The same incubations were repeated for lamb #2. The sera were allowed to bind to antigens for one hour, then strips were washed three times before secondary antibodies (goat anti-dog and rabbit anti -sheep) were applied to strips at a dilution of 1:150000 (Kirkegaard and Perry Laboratories). The secondary antibodies were allowed to bind for 90 minutes, then washed three times. For chemiluminescence, West Dura (Pierce) was used to expose the membrane strips after an incubation time of five minutes. Exposure on radiographic film was done for 30 sec., one min, one hour and over night.
SNAP® 4Dx® test (IDEXX Laboratories)
The sheep sera were tested with a SNAP 4Dx test developed for detection of Dirofilaria immitis antigen, Ehrlichia canis antibodies, Borrelia burgdorferi antibodies and Ap antibodies in dog serum, plasma or whole blood (IDEXX Laboratories Inc., Westbrook, ME). 15 serum samples from each of the two lambs (#1 and #2) including presera and post-peak sera collected during the three month long infection trial were tested on individual SNAP 4Dx tests. The test was performed according to the manufacturer’s instructions (IDEXX laboratories).
Synthetic peptides and dot blot analysis
The sequences of the predominant MSP2(P44) HVR peptides present in each different rickettsemic peak have been reported previously (Granquist et al., 2008). Analysis of these HVR sequences by PEPTIDESTRUCTURE and PLOTSIMILARITY (Wisconsin Package, Accelrys, Inc., San Diego, CA) showed three major regions of variability separated by amino acids conserved between all HVRs (Fig. 1). We hypothesized that these three hypervariable regions were likely to correspond to exposed epitopes recognized during infection as they also corresponded to regions predicted to be B-cell epitopes that were surface-exposed and hydrophilic. Seven different peptides were synthesized and designated pep1 through pep7. Pep1 through pep5 were 29-mers synthesised from the first hypervariable segment immediately preceding a conserved cysteine from each of the five rickettsemic peaks collected from lamb #1. Pep6 and pep7 were a 26 -and 17-mer respectively and were synthesised from the second and third HVR segment from the second peak in lamb #1 in order to test the responsiveness of serum to different antigenic regions (Fig. 1). These peptides are:
pep1, TPGSSEHFAIYKEETINEGSSTNKSEVAV;
pep2, NSSNSNKPAYGQYAEATGTKSNSDGNTAL;
pep3, TGGNKDKSFGKYAVKTTAKGTGNDNGTSL;
pep4, TENTGGKQGFGVYAETTNVQTSAKSDVAL;
pep5, GYAAVGTARGKEYAKVPDDTGGSKGHTSQ;
pep6, GDKGHTNSSAQAISAGHVNTPQVLRD;
pep7, TSTKKEGGPSDNKNDNA.
Fig. 1. Variant-specific synthetic peptides used for detection of antibody responses.
The amino acid sequences of the MSP2(P44) hypervariable regions (HVRs) from the major variants expressed in each peak of rickettsemia from lamb #1 (1._variants) and #2 (2._variants) are shown. The underlined areas define the synthetic peptide sequences produced from these variants and used in immunoblot assays for detection of peptide-specific antibody responses during infection. The percentage of each variant population in each rickettsemic peak is also shown and (below) a consensus sequence of this MSP2(P44) region. Lower case letters on the consensus line indicate: b, big; c, charged; h, hydrophobic; l, aliphatic; p, polar; s, small; t, tiny. Similar variants present in both lambs #1 and #2 are indicated by identical superscripts to the right of the sequences.
Serum responses were analyzed by performing a dot blot analysis. The analysis was conducted for both animals in order to compare the responses against shared HVR variants in the two animals. Two µl (10µg) from each of the seven synthetic peptides were spotted onto a nitrocellulose membrane together with two negative (C1 and C2) and one positive control peptides (C3). The negative control peptides were C1: DMNRKKSFEPKKQIGLRTNYC (sequence preceding the C residue is from MAP1-2 of Ehrlichia ruminantium), C2: DSSSAGGQQQESSVSSQSDQASTSSQLG (MSP1A of Anaplasma marginale) and the positive control peptide, C3 was: IGYERFKTKGIRDSGSKEDEADTC (sequence preceding the C residue was from the conserved region of Ap MSP2(P44), strongly predicted to include a B cell epitope). The sera were also tested against a whole NY-18 strain Ap antigen derived from infected HL-60 cell culture, prepared by solubilizing organisms in SDS gel sample buffer as for immunoblots. After blocking of non-specific binding using 5% BSA in TBS-T for one hour, the primary serum was applied to the spots. One preserum and 14 post sera corresponding to numbers of weeks after infection were tested against each peptide at a dilution of 1:167. HRP labelled secondary anti-sheep antibodies and Super Signal West Femto (Pierce Chemical, Rockford, IL) were used for detection of bound serum antibodies. An integrated densitogram was used for quantitative analysis of the dot blot (Image J, NIH, USA). The background was substracted and the rolling ball radius set to 25 pixels before the blot images were inverted. An area of 489 square pixels was measured for each dot.
Indirect immunofluorescence antibody assay
Briefly, an indirect immunofluorescence antibody assay (IFA) was used to determine the developing antibody titres to A. phagocytophilum (horse isolate) in the serum samples collected over time. Two-fold dilutions of sera were added to slides precoated with Ap antigen (Protatek International and Organon Teknika). Bound antibodies were visualized by fluorescein-isothiocyanate (FITC)-conjugated rabbit-anti-sheep immunoglobulin (Cappel, Organon Teknika). Sera were screened for antibodies at dilution 1:40. If positive, the serum was further diluted and retested. A titre of 40 or more was regarded as positive (Stuen and Bergstrom, 2001). The titre values were plotted in a graph for presentation.
Recombinant MSP5 ELISA
The ELISA assay was conducted as described (Alleman et al., 2006). Briefly, microtiter plates were coated with 100 µl per well of purified rMSP5 (2 µg/ml in 0.05 M carbonate-bicarbonate buffer, pH 9.6). The wells were blocked with 1% (wt/vol) bovine serum albumin (BSA), washed and incubated with test sera at 1:100 dilutions, washed again, incubated with alkaline phosphatase –conjugated anti-sheep IgG at a dilution of 1:5000 in 1% BSA. The wells were washed again and colour developed with 1mg/ml p-nitrophenylphosphate. Optical density was measured at 405 nm after a 30 minute incubation period. Each sample was analysed in duplicate and the final absorbance reading was recorded as a mean of the duplicated readings. Six negative control serum samples were used at dilutions identical to test samples in every assay to establish the cut off values for determining whether a serum sample was positive or negative. Samples were considered positive if the optical density was greater than 3 standard deviations above the mean of all negative serum samples used. This represents the upper limit of the 99% confidence interval of the mean optical density of the all negative controls. The OD values were plotted to demonstrate developing antibody response to the rMSP5 over time when using serum samples at a single dilution. This semi-quantitative analysis was done to determine if there was a persistent antibody response to a conserved antigen of A. phagocytophilum. Quantitative analysis using antibody titres to MSP5 was not performed.
Results
Animals and infection
As described previously (Granquist et al., 2008), the inoculated lambs (#1 and #2) that were observed for 100 days reacted with typical signs of infection. They showed inappetence and depression for one to two days and high fever (41.8°C and 41.5°C in lambs #1 and #2 respectively) at days four and five. They experienced neutropenia (<0.7 × 109 cells/litre) for 10 days from about day 13 post infection (p.i). The real time PCR analysis of the collected EDTA blood samples taken every second day during the whole experimental period showed a cyclic pattern with rickettsemia in the two infected animals. These peaks of rickettsemia were interrupted by negative samples (real-time PCR cycle threshold >35). Cloning and sequencing was performed from time points corresponding to five peaks in lamb #1 and four peaks in lamb #2. These data revealed the presence of multiple and different variants of MSP2(P44) in each peak, as described previously (Granquist et al., 2008).
Antibody reactions to synthetic peptides encoding the MSP2 (HVR)
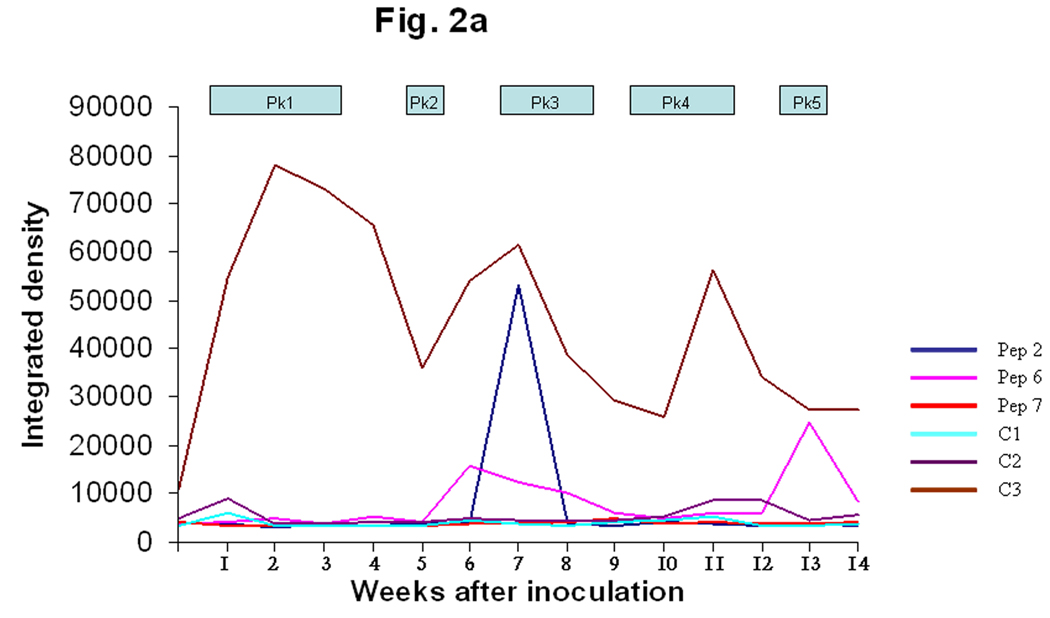
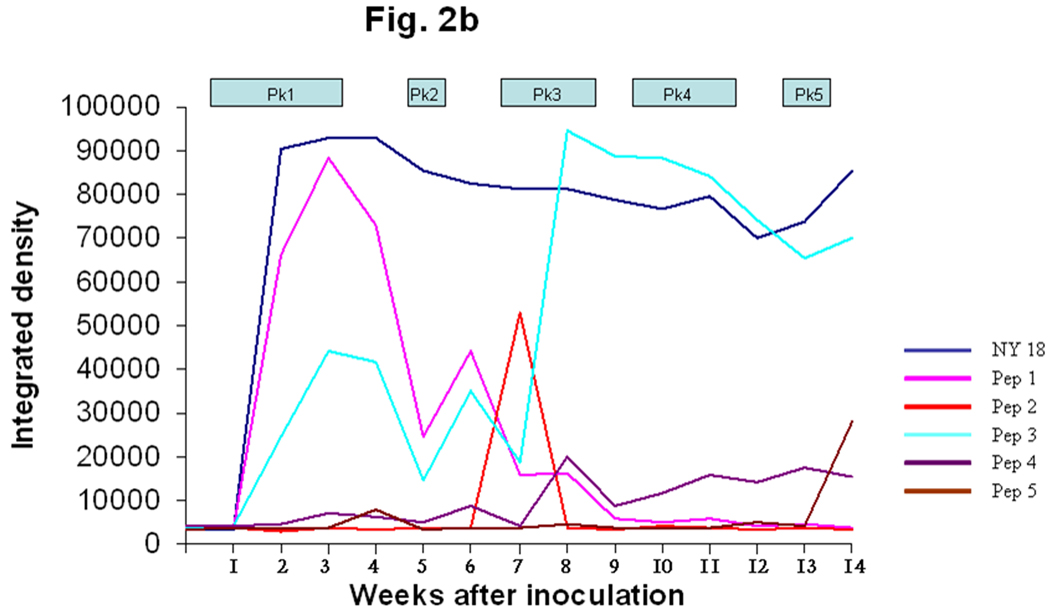
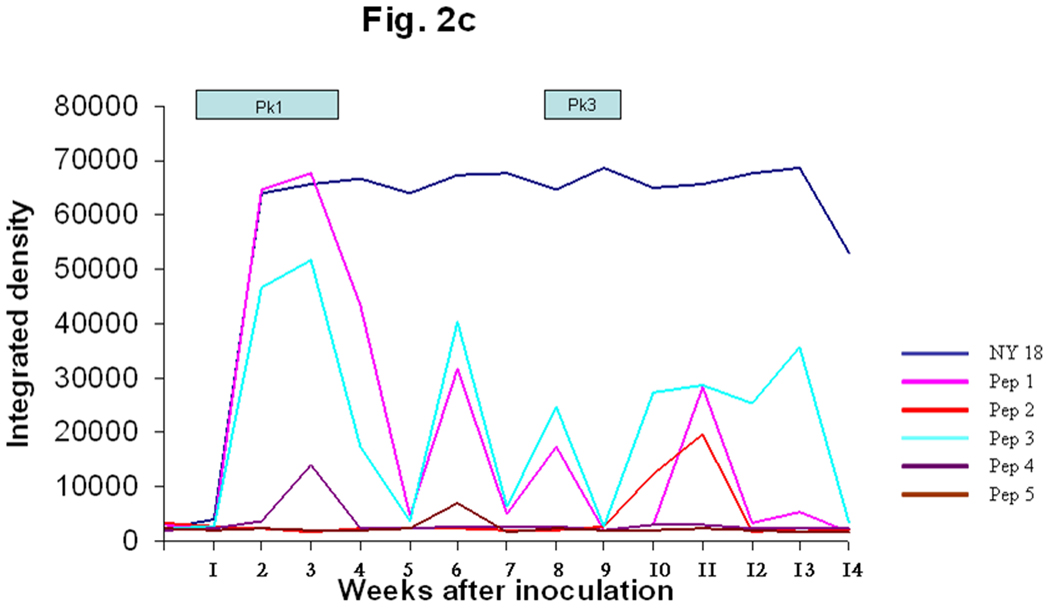
The N- and C termini of the MSP2(P44) sequences were relatively conserved and showed similarities to framework residues of US variants (Fig. 1) when compared with the GenBank database (Granquist et al., 2008). Three MSP2(P44) HVR segments were selected for peptide synthesis based on the likelihood of being both surface-exposed and a B-cell epitope. The first HVR segment was a 29 amino acid peptide preceding a conserved cysteine (peps 1–5). Two additional peptides were tested representing different segments of the HVR expressed in peak 2 (peps 6 and 7). The initial data from lamb #1 suggested that the first and longest HVR segment reacted most strongly with infection serum (Fig. 1 and Fig 2). A weak response to the second hypervariable region segment (pep6) was also observed from week seven through the end of the experiment (Fig. 2a). The negative controls (C1 and C2) did not react with the serum and a positive control peptide (C3), which was produced from an N-terminal conserved region of MSP2(P44), did react from week two (Fig. 2a), with a series of peaks. These data suggested that the conserved N-terminal region and the first hypervariable region segment preceding the conserved cysteine residue (Fig. 1) were likely to be recognized. Due to the different possible alignments of HVRs in this region and our desire to compare equivalent regions of each HVR it was decided to synthesize 29-mer peptides encoded by the 29 amino acids preceding the conserved cysteine in all cases, without allowing for gaps. The integrated densitogram analysis of the dot blots against each peptide (Fig. 2b and 2c) shows the serum response of both animals to the different peptides over time. There was a response detected to the HVR peptides denoted pep1 to pep5 which varied with time of infection. Peptide 1 from infected lamb #1 was expressed during the first, second and third week of infection in both lambs #1 and #2. Both animals responded to peptide 1 by two weeks after inoculation and the peak response was in week four (Figs 2b and 2c) of the infection. Both animals showed secondary reactions to this peptide later in infection also, possibly suggesting re-expression of the peptide at later time points. Peptide 2 was expressed during rickettsemic peak 2 (weeks 5– 6) in lamb #1 and during peak 3 (weeks 8–10) in lamb #2 (Fig. 1). Both animals responded to peptide 2 subsequent to its detection in rickettsemic peaks, before declining to baseline levels (Fig. 2b and 2c). Peptide 3 was expressed in rickettsemic peak 3 (week 7– 9) in lamb #1 and was not observed among the expression site clones from lamb #2 (Granquist et al., 2008). There was a moderate response towards peptide 3 during weeks 4–8 and a very strong response subsequent to its detection in rickettsemic peak 3 of lamb #1 (Fig. 2b). Despite lack of detection of peptide 3 in rickettsemic peaks, lamb #2 showed several peaks of response to this peptide (Fig. 2c). Only minor responses were observed against peptides 4 and 5 in both animals. For comparison, we tested the response of animals to SDS-solubilised Ap antigen in similar dot blots. There was no response to Ap antigen with application of presera, but both animals responded strongly to the NY18 Ap lysate antigen during and subsequent to the third week of infection (Fig 2b and 2c). The response was persistent until the end of the experiment with only minor decline in intensity of reaction towards the end of the experimental period. In summary, the strongest anti-variable region responses were to peptides expressed early in infection and the strongest responses occurred soon after the respective variants were detected in rickettsemic peaks, except for peptide 3 in lamb #2. It is possible that we did not detect expression of peptide 3 in lamb #2 because of the statistical predominance of other variable region clones at the times sampled.
Fig. 2. Variant-specific antibody responses of infected animals.
A. Serum responses of lamb #1 towards three different HVR sequences expressed during the second rickettsemic peak in lamb #1 are shown on an integrated densitogram produced from dot blots on a time scale corresponding to the duration of the experimental infection. Pep2 is the first HVR immediately preceding a conserved cysteine, pep6 and pep7 are the second and third HVR. The peptide sequences are as shown in Fig. 1, C1 and C2 are negative control peptides from E. ruminantium and A. marginale respectively, while C3 is a positive control peptide from the conserved region of A.phagocytophilum MSP2(P44). Sera were collected from lamb #1 every week during the experimental infection. The times when coding sequences for the respective variants were detected in the MSP2(P44) expression site are indicated by Pk1, Pk2 etc.
B The figure shows the immune response of lamb #1 towards the major HVR variants expressed during each peak of rickettsemia in lamb #1. The figure also shows the antibody responses towards NY 18 strain Ap antigen. Pep1 through 5 are synthesized from the first HVR of the major MSP2 (P44) species present in rickettsemic peaks 1 through 5, respectively.
C. The figure shows the immune response of lamb #2 towards the major HVR variants expressed in lamb #1. The figure also shows the antibody responses towards NY 18 strain Ap antigen. Pep1 was present in both animals during rickettsemic peak 1. Pep2 was expressed during the second rickettsemic peak in lamb #1 and the third peak of lamb #2. Peps 3–5 were not detected as expressed in lamb #2.
Antibody responses detected by Western blot
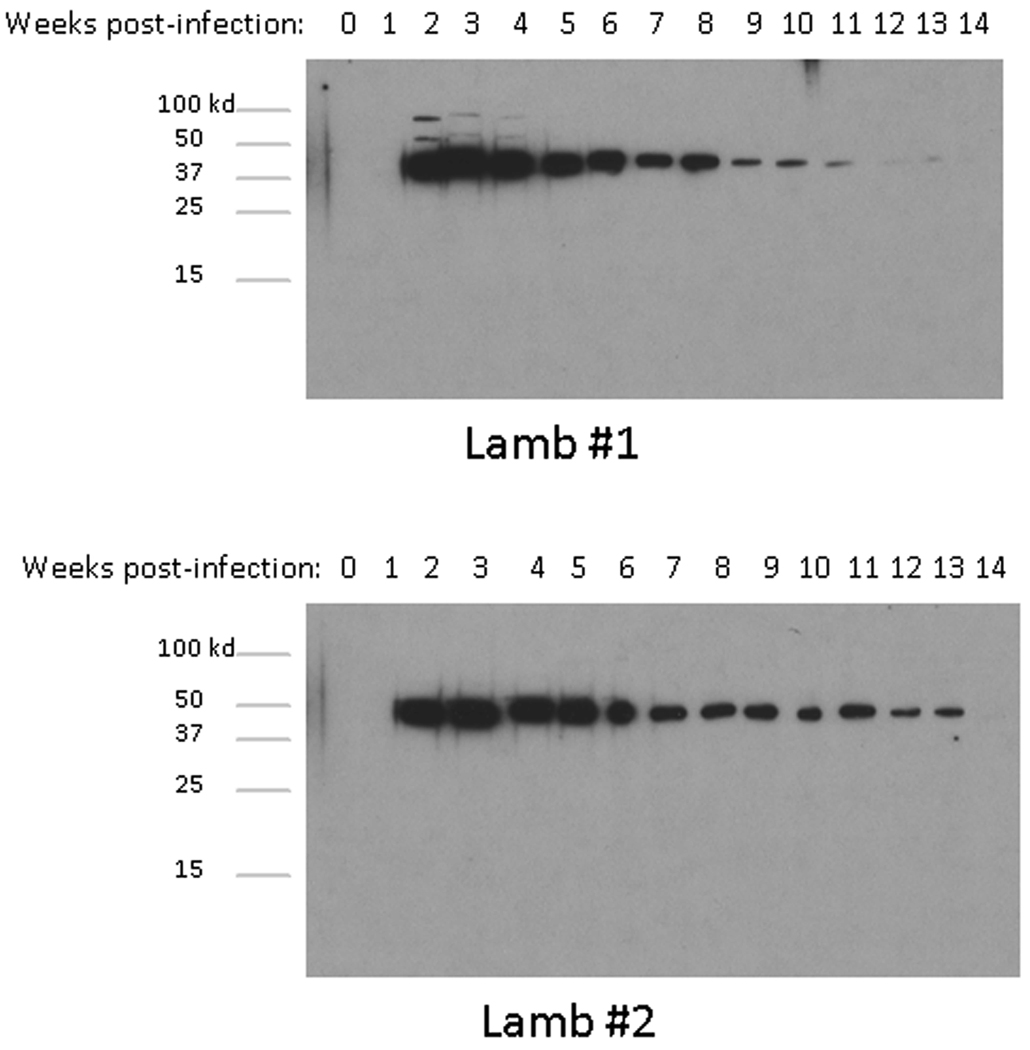
The Norwegian strain of Ap has not yet been successfully cultured in vitro. However, cultured Ap antigen is available from U.S. human strains. The 15 serum samples from animals #1 and #2 were tested against the NY 18 strain of Ap together with a preserum and a positive control serum from a dog experimentally infected with the NY 18 strain. The dog preserum showed no response against the antigen whereas the positive dog serum reacted with a protein of about 44 kDa, presumably MSP2 (P44), and two others between 75 and 150 kDa (data not shown). The sheep seroconverted in the third week of infection. The presera and the sera collected during the first week p.i. showed no reaction with the NY-18 strain. From day 14 p.i., the sera showed positive responses against the 44Kd protein, of the NY-18 extract in both lambs until the 13th week p.i. The response, although positive, gradually decreased towards the end of the experimental period (Fig.3). By 14 weeks p.i., it was not possible to detect a positive reaction, although the animals were still infected, as detected by real time PCR assays (data not shown). In addition to the 44-kDa band, the serum collected from lamb #1 during weeks two to four p.i reacted positively with two proteins between 50- and 75 kDa.
Fig. 3. A diminishing antibody response in immunoblots to MSP2(P44) with time of infection.
Immunoblots show the antibody responses to NY 18 Ap antigen from infected endothelial cell culture, using serum collected weekly from lamb #1 and #2 during the experimental infection. All sera were tested at a dilution of 1:1000.
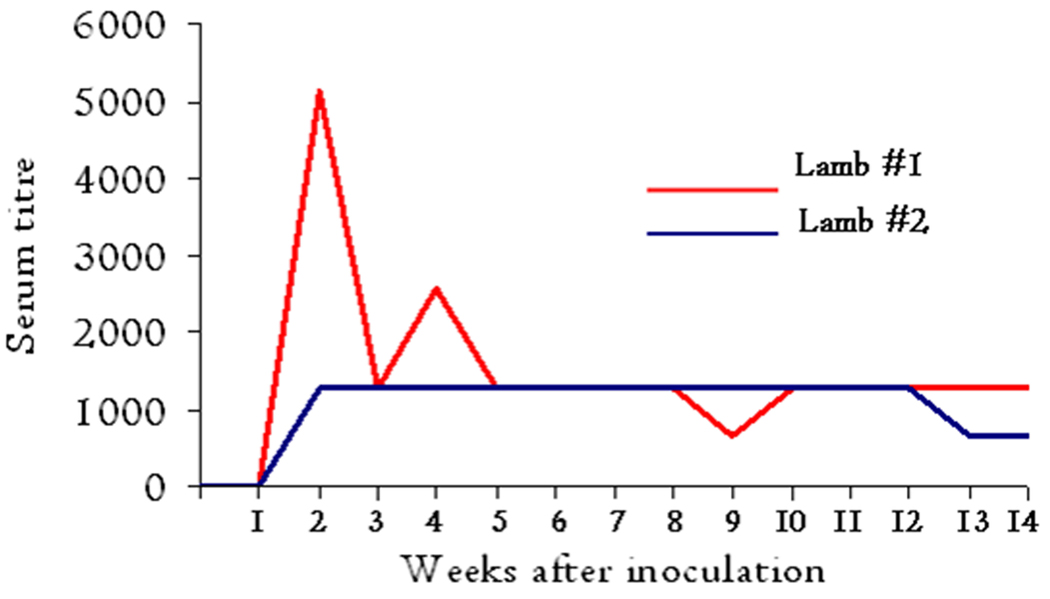
Immunofluorescence assay (IFA)
The assay showed no serum titre development with the presera and samples taken one week p.i. Both animals seroconverted during the 2nd week of infection and lamb #1 showed an elevated titre of 1:5120 at two weeks (Fig. 4), immediately after the appearance of the first peak of rickettsemia. A second antibody response appeared in lamb #1 at four weeks post infection. The serum collected from lamb #2 showed a titre of 1:1280 from the third week of infection until week 12 p.i., before declining to 1:640 until the end of the experimental period.
Fig. 4. Diminishing antibody responses to A. phagocytophilum in immunofluorescence assay.
Indirect fluorescent antibody test shows the development of serum titre responses to a horse isolate of A. phagocytophilum during the experimental infection of lambs #1 and #2.
SNAP 4Dx assay
The SNAP 4Dx test has been manufactured for detection of canine Ap infection, but has not yet been tested in sheep infections. The SNAP test was negative for both animals with the presera and sera collected during the second week of infection. A clear positive result was detected from the third through the sixth week of the infection. In the seventh week p.i., only lamb #1 was clearly positive. Both animals were negative by the SNAP test from week eight until the end of the experimental period (data not shown).
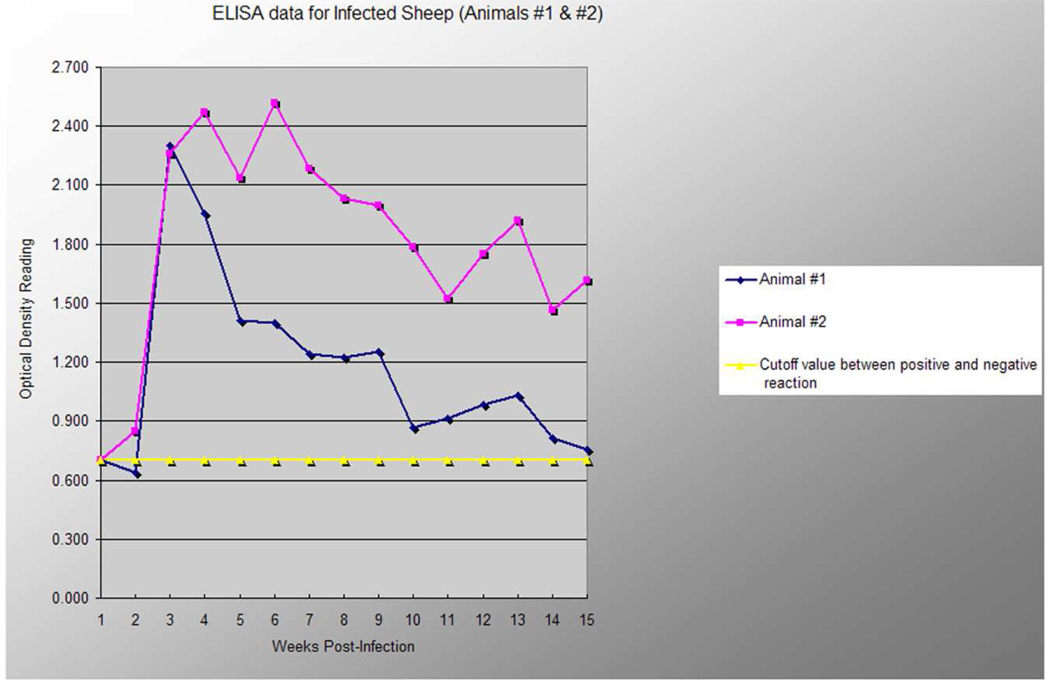
ELISA against recombinant MSP5
The assay was performed in order to analyze the temporal immune responses towards a conserved outer membrane protein that does not undergo antigenic variation during persistent infection and has been proposed for diagnosis of Ap infections of humans and dogs (Alleman et al., 2006). The assay showed an increasing serum response in both lambs during the second week after infection. High OD readings were obtained from the second week until week nine p.i with a decrease in response after week nine in lamb #1, towards the end of the experimental period (Fig. 5). Both animals remained seropositive during the entire trial period, however, the serum response was more sustained at the end of the experimental period in lamb #2.
Fig. 5. Diminishing antibody responses to rMSP5 of A. phagocytophilum with time of infection.
A line graph representing the rMSP5 ELISA optical density readings (y axis) on serum samples collected on day 0 through week 15 (x axis) from two lambs experimentally inoculated with viable A. phagocytophilum organisms. The horizontal line demarcates the optical density reading that represents the cutoff between a positive and negative reaction (upper limit of 3 standard deviations above the mean of all negative control samples).
Discussion
Experimental systems with lambs housed in controlled environments enable the study of persistent infection with Ap in a natural host. The Norwegian sheep strains of Ap are naturally persistent and it has previously been described that the Norwegian strains possess an msp2(p44) expression site syntenic to that of U.S. human strains and to the MSP2 of A. marginale (Barbet et al., 2003). Antigenic variation of MSP2 (P44) by combinatorial gene conversion mechanisms and insertion of functional pseudogenes into the expression site is believed to be a mechanism of evading the immune system in Ap as known for A. marginale (Barbet et al., 2000; Barbet et al., 2003; Granquist et al., 2008; Zhuang et al., 2007). The immune response to MSP2(P44) appears to be directed against both conserved and HVR segments of the molecule.. The response is strongest during the initial peaks of rickettsemia, and may decline during the persistent phase of infection. This is similar to previous results with A. marginale where immune responses to HVR sequences were highly dynamic and also strongest during the initial acute phase of infection (Zhuang et al., 2007). Webster and Mitchell have previously shown by counter immunoelectrophoresis that antibodies to Ap persisted for six to ten weeks after infection while Paxton and Scott developed an IFA test that showed a declining antibody response after 18 weeks (Paxton and Scott, 1989; Webster and Mitchell, 1988). These results suggest that differences in sensitivity of various immunological tests may give alternative impressions of the serological responses to this pathogen. Zhuang et al have previously suggested that immune reaction to MSP2(P44) depends on the position of the epitope within the HVR (Zhuang et al., 2007) as a position near to the N-terminus seems to bind more antibodies than those at the C-terminus (50% versus 6%). This is also suggested by the results from this study where two additional HVR regions (P6 and P7) were included that gave only moderate (P6) to no (P7) response with the serum collected from the two animals. The short-lived antibody responses do not appear to be associated with clearance of the organisms, as the 5th rickettsemic peak in lamb #1 occurred at 93 days p.i. (Granquist et al., 2008).
The present study reveals differences among immunodiagnostic tests that can be used for diagnosis of Ap infection. All tests agree that sero-conversion occurs during the 2nd week p.i. with a rapid increase in serum titres (Fig. 2a, Fig 3 and Fig 4) or serum response (Fig.5). When antigen from the in vitro cultured NY-18 strain is used in Western blots, a dominant response against a 44 kDa protein, presumably MSP2(P44), is observed. The reaction appears strongest soon after infection (week 3) and gradually diminishes as the persistent phase prevails. One explanation for this phenomenon may be that there is a very limited diversity of the MSP2(P44) in cultured Ap (Scorpio et al., 2004) whilst serum from infected sheep was responding to many rapidly changing antigens. However, data from other diagnostic tests including the SNAP 4DX test, the IFA, and, to a lesser extent, the rMSP5 ELISA also show diminishing responses with time of persistent infection indicating that there is an actual diminishing of serum responses towards the organism during persistent infection. It is known that Ap infection causes immune suppression by reduction of T cell populations in sheep (Woldehiwet, 1991). Whist et al have previously postulated that the impaired antibody response to both Ap antigen and other antigens can be due to reductions in B-cells, γδ T-cells, CD4+ T-cells and CD25 expression on lymphocytes (Whist et al., 2003). Another possible explanation of the short lived antibody response is that the infection stimulates production of short lived plasma cells that remain in the spleen rather than long lived plasma cells migrating to the bone marrow (Zhuang et al., 2007). The lack of proper antigen –or T cell stimulation of responsive mature IgM, IgD+ B cells may reduce isotype switching thus resulting in an early IgM response without an appropriate IgG response. This may be in part, due to the lack of LPS in the Ap cell wall or lack of CD4+ T cell responses as suggested previously (Chapes and Ganta, 2008; Lin and Rikihisa, 2003). Detailed description of the mechanisms of immunoglobulin secretion by B lymphocytes in response to Ap infection requires further study.
The dot blots show dynamic immune responses associated with the expression of novel MSP2 variants. The peak immune responses to the changing MSP2(P44) peptide sequences generally appeared following the detection of sequences encoding those peptides in the msp2(p44) expression site. These anti HVR peptide responses appear short-lived and fade rapidly thereafter. This shows that the humoral immune response adapts quickly to new variants of MSP2(P44), and specific immunoglobulins are produced against the continuously changing epitopes. Besides showing a fading character of serum responses towards a conserved epitope of the MSP2(P44) OMP, the SNAP 4Dx also reveals cross-reactivity between a US dog strain of Ap and the Norwegian sheep strain. The immunoblots demonstrate a similar serologic cross-reactivity in MSP2(P44) between the Norwegian sheep and a U.S. human strain. This underlines the fact that there are conserved epitopes in MSP2(P44) even between widely geographically separated strains infecting different animal species.
Conclusion
In summary, this study suggests that there are dominant serologic responses to MSP2(P44) in Norwegian sheep persistently infected with Ap, similar to those observed in U.S. human and dog infections. These responses are directed to both epitopes conserved between the different strains and to variable epitopes in the HVR of MSP2(P44). Responses to the HVR appear shortly after the respective MSP2(P44) variants are detected in rickettsemic peaks. This is consistent with the hypothesis that variation in MSP2(P44) is associated with antigenic variation and the cycles of rickettsemia observed during infection. In addition to these dynamic responses to the MSP2(P44) HVR, there is a diminishing response to Ap overall, during the course of infection, which is detected in most diagnostic assays utilising either whole Ap or the conserved recombinant MSP5 antigen.
Acknowledgement
This investigation received support from NIH grant AI 45580 and Haalands legat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleman AR, Barbet AF, Sorenson HL, Strik NI, Wamsley HL, Wong SJ, Chandrashaker R, Gaschen FP, Luckshander N, Bjoersdorff A. Cloning and expression of the gene encoding the major surface protein 5 (MSP5) of Anaplasma phagocytophilum and potential application for serodiagnosis. Veterinary clinical pathology / American Society for Veterinary Clinical Pathology. 2006;35:418–425. doi: 10.1111/j.1939-165x.2006.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554–560. doi: 10.1086/313948. [DOI] [PubMed] [Google Scholar]
- Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Annals of the New York Academy of Sciences. 2006;1078:236–247. doi: 10.1196/annals.1374.042. [DOI] [PubMed] [Google Scholar]
- Bakken JS, Krueth J, Tilden RL, Dumler JS, Kristiansen BE. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1996;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- Barbet AF, Lundgren A, Yi J, Rurangirwa FR, Palmer GH. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infection and immunity. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet AF, Lundgren AM, Alleman AR, Stuen S, Bjoersdorff A, Brown RN, Drazenovich NL, Foley JE. Structure of the expression site reveals global diversity in MSP2 (P44) variants in Anaplasma phagocytophilum. Infection and immunity. 2006;74:6429–6437. doi: 10.1128/IAI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet AF, Meeus PF, Belanger M, Bowie MV, Yi J, Lundgren AM, Alleman AR, Wong SJ, Chu FK, Munderloh UG, Jauron SD. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infection and immunity. 2003;71:1706–1718. doi: 10.1128/IAI.71.4.1706-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Knowles DP, McGuire TC, Palmer GH. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4130–4135. doi: 10.1073/pnas.071056298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Palmer GH, Lundgren A, Yi J, Barbet AF. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Molecular microbiology. 2002;43:1151–1159. doi: 10.1046/j.1365-2958.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- Chapes SK, Ganta RR. Defining the immune response to Ehrlichia species using murine models. Vet Parasitol. 2008;158:344–359. doi: 10.1016/j.vetpar.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Asanovich KM, Bakken JS. Analysis of genetic identity of North American Anaplasma phagocytophilum strains by pulsed-field gel electrophoresis. Journal of clinical microbiology. 2003;41:3392–3394. doi: 10.1128/JCM.41.7.3392-3394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. International journal of systematic and evolutionary microbiology. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45 Suppl 1:S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- Granick JL, Reneer DV, Carlyon JA, Borjesson DL. Anaplasma phagocytophilum infects cells of the megakaryocytic lineage through sialylated ligands but fails to alter platelet production. Journal of medical microbiology. 2008;57:416–423. doi: 10.1099/jmm.0.47551-0. [DOI] [PubMed] [Google Scholar]
- Granquist EG, Stuen S, Lundgren AM, Braten M, Barbet AF. Outer membrane protein sequence variation in lambs experimentally infected with Anaplasma phagocytophilum. Infection and immunity. 2008;76:120–126. doi: 10.1128/IAI.01206-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, Seshadri R, Ren Q, Wu M, Utterback TR, Smith S, Lewis M, Khouri H, Zhang C, Niu H, Lin Q, Ohashi N, Zhi N, Nelson W, Brinkac LM, Dodson RJ, Rosovitz MJ, Sundaram J, Daugherty SC, Davidsen T, Durkin AS, Gwinn M, Haft DH, Selengut JD, Sullivan SA, Zafar N, Zhou L, Benahmed F, Forberger H, Halpin R, Mulligan S, Robinson J, White O, Rikihisa Y, Tettelin H. Comparative genomics of emerging human ehrlichiosis agents. PLoS genetics. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infection and immunity. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, Kurtti TJ, Munderloh UG. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC genomics. 2008;9:364. doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton EA, Scott GR. Detection of antibodies to the agent of tick-borne fever by indirect immunofluorescence. Vet Microbiol. 1989;21:133–138. doi: 10.1016/0378-1135(89)90025-4. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Troese MJ, Kearns SA, Yang T, Reneer DV, Carlyon JA. Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells. Infection and immunity. 2008;76:2090–2098. doi: 10.1128/IAI.01594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorpio DG, Caspersen K, Ogata H, Park J, Dumler JS. Restricted changes in major surface protein-2 (msp2) transcription after prolonged in vitro passage of Anaplasma phagocytophilum. BMC microbiology. 2004;4:1. doi: 10.1186/1471-2180-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuen S. Anaplasma phagocytophilum - the most widespread tick-borne infection in animals in Europe. Veterinary research communications. 2007;31 Suppl 1:79–84. doi: 10.1007/s11259-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Stuen S, Bergstrom K. Serological investigation of granulocytic Ehrlichia infection in sheep in Norway. Acta veterinaria Scandinavica. 2001;42:331–338. doi: 10.1186/1751-0147-42-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuen S, Bergstrom K, Petrovec M, Van de Pol I, Schouls LM. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clinical and diagnostic laboratory immunology. 2003;10:692–695. doi: 10.1128/CDLI.10.4.692-695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rikihisa Y, Lai TH, Kumagai Y, Zhi N, Reed SM. Rapid sequential changeover of expressed p44 genes during the acute phase of Anaplasma phagocytophilum infection in horses. Infection and immunity. 2004;72:6852–6859. doi: 10.1128/IAI.72.12.6852-6859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KA, Mitchell GB. Use of counter immunoelectrophoresis in detection of antibodies to tickborne fever. Res Vet Sci. 1988;45:28–30. [PubMed] [Google Scholar]
- Whist SK, Storset AK, Johansen GM, Larsen HJ. Modulation of leukocyte populations and immune responses in sheep experimentally infected with Anaplasma (formerly Ehrlichia) phagocytophilum. Veterinary immunology and immunopathology. 2003;94:163–175. doi: 10.1016/s0165-2427(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z. Lymphocyte subpopulations in peripheral blood of sheep experimentally infected with tick-borne fever. Res Vet Sci. 1991;51:40–43. doi: 10.1016/0034-5288(91)90028-m. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z. Anaplasma phagocytophilum in ruminants in Europe. Annals of the New York Academy of Sciences. 2006;1078:446–460. doi: 10.1196/annals.1374.084. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z, Scaife H, Hart CA, Edwards SW. Purification of ovine neutrophils and eosinophils: Anaplasma phagocytophilum affects neutrophil density. Journal of comparative pathology. 2003;128:277–282. doi: 10.1053/jcpa.2002.0633. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Futse JE, Brown WC, Brayton KA, Palmer GH. Maintenance of antibody to pathogen epitopes generated by segmental gene conversion is highly dynamic during long-term persistent infection. Infection and immunity. 2007;75:5185–5190. doi: 10.1128/IAI.00913-07. [DOI] [PMC free article] [PubMed] [Google Scholar]