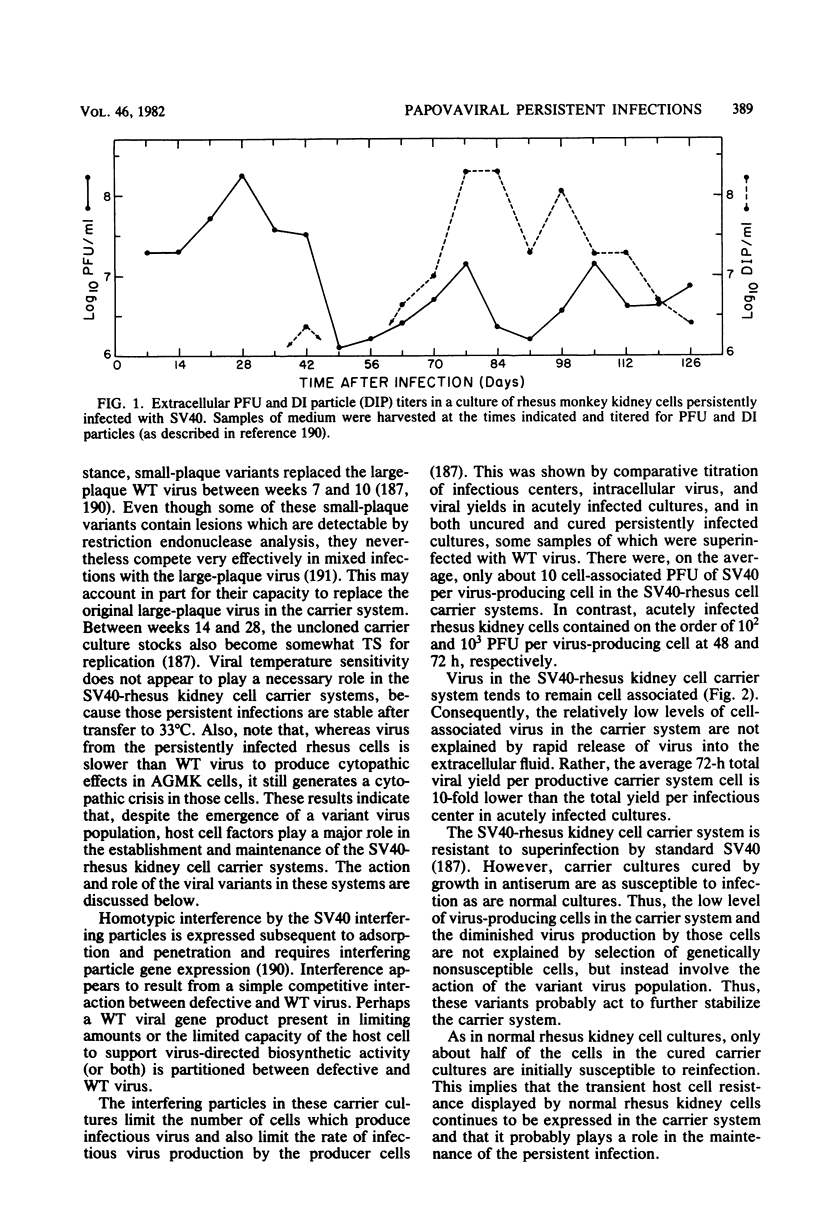
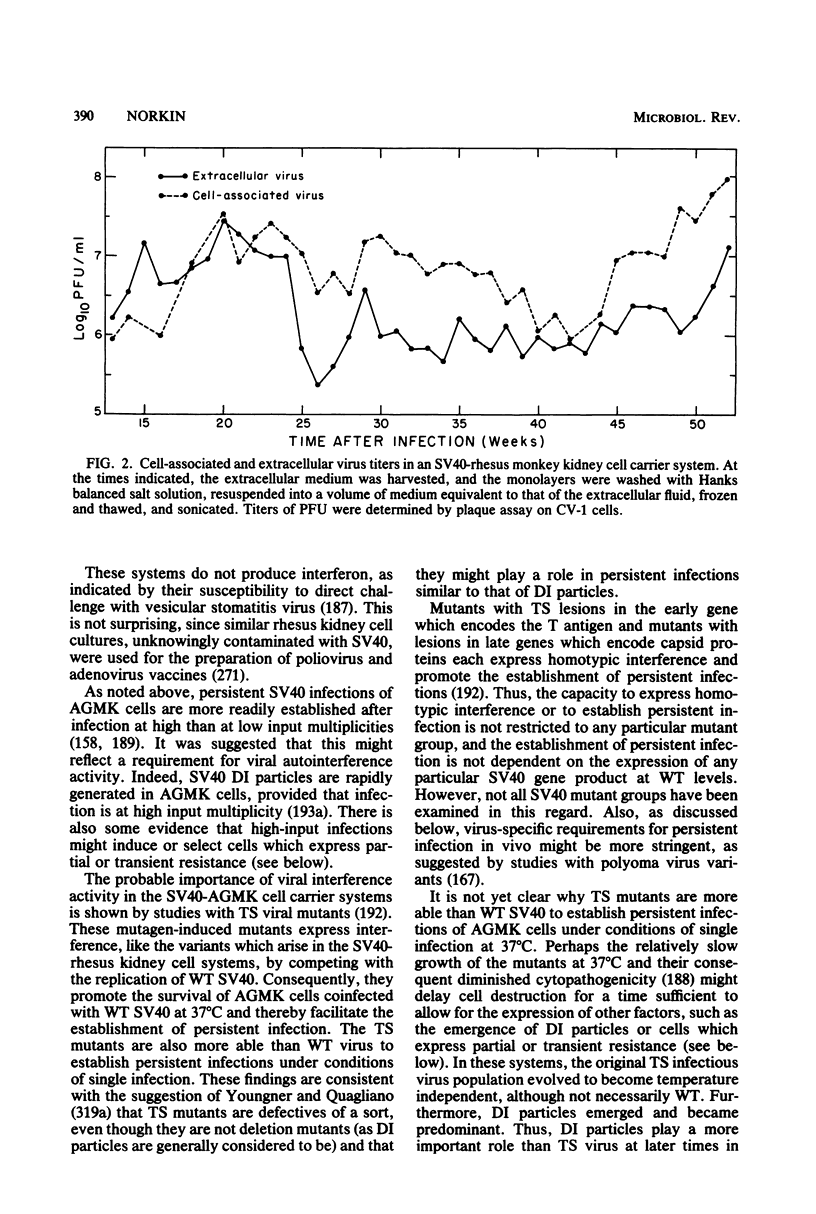
Full text
PDF







































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson-Anvret M., Lindahl T. Integrated viral DNA sequences in Epstein-Barr virus-converted human lymphoma lines. J Virol. 1978 Mar;25(3):710–718. doi: 10.1128/jvi.25.3.710-718.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Basilico C., Zouzias D., Della-Valle G., Gattoni S., Colantuoni V., Fenton R., Dailey L. Integration and excision of polyoma virus genomes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):611–620. doi: 10.1101/sqb.1980.044.01.064. [DOI] [PubMed] [Google Scholar]
- Beth E., Giraldo G., Schmidt-Ullrich R., Pater M. M., Pater A., di Mayorca G. BK virus-transformed inbred hamster brain cells. I. Status of the viral DNA and the association of BK virus early antigens with purified plasma membranes. J Virol. 1981 Oct;40(1):276–284. doi: 10.1128/jvi.40.1.276-284.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birg F., Dulbecco R., Fried M., Kamen R. State and organization of polyoma virus DNA sequences in transformed rat cell lines. J Virol. 1979 Feb;29(2):633–648. doi: 10.1128/jvi.29.2.633-648.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Brandner G., Burger A., Neumann-Haefelin D., Reinke C., Helwig H. Isolation of simian virus 40 from a newborn child. J Clin Microbiol. 1977 Feb;5(2):250–252. doi: 10.1128/jcm.5.2.250-252.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Tsai T., Gajdusek D. C. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am J Epidemiol. 1975 Oct;102(4):331–340. doi: 10.1093/oxfordjournals.aje.a112169. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Richardson L. S., Melnick J. L. Variation in properties of SV40-transformed simian cell lines detected by superinfection with SV40 and human adenoviruses. Virology. 1971 Dec;46(3):844–855. doi: 10.1016/0042-6822(71)90085-7. [DOI] [PubMed] [Google Scholar]
- COLE F. E., Jr, HETRICK F. M. PERSISTENT INFECTION OF HUMAN CONJUNCTIVA CELL CULTURES BY MYXOVIRUS PARAINFLUENZA 3. Can J Microbiol. 1965 Jun;11:513–521. doi: 10.1139/m65-068. [DOI] [PubMed] [Google Scholar]
- COOPER P. D., BELLETT A. J. A transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:485–497. doi: 10.1099/00221287-21-3-485. [DOI] [PubMed] [Google Scholar]
- Carroll D., Hansen J. L., Maryon E. B., O'Neill F. J. SV40 defectives selected during low multiplicity passage on A172 human glioblastoma cells. Virology. 1981 Jul 30;112(2):461–471. doi: 10.1016/0042-6822(81)90293-2. [DOI] [PubMed] [Google Scholar]
- Castaigne P., Rondot P., Escourolle R., Ribadeau dumas J. L., Cathala F., Hauw J. J. Leucoencéphalopathie multifocale progressive et "gliomes" multiples. Rev Neurol (Paris) 1974 Sep-Oct;130(9-10):379–392. [PubMed] [Google Scholar]
- Chartrand P., Gusew-Chartrand N., Bourgaux P. Integrated polyoma genomes in inducible permissive transformed cells. J Virol. 1981 Jul;39(1):185–195. doi: 10.1128/jvi.39.1.185-195.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman S. H., Black P. H., Rubin R. H., Cantell K., Hirsch M. S. Interferon and BK Papovavirus--clinical and laboratory studies. J Infect Dis. 1980 Feb;141(2):157–161. doi: 10.1093/infdis/141.2.157. [DOI] [PubMed] [Google Scholar]
- Chenciner N., Meneguzzi G., Corallini A., Grossi M. P., Grassi P., Barbanti-Brodano G., Milanesi G. Integrated and free viral DNA in hamster tumors induced by BK virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):975–979. doi: 10.1073/pnas.77.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Dailey L., Basilico C. Amplification of integrated viral DNA sequences in polyoma virus-transformed cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3850–3854. doi: 10.1073/pnas.77.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. 3. Interference and enrichment. J Mol Biol. 1973 May 25;76(3):345–361. doi: 10.1016/0022-2836(73)90509-3. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Gardner S. D., Field A. M. Human polyomavirus infection in renal allograft recipients. Br Med J. 1973 Aug 18;3(5876):371–375. doi: 10.1136/bmj.3.5876.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Mackenzie E. F., Gardner S. D., Poulding J. M., Amer B., Russell W. J. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978 Apr;31(4):338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Cecchetti M. G., Fabris G., Grossi M. P., Balboni P. G., Lanza G., Barbanti-Brodano G. Ependymomas, malignant tumors of pancreatic islets, and osteosarcomas induced in hamsters by BK virus, a human papovavirus. J Natl Cancer Inst. 1978 Sep;61(3):875–883. [PubMed] [Google Scholar]
- Corallini A., Barbanti-Brodano G., Portolani M., Balboni P. G., Grossi M. P., Possati L., Honorati C., La Placa M., Mazzoni A., Caputo A. Antibodies to BK virus structural and tumor antigens in human sera from normal persons and from patients with various diseases, including neoplasia. Infect Immun. 1976 Jun;13(6):1684–1691. doi: 10.1128/iai.13.6.1684-1691.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDHARE J., MORGAN H. R. POLYOMA VIRUS AND L CELL RELATIONSHIP. II. A CURABLE CARRIER SYSTEM NOT DEPENDENT ON INTERFERON. J Natl Cancer Inst. 1964 Nov;33:765–775. doi: 10.1093/jnci/33.5.765. [DOI] [PubMed] [Google Scholar]
- Dailey L., Colantuoni V., Fenton R. G., La Bella F., Zouzias D., Gattoni S., Basilico C. The evolution of polyoma-transformed rat cell lines during propagation in vitro. Virology. 1982 Jan 15;116(1):207–220. doi: 10.1016/0042-6822(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. An ultrastructural study of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Lab Invest. 1976 Aug;35(2):185–196. [PubMed] [Google Scholar]
- Daniel R., Shah K., Madden D., Stagno S. Serological Investigation of the possibility of congenital transmission of papovavirus JC. Infect Immun. 1981 Jul;33(1):319–321. doi: 10.1128/iai.33.1.319-321.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L., Monier R. Presence of free viral DNA in simian virus 40-transformed nonproducer cells. J Virol. 1978 Aug;27(2):307–312. doi: 10.1128/jvi.27.2.307-312.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecchi L., Gendron D., Bourgaux P. Inducible permissive cells transformed by a temperature-sensitive polyoma virus: superinfection does not allow excision of the resident viral genome. J Virol. 1981 Jul;39(1):196–206. doi: 10.1128/jvi.39.1.196-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Lai C. J., Khoury G. Nucleotide sequence of the DNA replication origin for human papovavirus BKV: sequence and structural homology with SV40. Cell. 1978 Feb;13(2):345–358. doi: 10.1016/0092-8674(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Diacumakos E. G., Gershey E. L. Uncoating and gene expression of simian virus 40 in CV-1 cell nuclei inoculated by microinjection. J Virol. 1977 Dec;24(3):903–906. doi: 10.1128/jvi.24.3.903-906.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. Pathology of measles virus infection of the nervous system: comparison with multiple sclerosis. Int Rev Exp Pathol. 1979;19:101–135. [PubMed] [Google Scholar]
- Dörries K., Johnson R. T., ter Meulen V. Detection of polyoma virus DNA in PML-brain tissue by (in situ) hybridization. J Gen Virol. 1979 Jan;42(1):49–57. doi: 10.1099/0022-1317-42-1-49. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., BERKELEY W. H., YOUNG R. D. Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts. Proc Soc Exp Biol Med. 1961 May;107:191–197. doi: 10.3181/00379727-107-26576. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., GRUBBS G. E., YOUNG R. D. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962 May;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Eggleton K. H., Norkin L. C. Cell killing by simian virus 40: the sequence of ultrastructural alterations leading to cellular degeneration and death. Virology. 1981 Apr 15;110(1):73–86. doi: 10.1016/0042-6822(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Einck K. H., Norkin L. C. Lysosome stability during lytic infection by simian virus 40. Intervirology. 1979;12(1):47–56. doi: 10.1159/000149068. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Fiori M., Di Mayorca G. Occurrence of BK virus DNA in DNA obtained from certain human tumors. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4662–4666. doi: 10.1073/pnas.73.12.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. The activation of virus synthesis in polyoma-transformed cells. Virology. 1969 Mar;37(3):327–334. doi: 10.1016/0042-6822(69)90216-5. [DOI] [PubMed] [Google Scholar]
- Fogh J., Loveless J. Simian virus 40 (SV40) production from SV40-transformed human amnion cells of established lines. J Natl Cancer Inst. 1978 Apr;60(4):895–898. [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Walker D. L. Transformation of primary hamster brain cells with JC virus and its DNA. J Virol. 1980 Jul;35(1):265–269. doi: 10.1128/jvi.35.1.265-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER P., KIRSCHSTEIN R. L. SV40-induced ependymomas in newborn hamsters. I. Virus-tumor relationships. Virology. 1962 Dec;18:582–588. doi: 10.1016/0042-6822(62)90061-2. [DOI] [PubMed] [Google Scholar]
- GROSS L. Neck tumors, or leukemia, developing in adult C3H mice following inoculation, in early infancy, with filtered (Berkefeld N), or centrifugated (144,000 X g), Ak-leukemic extracts. Cancer. 1953 Sep;6(5):948–958. doi: 10.1002/1097-0142(195309)6:5<948::aid-cncr2820060513>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gangemi J. D., Sharp D. G. Host-induced influences on the syntheses of defective vaccinia particles. Virology. 1976 Aug;73(1):165–172. doi: 10.1016/0042-6822(76)90070-2. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni S., Colantuoni V., Basilico C. Relationship between integrated and nonintegrated viral DNA in rat cells transformed by polyoma virus. J Virol. 1980 Jun;34(3):615–626. doi: 10.1128/jvi.34.3.615-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40 causes persistent infection of muntjac cells in the absence of virus transformation. J Gen Virol. 1981 Sep;56(Pt 1):33–40. doi: 10.1099/0022-1317-56-1-33. [DOI] [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40-host cell interaction during lytic infection. J Virol. 1979 Apr;30(1):76–83. doi: 10.1128/jvi.30.1.76-83.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Topp W. C., Botchan M. Retransformation of a simian virus 40 revertant cell line, which is resistant to viral and DNA infections, by microinjection of viral DNA. J Virol. 1979 Dec;32(3):989–994. doi: 10.1128/jvi.32.3.989-994.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee J. E., Narayan O., Johnson R. T., Herndon R. M. Induction of brain tumors in hamsters with BK virus, a human papovavirus. Lab Invest. 1977 Jun;36(6):636–641. [PubMed] [Google Scholar]
- Gribble D. H., Haden C. C., Schwartz L. W., Henrickson R. V. Spontaneous progressive multifocal leukoencephalopathy (PML) in macaques. Nature. 1975 Apr 17;254(5501):602–604. doi: 10.1038/254602a0. [DOI] [PubMed] [Google Scholar]
- Gutai M. W., Nathans D. Evolutionary variants of simian virus 40: Cellular DNA sequences and sequences at recombinant joints of substituted variants. J Mol Biol. 1978 Dec 5;126(2):275–288. doi: 10.1016/0022-2836(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Hahn E. C., Sauer G. Initial stage of transformation of permissive cells by simian virus 40: development of resistance to productive infection. J Virol. 1971 Jul;8(1):7–16. doi: 10.1128/jvi.8.1.7-16.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C. The development of CV-1 cells resistant to SV 40. Arch Gesamte Virusforsch. 1972;37(1):34–44. doi: 10.1007/BF01241148. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Experimental measles encephalitis: a genetic analysis. Infect Immun. 1975 Oct;12(4):785–790. doi: 10.1128/iai.12.4.785-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Topp W. C., Rifkin D. B., Moreau P. E. Transformation of rat embryo fibroblasts by cloned polyoma virus DNA fragments containing only part of the early region. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3978–3982. doi: 10.1073/pnas.77.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw J. J., Esourolle R. Filamentous and multilamellated cytoplasmic inclusions in progressive multifocal leukoencephalopathy. Acta Neuropathol. 1977 Mar 31;37(3):263–265. doi: 10.1007/BF00686889. [DOI] [PubMed] [Google Scholar]
- Help G. I., Coto C. E. Génesis de partículas interferentes durante la multiplicacíon del virus Junin in vivo. Medicina (B Aires) 1980 Sep-Oct;40(5):531–536. [PubMed] [Google Scholar]
- Henry B. E., Newcomb W. W., O'Callaghan D. J. Biological and biochemical properties of defective interfering particles of equine herpesvirus type 1. Virology. 1979 Jan 30;92(2):495–506. doi: 10.1016/0042-6822(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Hirai K. Characterization of simian virus 40 transformed African green monkey cells (CV-1). I. Defective virion and viral genome. Microbiol Immunol. 1977;21(5):267–278. doi: 10.1111/j.1348-0421.1977.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J., Murphy D., Defendi V. Amplification and rearrangement of integrated SV40 DNA sequences accompany the selection of anchorage-independent transformed mouse cells. Cell. 1980 Nov;22(2 Pt 2):535–543. doi: 10.1016/0092-8674(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Ho M., Suwansirikul S., Dowling J. N., Youngblood L. A., Armstrong J. A. The transplanted kidney as a source of cytomegalovirus infection. N Engl J Med. 1975 Nov 27;293(22):1109–1112. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Takemoto K. K., Martin M. A. Polynucleotide sequences common to the genomes of simian virus 40 and the human papovaviruses JC and BK. Virology. 1976 Aug;73(1):303–307. doi: 10.1016/0042-6822(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huebner K., Croce C. M., Koprowski H. Isolation of defective viruses from SV40-transformed human and hamster cells. Virology. 1974 Jun;59(2):570–573. doi: 10.1016/0042-6822(74)90467-x. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970 Jul;6(1):87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel F., Sokol F. Integration of progeny simian virus 40 DNA into the host cell genome. J Mol Biol. 1974 Apr 15;84(3):423–444. doi: 10.1016/0022-2836(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Martin M. A., Takemoto K. K., Howley P. M., Aaronson S. A., Solomon D., Khoury G. Evaluation of normal and neoplastic human tissue for BK virus. Virology. 1978 Oct 15;90(2):187–196. doi: 10.1016/0042-6822(78)90302-1. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Vanderryn D. F., Meltzer M. L., Martin M. A. Characterization of polyoma viral DNA sequences in polyoma-induced hamster tumor cell lines. J Biol Chem. 1980 Apr 25;255(8):3798–3805. [PubMed] [Google Scholar]
- JOHNSON R. T. VIRUS INVASION OF THE CENTRAL NERVOUS SYSTEM: A STUDY OF SINDBIS VIRUS INFECTION IN THE MOUSE USING FLUORESCENT ANTIBODY. Am J Pathol. 1965 Jun;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- Joncas J. H. Persistence, reactivation, and cell transformation by human herpeviruses: herpes simplex 1, 2 (HSV-1, HSV-2), cytomegalovirus (CMV), varicella-zoster (VZV), Epstein-Barr virus (EBV). Can J Microbiol. 1979 Mar;25(3):254–260. doi: 10.1139/m79-041. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M., Krech U., Price P. C., Pyndiah M. N. Evidence of chronic persistent infections with polyomaviruses (BK type) in renal transplant recipients. Arch Virol. 1975;47(1):39–46. doi: 10.1007/BF01315591. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- KILHAM L., MURPHY H. W. A pneumotropic virus isolated from C3H mice carrying the Bittner Milk Agent. Proc Soc Exp Biol Med. 1953 Jan;82(1):133–137. doi: 10.3181/00379727-82-20044. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Hirai K. Characterization of simian virus 40-transformed African green monkey cells (CV-1). II. Semipermissive character for viral replication. Microbiol Immunol. 1977;21(8):475–479. doi: 10.1111/j.1348-0421.1977.tb00313.x. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A., O'Brien P., Osoba D. "Spontaneous" progressive multifocal leukoencephalopathy. Immunologic aspects. Ann Intern Med. 1972 Aug;77(2):229–233. doi: 10.7326/0003-4819-77-2-229. [DOI] [PubMed] [Google Scholar]
- Knight P., Duff R., Rapp F. Latency of human measles virus in hamster cells. J Virol. 1972 Nov;10(5):995–1001. doi: 10.1128/jvi.10.5.995-1001.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Warren K. G. Can a defective herpes simplex virus cause multiple sclerosis? Perspect Biol Med. 1978 Autumn;22(1):10–18. [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Kreth W. H., Käckell M. Y., ter Meulen V. Demonstration of in vitro lymphocyte-mediated cytotoxicity against measles virus in SSPE. J Immunol. 1975 Mar;114(3):1042–1046. [PubMed] [Google Scholar]
- Kriegler M. P., Griffin J. D., Livingston D. M. Phenotypic complementation of the SV40 tsA mutant defect in viral DNA synthesis following microinjection of SV40 T antigen. Cell. 1978 Aug;14(4):983–994. doi: 10.1016/0092-8674(78)90352-5. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Watts S. L., Anderson D. L., Faras A. J., Pass F. Anogenital warts contain several distinct species of human papillomavirus. J Virol. 1980 Oct;36(1):236–244. doi: 10.1128/jvi.36.1.236-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R., Hwang S. P., Shimizu N., McDougall J. K., Botchan M. R. Another chromosomal assignment for a simian virus 40 integration site in human cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4460–4464. doi: 10.1073/pnas.75.9.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster J. M., Mora P. T., Brown M., Khoury G. Immunologic selection against simian virus-40 transformed cells: concomitnat loss of viral antigens and early viral gene sequences. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4796–4800. doi: 10.1073/pnas.74.11.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL J. D., JAKOBOVITS M., EATON M. D. Polyoma disease and tumors in mice: the distribution of viral antigen detected by immunofluorescence. Virology. 1962 Mar;16:314–319. doi: 10.1016/0042-6822(62)90252-0. [DOI] [PubMed] [Google Scholar]
- Lania L., Boast S., Fried M. Excision of polyoma virus genomes from chromosomal DNA by homologous recombination. Nature. 1982 Jan 28;295(5847):349–350. doi: 10.1038/295349a0. [DOI] [PubMed] [Google Scholar]
- Lania L., Hayday A., Fried M. Loss of functional large T-antigen and free viral genomes from cells transformed in vitro by polyoma virus after passage in vivo as tumor cells. J Virol. 1981 Aug;39(2):422–431. doi: 10.1128/jvi.39.2.422-431.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. Evolutionary variants of simian virus 40: replication and encapsidation of variant DNA. Virology. 1979 Jan 30;92(2):291–298. doi: 10.1016/0042-6822(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Lief F. S., Verini M. A., Pienkowski M. M., ter Meulen V., Koprowski H. Analysis of a viral agent isolated from multiple sclerosis brain tissue: characterization as a parainfluenzavirus type 1. J Virol. 1974 May;13(5):1037–1045. doi: 10.1128/jvi.13.5.1037-1045.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature. 1980 Jun 12;285(5765):490–492. doi: 10.1038/285490a0. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi P. S., Balduzzi P., Hare J. D., Morgan H. R. Mechanism of the development of a stable carrier system of L cells with polyoma virus. J Natl Cancer Inst. 1970 Jul;45(1):171–178. [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Hayes E. C., Gollobin S. D., Westfall L. K., Zweerink H. J. Antibodies against the measles matrix polypeptide after clinical infection and vaccination. Infect Immun. 1980 Mar;27(3):817–825. doi: 10.1128/iai.27.3.817-825.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman W., Levine A. J. Viruses as probes for development and differentiation. Adv Virus Res. 1981;26:65–116. doi: 10.1016/s0065-3527(08)60421-2. [DOI] [PubMed] [Google Scholar]
- Manteuil S., Pages J., Stehelin D., Girard M. Replication of simian virus 40 deoxyribonucleic acid: analysis of the one-step growth cycle. J Virol. 1973 Jan;11(1):98–106. doi: 10.1128/jvi.11.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Marriott P. J., O'Brien M. D., Mackenzie I. C., Janota I. Progressive multifocal leucoencephalopathy: remission with cytarabine. J Neurol Neurosurg Psychiatry. 1975 Mar;38(3):205–209. doi: 10.1136/jnnp.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Gelb L. D., Garon C., Takemoto K. K., Lee T. N., Sack G. H., Jr, Nathans D. Characterization of "heavy" and "light" SV40-like particles from a patient with PML. Virology. 1974 May;59(1):179–189. doi: 10.1016/0042-6822(74)90214-1. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Jr, Takemoto K. K. Transformation of rabbit kidney cells by BKV(MM) human papovavirus. Int J Cancer. 1977 Mar 15;19(3):391–395. doi: 10.1002/ijc.2910190317. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Takemoto K. K. Complementation between BK human papovavirus and a simian virus 40 tsA mutant. J Virol. 1976 Mar;17(3):1060–1062. doi: 10.1128/jvi.17.3.1060-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., Daniel W. A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966 Oct;30(2):242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- May G., Fischer H., Zang K. D. SV40-related T-antigen expression in human meningiomas with normal and G-22-monosomic karyotype. J Gen Virol. 1979 Jun;43(3):697–700. doi: 10.1099/0022-1317-43-3-697. [DOI] [PubMed] [Google Scholar]
- McCance D. J. Growth and persistence of polyoma early region deletion mutants in mice. J Virol. 1981 Sep;39(3):958–962. doi: 10.1128/jvi.39.3.958-962.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Reactivation of polyoma virus in kidneys of persistently infected mice during pregnancy. Infect Immun. 1979 Sep;25(3):998–1002. doi: 10.1128/iai.25.3.998-1002.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Transplacental transmission of polyoma virus in mice. Infect Immun. 1977 Oct;18(1):196–202. doi: 10.1128/iai.18.1.196-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick W. F., Schochet S. S., Jr, Sarles H. E., Calverley J. R. Progressive multifocal leukoencephalopathy in renal transplant recipients. Arch Intern Med. 1976 Jul;136(7):829–834. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B. Immunology of a persistent retrovirus infection--equine infectious anemia. Adv Vet Sci Comp Med. 1979;23:137–159. [PubMed] [Google Scholar]
- Mehta P. D., Kane A., Thormar H. Quantitation of measles virus-specific immunoglobulins in serum, CSF, and brain extract from patients with subactue sclerosing panencephalitis. J Immunol. 1977 Jun;118(6):2254–2261. [PubMed] [Google Scholar]
- Melnick J. L., Allison A. C., Butel J. S., Eckhart W., Eddy B. E., Kit S., Levine A. J., Miles J. A., Pagano J. S., Sachs L. Papovaviridae. Intervirology. 1974;3(1-2):106–120. doi: 10.1159/000149746. [DOI] [PubMed] [Google Scholar]
- Mendelsohn E., Baran N., Neer A., Manor H. Integration site of polyoma virus DNA in the inducible LPT line of polyoma-transformed rat cells. J Virol. 1982 Jan;41(1):192–209. doi: 10.1128/jvi.41.1.192-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A. Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J Pathol Bacteriol. 1966 Apr;91(2):395–402. doi: 10.1002/path.1700910214. [DOI] [PubMed] [Google Scholar]
- Minagawa T. Studies on the persistent infection with measles virus in HeLa cells. I. Clonal analysis of cells of carrier cultures. Jpn J Microbiol. 1971 Jul;15(4):325–331. doi: 10.1111/j.1348-0421.1971.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Yoshiike K., Takemoto K. K. Characterization of JC papovavirus adapted to growth in human embryonic kidney cells. J Virol. 1980 Aug;35(2):498–504. doi: 10.1128/jvi.35.2.498-504.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H. M., Jarrett W. F. Unintegrated viral DNA sequences in a hamster tumor induced by bovine papilloma virus. J Virol. 1981 Sep;39(3):945–949. doi: 10.1128/jvi.39.3.945-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Stinski M. F. Persistence of the cytomegalovirus genome in human cells. J Virol. 1979 Sep;31(3):761–775. doi: 10.1128/jvi.31.3.761-775.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mázló M., Tariska I. Morphological demonstration of the first phase of polyomavirus replication in oligodendroglia cells of human brain in progressive multifocal leukoencephalopathy (PML). Acta Neuropathol. 1980;49(2):133–143. doi: 10.1007/BF00690753. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Meurman O. H., Vihma L., Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973 Oct;5(5):283–287. [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Penney J. B., Jr, Johnson R. T., Herndon R. M., Weiner L. P. Etiology of progressive multifocal leukoencephalopathy. Identification of papovavirus. N Engl J Med. 1973 Dec 13;289(24):1278–1282. doi: 10.1056/NEJM197312132892405. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Panitch H., Palsson P. A., Petursson G., Georgsson G. Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab Invest. 1976 Nov;35(5):444–451. [PubMed] [Google Scholar]
- Newell N., Shah K. V., Kelly T. J., Jr Evolutionary relationships of the primate papovaviruses: base sequence homology among the genomes of simian virus 40, stump-tailed macaque virus, and SA12 virus. J Virol. 1979 May;30(2):624–636. doi: 10.1128/jvi.30.2.624-636.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Cell killing by simian virus 40: impairment of membrane formation and function. J Virol. 1977 Mar;21(3):872–879. doi: 10.1128/jvi.21.3.872-879.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Effect of input multiplicity on the establishment of simian virus 40 persistent infections in rhesus monkey kidney cells. Infect Immun. 1977 Dec;18(3):868–871. doi: 10.1128/iai.18.3.868-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Ouellette J. Cell killing by simian virus 40: variation in the pattern of lysosomal enzyme release, cellular enzyme release, and cell death during productive infection of normal and simian virus 40-transformed simian cell lines. J Virol. 1976 Apr;18(1):48–57. doi: 10.1128/jvi.18.1.48-57.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Persistent infections of green monkey kidney cells initiated with temperature-sensitive mutants of simian virus 40. Virology. 1980 Dec;107(2):375–388. doi: 10.1016/0042-6822(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Norkin L. C. Rhesus monkeys kidney cells persistently infected with Simian Virus 40: production of defective interfering virus and acquisition of the transformed phenotype. Infect Immun. 1976 Sep;14(3):783–792. doi: 10.1128/iai.14.3.783-792.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Small plaque variants of simian virus 40 from a persistent infection of rhesus monkey kidney cells. Virology. 1979 Aug;97(1):201–206. doi: 10.1016/0042-6822(79)90388-x. [DOI] [PubMed] [Google Scholar]
- Norkin L. C. The emergence of simian virus 40 variants in a persistent infection of rhesus monkey kidney cells and their interaction with standard simian virus 40. Virology. 1979 Jun;95(2):598–603. doi: 10.1016/0042-6822(79)90515-4. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Wojcik J. B., Goguen C. A. Effect of the host cell on the generation of defective Simian Virus 40 during undiluted serial passages and persistent infection. Virology. 1981 Jan 30;108(2):525–530. doi: 10.1016/0042-6822(81)90462-1. [DOI] [PubMed] [Google Scholar]
- Olive D. M., Lampert M., Major E. O. Comparison of wild-type BK virus DNA and BK virion DNA rescued from virus-transformed BHK cells. Virology. 1980 May;103(1):1–10. doi: 10.1016/0042-6822(80)90121-x. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., ZuRhein G. M., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: restriction endonuclease digestion and gel electrophoresis of resultant fragments. J Virol. 1974 Mar;13(3):614–622. doi: 10.1128/jvi.13.3.614-622.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster-Granite M. L., Narayan O., Johnson R. T., Herndon R. M. Studies of cultured human and simian fetal brain cells. II. Infections with human (BK) and simian (SV40) papovaviruses. Neuropathol Appl Neurobiol. 1978 Nov-Dec;4(6):443–455. doi: 10.1111/j.1365-2990.1978.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Hodach A. E., Chou S. M. JC Papovavirus in progressive multifocal leukoencephalopathy. J Infect Dis. 1976 Jun;133(6):686–690. doi: 10.1093/infdis/133.6.686. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Varakis J. N. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977 Mar;37(3):718–720. [PubMed] [Google Scholar]
- Pagano J. S. Diseases and mechanisms of persistent DNA virus infection: latency and cellular transformation. J Infect Dis. 1975 Aug;132(2):209–223. doi: 10.1093/infdis/132.2.209. [DOI] [PubMed] [Google Scholar]
- Pages J., Manteuil S., Stehelin D., Fiszman M., Marx M., Girard M. Relationship between replication of simian virus 40 DNA and specific events of the host cell cycle. J Virol. 1973 Jul;12(1):99–107. doi: 10.1128/jvi.12.1.99-107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Kakulas B. A., Sadka M. Virus-like particles in proximity to myelin in a case of progressive multifocal leukoencephalopathy. Proc Aust Assoc Neurol. 1966;4:133–140. [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Pfau C. J. Biochemical and biophysical properties of the arenaviruses. Prog Med Virol. 1974;18(0):64–80. [PubMed] [Google Scholar]
- Popescu M., Lehmann-Grube F. Defective interfering particles in mice infected with lymphocytic choriomeningitis virus. Virology. 1977 Mar;77(1):78–83. doi: 10.1016/0042-6822(77)90407-x. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Portolani M., Barbanti-Brodano G., Placa M. L. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975 Feb;15(2):420–422. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. Nonintegrated viral DNA in rat cells doubly transformed by SV40 and polyoma virus. Virology. 1978 Mar;85(1):328–331. doi: 10.1016/0042-6822(78)90439-7. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Prineas J. Pathology of the early lesion in multiple sclerosis. Hum Pathol. 1975 Sep;6(5):531–554. doi: 10.1016/s0046-8177(75)80040-2. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Fareed G. C. Transformation of human embryonic kidney cells by human papovarirus BK. J Virol. 1979 Feb;29(2):763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON E. P., Jr Progressive multifocal leukoencephalopathy. N Engl J Med. 1961 Oct 26;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J. E., HENLE W. STUDIES ON PERSISTENT INFECTIONS OF TISSUE CULTURES. V. THE INITIAL STAGES OF INFECTION OF L(MCN) CELLS BY NEWCASTLE DISEASE VIRUS. J Exp Med. 1964 Jan 1;119:895–921. doi: 10.1084/jem.119.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P. The epidemiology of mouse polyoma virus infection. Bacteriol Rev. 1961 Mar;25:18–31. doi: 10.1128/br.25.1.18-31.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S. G., Dal Canto M. C., Johnson T. C. Comparison of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1976 Apr;13(4):1242–1249. doi: 10.1128/iai.13.4.1242-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty K. A., Jr, Ruben R. L., Young S. K. A virus-producing cell line developed by transformation of human parapharyngeal cells with SV40. In Vitro. 1978 Feb;14(2):227–235. doi: 10.1007/BF02618227. [DOI] [PubMed] [Google Scholar]
- Rao D. D., Huang A. S. Interference among defective interfering particles of vesicular stomatitis virus. J Virol. 1982 Jan;41(1):210–221. doi: 10.1128/jvi.41.1.210-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reese J. M., Reissing M., Daniel R. W., Shah K. V. Occurrence of BK virus and BK virus-specific antibodies in the urine of patients receiving chemotherapy for malignancy. Infect Immun. 1975 Jun;11(6):1375–1381. doi: 10.1128/iai.11.6.1375-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig M., Kelly T. J., Jr, Daniel R. W., Rangan S. R., Shah K. V. Identification of the stumptailed macaque virus as a new papovavirus. Infect Immun. 1976 Jul;14(1):225–231. doi: 10.1128/iai.14.1.225-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentier-Delrue F., Lubiniecki A., Howley P. M. Analysis of JC virus DNA purified directly from human progressive multifocal leukoencephalopathy brains. J Virol. 1981 May;38(2):761–769. doi: 10.1128/jvi.38.2.761-769.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Berg P. Does simian virus 40 DNA integrate into cellular DNA during productive infection? J Virol. 1978 Nov;28(2):475–489. doi: 10.1128/jvi.28.2.475-489.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima R. K., Martin S. J. Persistent infection of tissue culture cells by RNA viruses. Med Microbiol Immunol. 1976 Jun 1;162(2):89–119. doi: 10.1007/BF02121320. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Rockwell D., Ruben F. L., Winkelstein A., Mendelow H. Absence of imune deficiencies in a case of progressive multifocal leukoencephalopathy. Am J Med. 1976 Sep;61(3):433–436. doi: 10.1016/0002-9343(76)90383-1. [DOI] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr, Narayan O., Danna K. J., Weiner L. P., Nathans D. The nucleic acid of an SV40-like virus isolated from a patient with progressive multifocal leukoencephalopathy. Virology. 1973 Feb;51(2):345–350. doi: 10.1016/0042-6822(73)90433-9. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schwöbel W., Ahl R. Peristence of sindbis virus in BHK-21 cell cultures. Arch Gesamte Virusforsch. 1972;38(1):1–10. doi: 10.1007/BF01241350. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Brockman W. W., Nathans D. Biological activities of deletion mutants of simian virus 40. Virology. 1976 Dec;75(2):319–334. doi: 10.1016/0042-6822(76)90031-3. [DOI] [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. I Interferon-inducing defective-interfering particles as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1978 Mar;85(1):175–186. doi: 10.1016/0042-6822(78)90422-1. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Kelly T. J., Jr Immunological relatedness of papovaviruses of the simian virus 40-polyoma subgroup. Infect Immun. 1977 Nov;18(2):558–560. doi: 10.1128/iai.18.2.558-560.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Stone K. R., Elliott A. Y. Investigation of human urogenital tract tumors of papovavirus etiology: brief communication. J Natl Cancer Inst. 1978 Mar;60(3):579–582. doi: 10.1093/jnci/60.3.579. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Strandberg J. D. Sarcoma in a hamster inoculated with BK virus, a human papovavirus. J Natl Cancer Inst. 1975 Apr;54(4):945–950. [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Rangan S. R., Reissig M., Daniel R. W., Bellhan F. Z. Congenital transmission of a papovavirus of the stump-tailed macaque. Science. 1977 Jan 28;195(4276):404–406. doi: 10.1126/science.401546. [DOI] [PubMed] [Google Scholar]
- Shah K., Daniel R., Madden D., Stagno S. Serological investigation of BK papovavirus infection in pregnant women and their offspring. Infect Immun. 1980 Oct;30(1):29–35. doi: 10.1128/iai.30.1.29-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shein H. M. Transformation of astrocytes and destruction of spongioblasts induced by a simian tumor virus (SV40) in cultures of human fetal neuroglia. J Neuropathol Exp Neurol. 1967 Jan;26(1):60–76. doi: 10.1097/00005072-196701000-00005. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Iinuma M. Recovery of infectious proviral DNA from mammalian cells infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3230–3234. doi: 10.1073/pnas.72.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Defendi V., Wigglesworth N. M. The effect of dibutyryl cyclic AMP on transformation by oncogenic viruses. Virology. 1973 Jan;51(1):230–232. doi: 10.1016/0042-6822(73)90383-8. [DOI] [PubMed] [Google Scholar]
- Soriano F., Shelburne C. E., Gökcen M. Simian virus 40 in a human cancer. Nature. 1974 May 31;249(456):421–424. doi: 10.1038/249421a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Siddell S. G., Meulen V. T. Persistent and lytic infections with SSPE virus: a comparison of the synthesis of virus-specific polypeptides. J Gen Virol. 1981 Nov;57(Pt 1):191–197. doi: 10.1099/0022-1317-57-1-191. [DOI] [PubMed] [Google Scholar]
- Straver P. J., van Bekkum J. G. Plaque production by carrier strains of foot-and-mouth disease virus in BHK-monolayers incubated at different temperatures. Arch Gesamte Virusforsch. 1972;37(1):12–18. doi: 10.1007/BF01241146. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Integrated simian virus 40 DNA: nucleotide sequences at cell-virus recombinant junctions. J Virol. 1981 May;38(2):671–679. doi: 10.1128/jvi.38.2.671-679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. E., Friedrich T. D., Lehman J. M. Resistance of teratocarcinoma stem cells to infection with simian virus 40: early events. J Cell Physiol. 1977 Oct;93(1):25–30. doi: 10.1002/jcp.1040930105. [DOI] [PubMed] [Google Scholar]
- Tabuchi K., Kirsch W. M., Van Buskirk J. J. Immunocytochemical evidence of SV 40-related T antigen in two human brain tumours of ependymal origin. Acta Neurochir (Wien) 1978;43(3-4):239–249. doi: 10.1007/BF01587959. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Nagaki D., Saito M., Haruyama C., Iwasaki K. Transplacental transmission of BK virus in human. Jpn J Microbiol. 1975 Oct;19(5):395–398. doi: 10.1111/j.1348-0421.1975.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Bond S. B., Haase A. T., Ting R. C. Polyoma virus-human cell interactions: persistence of T-antigen in two cell lines with and without transformation. J Virol. 1978 Jan;25(1):326–330. doi: 10.1128/jvi.25.1.326-330.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Howley P. M., Miyamura T. JC human papovavirus replication in human amnion cells. J Virol. 1979 Apr;30(1):384–389. doi: 10.1128/jvi.30.1.384-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Thorne H. V. Cyclic variation in susceptibility of Balb-c 3T3 cells to polyoma virus. J Gen Virol. 1973 Feb;18(2):163–169. doi: 10.1099/0022-1317-18-2-163. [DOI] [PubMed] [Google Scholar]
- Topp W., Hall J. D., Rifkin D., Levine A. J., Pollack R. The characterization of SV40-transformed cell lines derived from mouse teratocarcinoma: growth properties and differentiated characteristics. J Cell Physiol. 1977 Nov;93(2):269–276. doi: 10.1002/jcp.1040930212. [DOI] [PubMed] [Google Scholar]
- Twist E. M., Clark H. F., Aden D. P., Knowles B. B., Plotkin S. A. Integration pattern of hepatitis B virus DNA sequences in human hepatoma cell lines. J Virol. 1981 Jan;37(1):239–243. doi: 10.1128/jvi.37.1.239-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979 Jul;63(1):119–126. [PubMed] [Google Scholar]
- Upcroft P., Skolnik H., Upcroft J. A., Solomon D., Khoury G., Hamer D. H., Fareed G. C. Transduction of a bacterial gene into mammalian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2117–2121. doi: 10.1073/pnas.75.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valis J. D., Newell N., Reissig M., Malherbe H., Kaschula V. R., Shah K. V. Characterization of SA12 as a simian virus 40-related papovavirus of chacma baboons. Infect Immun. 1977 Oct;18(1):247–252. doi: 10.1128/iai.18.1.247-252.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L., HINZE H. C. A carrier state of mumps virus in human conjunctiva cells. I. General characteristics. J Exp Med. 1962 Nov 1;116:739–750. doi: 10.1084/jem.116.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISBERG R. A. VIRUS MULTIPLICATION AND CELL KILLING IN POLYOMA-INFECTED MOUSE EMBRYO CULTURES. Virology. 1963 Dec;21:658–661. doi: 10.1016/0042-6822(63)90241-1. [DOI] [PubMed] [Google Scholar]
- Wakamiya T., McCutchan T., Rosenberg M., Singer M. Structure of simian virus 40 recombinants that contain both host and viral DNA sequences. I. The structure of variant CVPS/1/P2 (EcoRI res). J Biol Chem. 1979 May 10;254(9):3584–3591. [PubMed] [Google Scholar]
- Walker D. L., Chang P. P., Northrop R. L., Hinze H. C. Persistent, noncytocidal viral infection: nonsynchrony of viral and cellular multiplication. J Bacteriol. 1966 Oct;92(4):983–989. doi: 10.1128/jb.92.4.983-989.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Differences between the intracellular polypeptides of measles and subacute sclerosing panencephalitis virus. Nature. 1978 Mar 30;272(5652):458–460. doi: 10.1038/272458a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Integrated genomes of animal viruses. Annu Rev Biochem. 1980;49:197–226. doi: 10.1146/annurev.bi.49.070180.001213. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Herndon R. M., Narayan O., Johnson R. T., Shah K., Rubinstein L. J., Preziosi T. J., Conley F. K. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N Engl J Med. 1972 Feb 24;286(8):385–390. doi: 10.1056/NEJM197202242860801. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Johnson R. T., Herndon R. M. Viral infections and demyelinating diseases. N Engl J Med. 1973 May 24;288(21):1103–1110. doi: 10.1056/NEJM197305242882106. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Weiss A. F., Portmann R., Fischer H., Simon J., Zang K. D. Simian virus 40-related antigens in three human meningiomas with defined chromosome loss. Proc Natl Acad Sci U S A. 1975 Feb;72(2):609–613. doi: 10.1073/pnas.72.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Lampert P. W., Oldstone M. B. Prevention of virus-induced cerebellar diseases by defective-interfering lymphocytic choriomeningitis virus. J Infect Dis. 1977 Sep;136(3):391–399. doi: 10.1093/infdis/136.3.391. [DOI] [PubMed] [Google Scholar]
- Willoughby E., Price R. W., Padgett B. L., Walker D. L., Dupont B. Progressive multifocal leukoencephalopathy (PML): in vitro cell-mediated immune responses to mitogens and JC virus. Neurology. 1980 Mar;30(3):256–262. doi: 10.1212/wnl.30.3.256. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., DePamphilis M., Berg P. Simian virus 40-permissive cell interactions: selection and characterization of spontaneously arising monkey cells that are resistant to simian virus 40 infection. J Virol. 1976 Nov;20(2):391–399. doi: 10.1128/jvi.20.2.391-399.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Green M., Mackey J. K., Martin J. D., Padgett B. L., Walker D. L. Integration pattern of human JC virus sequences in two clones of a cell line established from a JC virus-induced hamster brain tumor. J Virol. 1980 Mar;33(3):1225–1228. doi: 10.1128/jvi.33.3.1225-1228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Mackey J. K., Brackmann K. H., Takemori N., Rigden P., Green M. Analysis of human tumors and human malignant cell lines for BK virus-specific DNA sequences. Proc Natl Acad Sci U S A. 1978 Jan;75(1):454–458. doi: 10.1073/pnas.75.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth-Gutai M. Recombination in SV40-infected cells: nucleotide sequences at viral-viral recombinant joints in naturally arising variants. Virology. 1981 Mar;109(2):344–352. doi: 10.1016/0042-6822(81)90505-5. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Revel M., Winocour E. Inhibition of simian virus 40 replication by interferon treatment late in the lytic cycle. Virology. 1977 Jul 1;80(1):225–228. doi: 10.1016/0042-6822(77)90397-x. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Furuno A., Nozawa A., Uchida S. Organization of viral genome in a T antigen-negative hamster tumor induced by human papovavirus BK. J Virol. 1981 May;38(2):556–563. doi: 10.1128/jvi.38.2.556-563.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Furuno A., Watanabe S., Yoshiike K. Occurrence of free, defective viral DNA in a hamster tumor induced by human papovavirus BK. Virology. 1980 May;103(1):241–244. doi: 10.1016/0042-6822(80)90143-9. [DOI] [PubMed] [Google Scholar]
- Yoshiike K., Miyamura T., Chan H. W., Takemoto K. K. Two defective DNAs of human polyomavirus JC adapted to growth in human embryonic kidney cells. J Virol. 1982 May;42(2):395–401. doi: 10.1128/jvi.42.2.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshike K., Defendi V. Addition of extra DNA sequences to simian virus 40 DNA in vivo. J Virol. 1977 Aug;23(2):323–337. doi: 10.1128/jvi.23.2.323-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanov V. M. Integration of viral genomes. Nature. 1975 Aug 7;256(5517):471–473. doi: 10.1038/256471a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Glaser R., Rapp F. Effect of dibutyryl cyclic AMP on the induction of Epstein-Barr virus in hybrid cells. J Virol. 1973 Dec;12(6):1442–1445. doi: 10.1128/jvi.12.6.1442-1445.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Rhein G. M. Association of papova-virions with a human demyelinating disease (progressive multifocal leukoencephalopathy). Prog Med Virol. 1969;11:185–247. [PubMed] [Google Scholar]
- Zu Rhein G. M., Varakis J. N. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC). Natl Cancer Inst Monogr. 1979 May;(51):205–208. [PubMed] [Google Scholar]
- Zu Rhein G., Padgett B. L., Walker D. L., Chun R. W., Horowitz S. D., Hong R. Pituitary function after removal of microadenomas for Cushing's disease. N Engl J Med. 1978 Aug 3;299(5):256–257. doi: 10.1056/NEJM197808032990517. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Hall W. W. Slow virus infections of the nervous system: virological, immunological and pathogenetic considerations. J Gen Virol. 1978 Oct;41(1):1–25. doi: 10.1099/0022-1317-41-1-1. [DOI] [PubMed] [Google Scholar]
- ter Schegget J., Voves J., van Strien A., van der Noordaa J. Free viral DNA in BK virus-induced hamster tumor cells. J Virol. 1980 Aug;35(2):331–339. doi: 10.1128/jvi.35.2.331-339.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]


