Abstract
Oscillatory tip growth in pollen tubes depends on prodigious amounts of energy. We have tested the hypothesis that oscillations in the electron transport chain lead to growth oscillations in lily (Lilium formosanum). Using three respiratory inhibitors, oligomycin, antimycin A, and cyanide, we find that pollen tube growth is much less sensitive to respiratory inhibition than respiration is. All three block respiration at concentrations severalfold lower than necessary to inhibit growth. Mitochondrial NAD(P)H and potentiometric JC-1 fluorescence, employed as markers for electron transport chain activity, rise rapidly in response to oligomycin, as expected. Pollen tube growth stops for several minutes before resuming. Subsequent growth has a lower mean rate, but continues to oscillate, albeit with a longer period. NAD(P)H fluorescence no longer exhibits coherent oscillations, and mitochondria no longer congregate directly behind the apex: they distribute evenly throughout the cell. Postinhibition growth relies on aerobic fermentation for energy production as revealed by an increase in ethanol in the media. These data suggest that oscillatory growth depends not on a single oscillatory pacemaker but rather is an emergent property arising from a number of stable limit cycles.
As a model system for studying tip growth, the lily (Lilium formosanum) pollen tube stands out. Its rapid growth puts it among the fastest growing cells known to science. At 15 to 20 μm across, its large diameter makes observations of the cytoplasm particularly gratifying. One of the most compelling features of this and other pollen tubes is the phenomenon of oscillatory tip growth. Pollen tubes of many species oscillate in growth rate (Chebli and Geitmann, 2007). This striking feature has drawn much attention in recent years in part because it represents an excellent means of exploring causal connections (for review, see Hepler et al., 2001, 2006; Robinson and Messerli, 2002; Chebli and Geitmann, 2007; Cheung and Wu, 2008). The search for the factors leading to oscillatory growth has led to the observation that several underlying cellular processes also oscillate in the growing pollen tube (Holdaway-Clarke et al., 1997, 2003; Moreno et al., 2007).
Among the oscillatory processes that have garnered the most attention are Ca2+ (Pierson et al., 1996; Holdaway-Clarke et al., 1997; Messerli et al., 2000), G-actin/F-actin (Cárdenas et al., 2005; Hwang et al., 2005), the alkaline band (Lovy-Wheeler et al., 2006), apical cell wall thickening (McKenna et al., 2009), NAD(P)H/NAD(P)+ (Cárdenas et al., 2006), Cl− efflux (Zonia et al., 2002), and Rop/Ric dynamics (Gu et al., 2005; Hwang et al., 2005). To discern causality, two basic approaches have been used to clarify the order of events. One approach has been to rank the oscillatory phenomenon through cross-correlation analysis. This procedure exploits variations in the amplitude and periodicity to determine which processes precede growth and which follow (Holdaway-Clarke et al., 1997; Cárdenas et al., 2006, 2008; Lovy-Wheeler et al., 2006). A second approach has been to show that oscillations in growth cannot continue when some other oscillator has been inhibited or curtailed (Wu et al., 2001; Gu et al., 2003, 2005, 2006; Hwang et al., 2005; Cárdenas et al., 2006; Lovy-Wheeler et al., 2006). Despite much effort, a central regulator has not been identified, though many have been proposed.
Recent work showed that oscillations in NAD(P)H/NAD(P)+ fluorescence lead growth oscillations in lily pollen tubes by 50° to 110° (Cárdenas et al., 2006). The reduced species, NAD(P)H, has greater fluorescence; thus, troughs in the oscillatory signal can be interpreted as NAD(P)+, whereas peaks are NAD(P)H (Møller, 2001; Rasmusson et al., 2004; Cárdenas et al., 2006). It was proposed that these oscillations might lead to oscillations in ATP concentration. As ATP is necessary for exocytosis, protein synthesis, ion pumping, and many other processes necessary for tip growth, Cárdenas et al. (2006) suggested that these oscillations in energy production might in turn lead to growth oscillations. Oscillations in NAD(P)H fluorescence and, indeed, ATP have been demonstrated in other organisms (Ghosh and Chance, 1964; Yoshimoto et al., 1981; O'Rourke et al., 1994; Ainscow and Rutter, 2002; Kennedy et al., 2002; Yang et al., 2008; Olsen et al., 2009). The most studied have been the glycolytic oscillations following perturbations of the electron transport chain (ETC) in yeast (Ghosh and Chance, 1964; Danø et al., 1999; Yang et al., 2008; Olsen et al., 2009). Similar glycolytic oscillations coupled to mitochondrial membrane potential have been linked to ATP changes in mammalian islet β-cells and have been shown to occur without perturbation (Ainscow and Rutter, 2002; Kennedy et al., 2002). Work in cardiac myocytes has also demonstrated that NAD(P)H/NAD(P)+ oscillates along with membrane potential and reactive oxygen species under defined growth conditions (O'Rourke et al., 1994; Aon et al., 2003). Combined with the data from other organisms, it has seemed plausible that oscillations in the mitochondrial ETC might be a good candidate for the oscillatory pacemaker (Cárdenas et al., 2006).
Lending support to the hypothesis that mitochondria play a role close to the oscillatory pacemaker has been the observation that these organelles congregate toward the tip, compellingly near the site of the alkaline band and the actin fringe (Lovy-Wheeler et al., 2005, 2006; Cárdenas et al., 2006). Other cells also position mitochondria near sites of high energy demand. In mammalian sperm cells, mitochondria localize to the tail, directly at the site of ATP utilization (Turner, 2003). In neurons, evidence suggests that mitochondria with particularly high ΔΨ values localize to sites of growth and great energy needs (Miller and Sheetz, 2004; Hollenbeck and Saxton, 2005; Lee and Peng, 2006, 2008). The pollen tube, like the neuron, is a long, polarized cell. Its rapid growth consumes tremendous amounts of energy. Pollen grains have been shown to have roughly 20 times the level of mitochondria as other plant tissues, a possible adaptation to the high energy demand in pollen coupled to the rapid growth of the pollen tube (Lee and Warmke, 1979). Furthermore, the FOF1-ATPase activity of lily pollen is several times higher than bulb and leaf tissues (Itoh and Sekiya, 1994). However, no direct evidence has been presented that mitochondrial energy production leads to, or is necessary for, growth oscillations in pollen tubes.
We ultimately wish to elucidate the causal events leading to oscillatory tip growth. However, the elusive pacemaker may prove to be a red herring. The rich literature concerning biological oscillators covers cycles as diverse as Canadian lynx/hare relationships (Krebs et al., 1995), fungal mycelia nutrient allocation (Tlalka et al., 2007), cardiac cell energetics (Aon et al., 2003, 2008a, 2008b), yeast energetics (Ghosh and Chance, 1964; Aon et al., 2003, 2008a, 2008b; Xu and Tsurugi, 2007), and of course circadian rhythms (Hastings et al., 2008; Johnson et al., 2008). It has become clear that in many of these systems a central pacemaker does not govern oscillations. Rather, coupled oscillations in limit cycles or a few distinct oscillations together form the observed emergent phenomenon (Strogatz, 2001; De La Fuente et al., 2008; Johnson et al., 2008). In the photosynthetic cyanobacteria Synechococcus elongatus, circadian rhythms develop from two separate oscillatory phenomenon: an intrinsic proteinaceous oscillator termed KaiABC and a transcription/translation feedback loop for the entire genome (Johnson et al., 2008). It has been argued that what appears to be a coherent oscillator can emerge as a property from many different independently oscillating processes (Strogatz, 2001; De La Fuente et al., 2008). It remains unclear whether the oscillatory phenomena observed in pollen tubes stem from a single pacemaker or are the emergent property of a system of cycles (Moreno et al., 2007).
In this report, we investigated whether oscillations in the ETC lead to growth oscillations. We present evidence that lily pollen tubes cannot only grow, but oscillate in the absence of aerobic respiration. We show that aerobic fermentation takes over the role of energy producer. Interestingly, although oscillations continue, the period is altered. When pollen tubes are growing normally, we suggest that despite the fact that oscillations in mitochondrial NAD(P)H/NAD(P)+ fluorescence are anticipatory, they are not causal. Rather, the oscillations occur because of an emergent system of stable limit cycles.
RESULTS
Respiratory Inhibitors Alter Growth in a Dose-Dependent Manner
To test the hypothesis that NAD(P)H/NAD(P)+ oscillations were leading to growth oscillations, we examined the effects of three inhibitors of the major complexes of the ETC. We chose inhibitors targeted to cytochrome bc1 (complex III), cytochrome oxidase (complex IV), and the FOF1-ATPase (complex V) of the mitochondrial ETC. These complexes were chosen because specific, well-characterized inhibitors are available and effective in plants. Antimyin A targets complex III and has been shown to stop electron transport and, therefore, proton pumping and ATP production. We inhibited complex IV using cyanide. Cyanide binds reversibly to cytochrome oxidase and blocks the final reduction of oxygen to water, thus preventing ATP production. Finally, we employed oligomycin, which targets the FO subunit of the FOF1-ATPase and binds irreversibly, thus blocking ATP production.
We examined the effect that increasing concentrations of each inhibitor had on growing pollen tubes. Experiments in the past have shown that doses of oligomycin in the 0.5 to 2 μm range are effective at slowing respiration in lily (Dickinson, 1965). Antimycin has been used at 2 μm in pollen to inhibit respiration (Tadege and Kuhlemeier, 1997). Experiments using cyanide have not been performed in pollen, but they have been performed on other plant tissues. A wide range of concentrations has been used, from 200 μm (Zabalza et al., 2009) to 2 mm (Yoshida et al., 2008).
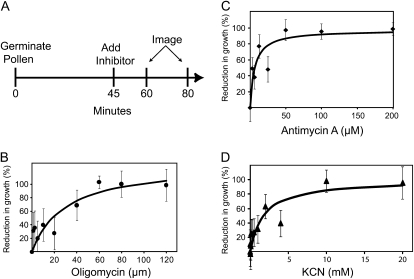
We germinated pollen and allowed it to grow to approximately 0.5 mm (Pierson et al., 1996) and then treated it with increasing doses of the inhibitors. Figure 1 shows the growth response to each inhibitor, including a dose-dependent decrease in growth and a complete cessation of growth at high concentrations. Pollen tube growth proved quite resistant to KCN up to 5 mm, showing only an approximately 50% reduction in growth at nearly twice the standard dosage. Cells grown in antimycin A showed 60% inhibition in concentrations as high as 25 μm. Oligomycin was surprisingly ineffective with 50% inhibition at 40 μm, which is far higher than the dose used to curtail respiration by Dickinson (1965; 0.5–2 μm). Even at 120 μm oligomycin, many cells are still growing. These data show that pollen tube growth appears to be remarkably resistant to respiratory ETC inhibitors.
Figure 1.
Inhibitors of aerobic respiration inhibit growth of lily pollen tubes. A, A schematic of the experimental outline. Subsequent panels show the reduction in growth of lily pollen tubes treated with oligomycin (B), Antimycin A (C), and KCN (D). A line was fitted to each data set using a nonlinear regression model, and the error bars represent se for four replicates (n > 8 per replicate).
Oxygen Uptake Is More Sensitive to Mitochondrial Inhibitors Than Growth
Previous studies showed a significant reduction (54%) in respiration using oligomycin at concentrations <2 μm (Dickinson, 1966). To test respiration in our system, we used a Clarke electrode to examine oxygen consumption of cells in KCN, oligomycin, and antimycin A. In many plant tissues, the alternative oxidase (AOX) allows respiration but not ATP synthesis to continue when complex III or IV is blocked. However, recent studies suggest that AOX is not present in pollen (Fujii et al., 2007; Fujii and Toriyama, 2008). Furthermore, if respiration is effectively curtailed by oligomycin, antimycin A, and KCN, it would be reasonable to conclude that AOX is not active in lily pollen.
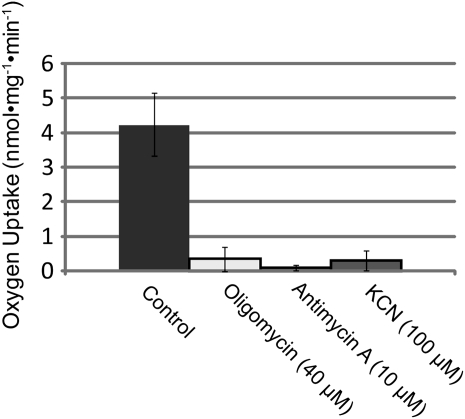
We grew cells to the oscillatory stage and then added inhibitor. We measured oxygen consumption and compared rates before and after inhibition. Surprisingly, at concentrations of inhibitor in which cells are still growing (Fig. 1), respiration had ceased or been dramatically reduced (Fig. 2). At 40 μm oligomycin, we saw a reduction in respiration rates ranging from 0.09 nmol mg−1 min−1 to near the detection limit (Fig. 2). This result is consistent with measurements performed previously, which showed that 2 μm oligomycin dropped respiration approximately 50% (Dickinson, 1965). Cells in 40 μm oligomycin grow at roughly 40% of the uninhibited growth rate (Fig. 1). In both Antimycin A- and KCN-treated cells, at 10 and 100 μm, respectively, oxygen consumption dropped to near the detection limit (Fig. 2). These results are particularly surprising given the data presented above showing that at 100 μm KCN and 10 μm Antimycin A, cells grow at or near uninhibited rates (Fig. 1, C and D). Taken together, the data in Figures 1 and 2 suggest that robust growth in lily pollen tubes can occur in the absence of aerobic respiration.
Figure 2.
Oxygen consumption in response to ETC inhbitors. A Clarke electrode was used to measure oxygen consumption in response to 40 μm oligomycin, 10 μm Antimycin A, and 100 μm KCN. The control shows an average of all trials. All experiments were performed in triplicate. Error bars represent sd.
The NAD(P)H Signal Reflects Mitochondrial NAD(P)H Utilization
Previously, Cárdenas et al. (2006) showed that the NAD(P)H signal and mitochondria colocalized in the pollen tube apex. However, our results indicating that growth was not inhibited by respiratory inhibitors led us to question whether the NAD(P)H signal reflects oxidation of the reductant in the mitochondria. Therefore, we investigated the effect of respiratory inhibitors upon NAD(P)H fluorescence. We reasoned that if the NAD(P)H signal represented mitochondrial reductant, we would see an increase in fluorescence upon treatment with inhibitor (Fisher et al., 1976; Kahraman and Fiskum, 2007). We tried all three inhibitors and found similar results with each; to remain concise, we will present the results only from oligomycin (Supplemental Fig. S1).
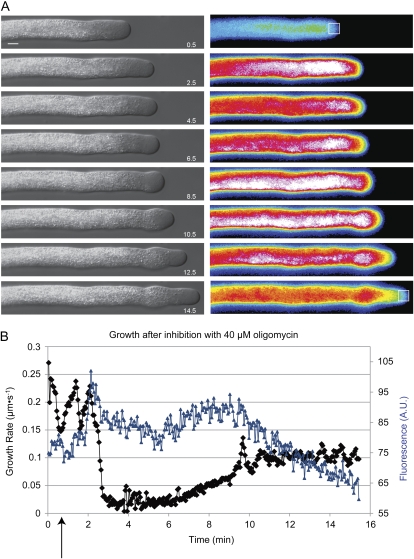
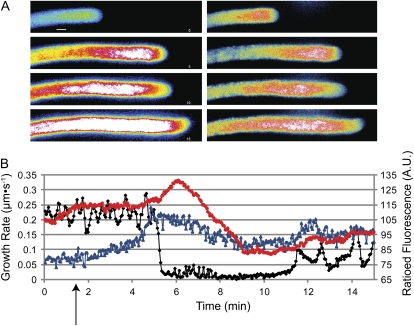
Given the results shown in Figure 1, we expected cells to slow down but to continue growing upon inhibition with 40 μm oligomycin. We also expected to see a rise in NAD(P)H fluorescence. In every trial, cells stopped growing as the NAD(P)H signal rose rapidly (Fig. 3, A and B, compare min 2.5–6.5; Supplemental Movies S1 and S2). In the pollen tube shown in Figure 3A, from 2.5 to 6.5 min, little measurable growth occurred. Cells then began growing again (Fig. 3A, compare 6.5 and 10.5 min). We observed this characteristic phenomenon with all three inhibitors tested and at a wide variety of concentrations (Supplemental Fig. S1). As is clear in Supplemental Movie S1, cytoplasmic streaming never ceases. Although the tip often swells during growth stoppage, the gross pollen tube morphology returns after growth resumption, including the apical clear zone (Fig. 3A, left column). As cells began growing again, the point of stoppage remains clear even after the pollen tube begins growing again (Fig. 3A; Supplemental Movies S1 and S2); the pollen tube is wider and the high NAD(P)H signal peak temporarily remains in this region. Figure 3B shows the growth rate of the pollen tube tip in black and the average fluorescence of a 10 μm box at the tip in blue. The NAD(P)H signal rises sharply and then as the tip begins growing again, the window of measurement follows the tip and the NAD(P)H signal goes down. The characteristics of the growth after recovery are considered below in Figures 5 and 6.
Figure 3.
NAD(P)H fluorescence responds to oligomycin. A, DIC (left) and pseudocolored NAD(P)H fluorescence images (right) of a pollen tube during 40 μm oligomycin treatment. The numbers in the bottom right-hand corners of the DIC images indicate time in minutes from the beginning of the experiment. The white boxes in the first and last fluorescence images correspond to the areas averaged for the chart in B. The NAD(P)H signal spikes especially in the area with the highest density of mitochondria. This peak stays at the spatial location when inhibition first occurred and remains at this place even as the cell begins growing again. Note that the fluorescence of the first image appears to be very low because the look-up table is relative to all the values and the intensity after inhibition is very high. Bar = 10 μm. B, The black line represents a trace of the tip of the pollen tube in A over time showing oscillations followed by growth cessation and recovery. The blue line represents the average fluorescence of a 10-μm box immediately behind the tip. The black arrow shows where oligomycin was added with a peristaltic pump. A.U., Arbitrary units.
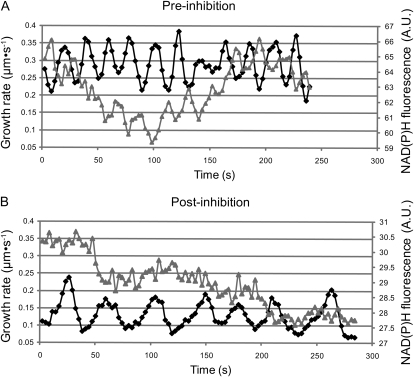
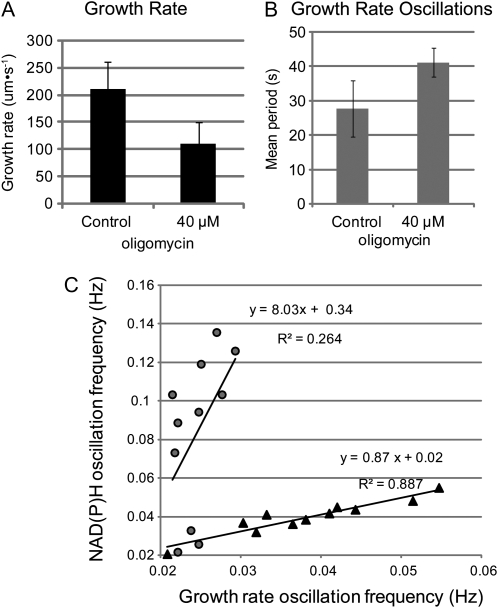
Figure 5.
Oscillatory growth before and after treatment with oligomycin. A, A control pollen tube grown in LPGM. Black trace represents growth of the tip, and gray represents the NAD(P)H fluorescence in a 10-μm box immediately behind the tip. Left y axis shows growth rate, whereas the right y axis shows average NAD(P)H fluorescence. Note that cross-correlation analysis reveals that the troughs of fluorescence precede the peaks in rate. B, A pollen tube after treatment with 40 μm oligomycin for approximately 10 min. The growth rate still oscillates though the period has changed (black trace). The fluorescence trace has lost coherence and no longer appears to oscillate (gray trace). A.U., Arbitrary units.
Figure 6.
Quantification of differences in growth and fluorescence between control and inhibited pollen tubes grown in oligomycin from 10 to 20 min. A, Mean growth rate of pollen tubes before (control) or after (40 μm oligomycin) inhibition with oligomycin. Growth rate after is significantly slower approximately 50% (t test of paired samples P < 0.0001). B, The period of growth rate oscillations were determined and the mean for pre (Control) and post (40 μm oligomycin) inhibition were determined. The period of growth rate increases significantly (Student's t test of paired samples P < 0.001). C, Comparison of the growth oscillation frequency (x axis) to the NAD(P)H oscillation frequency (y axis) as determined using the multitaper method (see “Materials and Methods”) of preinhibition cells (black triangles) to the same cells after inhibition (gray circles). A line has been fitted to each data set and the equation, and R2 value are shown. The preinhibition frequencies correlate quite well (P < 0.001); the regression line has a slope of nearly 1, whereas postinhibition, no significant correlation is detected (P = 0.1).
Mitochondrial Membrane Potential Responds along with NAD(P)H Fluorescence
To directly measure the mitochondrial membrane potential, we employed the potentiometric dye JC-1 (Reers et al., 1991; Smiley et al., 1991). At low ΔΨ levels, the dye exists as a monomer with an emission of approximately 525 nm. At high ΔΨ, the dye forms so-called J-aggregates with an emission of approximately 590 nm. By ratioing the two, the relative mitochondrial delta ΔΨ can be determined (Smiley et al., 1991).
We sequentially collected JC-1 fluorescence (at both 525 and 590 nm), NAD(P)H fluorescence, and differential interference contrast (DIC) images on growing pollen tubes before and during inhibition with oligomycin. Before analyzing the data, we ratioed the JC-1 emission at 590 nm to its emission at 525 nm. Figure 4A shows the response of the NAD(P)H signal in the left hand column and JC-1 on the right. As expected, both signals rise in tandem in response to oligomycin. Figure 4B shows the average signal in a 10 μm box at the pollen tube tip for NAD(P)H (blue) and ratioed JC-1 (red) along with the growth rate (black). Though both JC-1 and NAD(P)H rise in response to oligomycin addition, JC-1 responds somewhat more slowly. As the pollen tube begins growing again, both signals go down. This is largely due to the pollen tube tip moving away from the peak levels at the stopping point. We were not able to observe coherent oscillations in the JC-1 signal. This is likely because oscillations in the ETC are faster than the JC-1 response time. Feeney et al. (2003) showed that JC-1 response to carbonyl cyanide-p-trifluoromethoxyphenylhydrazone is on the order of minutes, whereas oscillations in the pollen tube are much faster: from 20 to 50 s.
Figure 4.
JC-1 and NAD(P)H both react to oligomycin. A, The left-hand column presents pseudocolored NAD(P)H fluorescence images taken at 5-min intervals during inhibition with 10 μm oligomycin. The right-hand column shows JC-1 images from the same tube collected simultaneously. The JC-1 is less dramatic but reports the same change as the NAD(P)H signal. B, Traces of tip growth (black), JC-1 ratioed fluorescence (red), and NAD(P)H fluorescence (blue) of the same pollen tube shown in A. Oligomycin was added as shown by the black arrow. A.U., Arbitrary units.
Growth Oscillations Occur in the Presence of 40 μm Oligomycin
To more thoroughly examine the characteristics of growth after respiratory inhibition, we examined the NAD(P)H signal and growth rate oscillations after treatment with oligomycin and compared them to those in untreated pollen tubes. Oligomycin was added to cells oscillating in growth rate in a flow through chamber. As expected, and as shown in Figure 3, growth stopped, streaming continued, and then, after several minutes, cells resumed growing. Before and during inhibition, we collected sequential DIC and NAD(P)H fluorescence images; Figure 5 shows traces from a representative cell before and during inhibition. Figure 5A shows a cell before inhibition, the black line represents the rate of growth of the tip, and the gray line represents the average fluorescence in a 10-μm square at the tip. Both NAD(P)H signal and growth rate oscillate at roughly the same period, though they are out of phase, with the trough of the NAD(P)H signal preceding the growth peaks as reported by Cárdenas et al. (2006). Figure 5B shows the same pollen tube after treatment with 40 μm oligomycin after growth had resumed. The NAD(P)H fluorescence has lost much of its coherence, and the period of the growth rate oscillation has increased. The pollen tube is growing at roughly 50% of the preinhibition rate, in line with our bulk growth study (Fig. 1B).
To quantify the observations presented in Figure 5, we monitored several cells before and during treatment with 40 μm oligomycin. We measured the NAD(P)H fluorescence in a 10-μm box at the tip and determined the growth rate. Figure 6A shows that the mean growth rate had decreased by 50% in line with our earlier observations (Fig. 1B). We next identified the primary frequencies for the growth rate and NAD(P)H fluorescence signals using a multitaper method (see “Materials and Methods” and Supplemental Materials S2). We found that the period of the growth rate oscillations had increased from 28 to 40 s for pollen treated with 40 μm oligomycin. This represents a significant increase of approximately 40% (P < 0.005). Before inhibition, the frequency of the NAD(P)H fluorescence oscillations for individual pollen tubes was generally the same or very close to the growth rate oscillation frequency (Fig. 6C, triangles). The slope of a line fitted to the data is nearly 1 (0.87, R2 = 0.887), indicating that the correlation between growth rate and NAD(P)H oscillation frequencies is excellent. After inhibition and growth reinitiation, the signal is much noisier, and the primary frequency identified using the multitaper method is quite variable and has no correlation to the growth rate oscillation (Fig. 6C, circles). These findings reveal a substantial change in periodicity and indicate that the NAD(P)H signal no longer correlates with growth. Indeed, cross-correlation analysis was not possible.
Mitochondria Redistribute in the Absence of Aerobic Metabolism
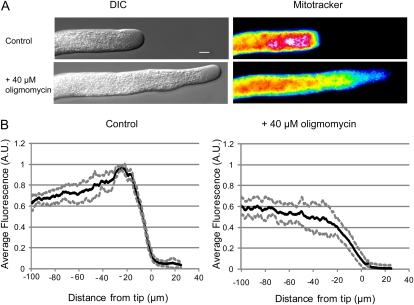
Previous studies have shown that mitochondria are not distributed evenly throughout the cell. Although observed through the length of the pollen tube, mitochondria congregate in large numbers directly behind the clear zone in the cell apex (Cárdenas et al., 2006; Lovy-Wheeler et al., 2007). It was suggested that the cell actively maintains this mitochondrial peak because of the organelle's contribution to the energy requirements of growth. We wished to investigate whether mitochondrial distribution would change after a shift in their energy status. If redistribution occurred, it would suggest that the pollen tube actively maintains the mitochondrial clusters observed previously.
We grew pollen tubes in lily pollen growth media (LPGM) supplemented with Mitotracker Green FM to visualize mitochondria then added 40 μm oligomycin. Figure 7A shows DIC and pseudocolored epifluorescence images of a representative pollen tube before and during inhibition. Note that the characteristic aggregation has disappeared in the inhibited pollen tube, indicating that the mitochondria have redistributed. To quantify this observation, we collected images of five pollen tubes before and during inhibition with 40 μm oligomycin and chose a representative image before inhibition and 5 to 10 min after growth had resumed. The difference between the distribution before and after inhibition is striking. The left panel of Figure 7B shows the characteristic mitochondrial peak behind the apex of the pollen tube. The black line represents the average value, and the gray lines show the sd at each point. On the right, the mitochondrial distribution after inhibition is shown. Again, the black lines represent the mean value at each point. The mitochondrial distribution is now roughly even along the length of the pollen tube. Clearly, the mitochondria have spread from a peak 20 μm behind the apex to a more uniform distribution throughout the pollen tube.
Figure 7.
Mitochondria redistribute in response to oligomycin. A, A DIC (left column) and pseudocolored epifluorescence (right column) image of a pollen tube treated with mitotracker Green-FM before (top two panels) and after (bottom two panels) treatment with 40 μm oligomycin. The intense peak directly behind the cell apex has dispersed, and the mitochondria are now evenly distributed. B, The average intensity along a line scan down the center of five pollen tubes was averaged and normalized. The black line represents the average value, and the gray lines represent the sd. The left-hand side shows the average prior to inhibition, and the right-hand side shows the average of the pollen tubes after inhibition. Note that though the average fluorescence is similar, there is now no apparent subapical peak and the values are more variable. A.U., Arbitrary units.
Fermentation Provides the Energy for Growth in the Absence of Aerobic Respiration
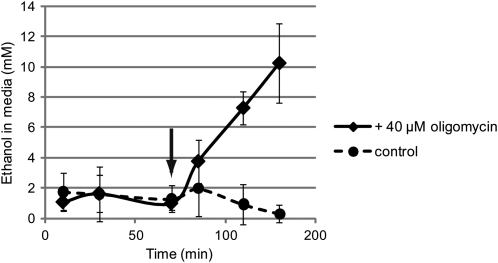
Plant cells of many types rely on ethanolic fermentation under stressful conditions and in conditions of low oxygen (Perata and Alpi, 1991; Kato-Noguchi and Yasuda, 2007; Zabalza et al., 2009). In tobacco (Nicotiana tabacum) pollen, aerobic fermentation has been well characterized in atmospheric oxygen (Bucher et al., 1995; Tadege and Kuhlemeier, 1997). Though similar experiments have not been performed in lily, it seemed reasonable to expect that the cell could use fermentation to regenerate NAD+ so that glycolysis could support ATP production and growth. Because ethanol produced by pollen tubes can be measured in the medium (Bucher et al., 1995), we investigated whether lily pollen tubes inhibited by oligoymycin produced ethanol.
We grew pollen in LPGM to the oscillatory stage while collecting samples of the medium every 30 min. At 70 min, oligomycin was added to a final concentration 40 μm (Fig. 8, arrow), and a sample was collected after 10 min. Subsequent samples were collected every 30 min. We chose this time frame because it encompasses those used in our growth experiments. Figure 8 shows that a rapid increase in ethanol concentration began shortly after the addition of oligomycin. After 2 h, the concentration of ethanol in the media had risen to 10 mm. The data indicate that a low level of ethanol production may occur in untreated lily pollen tubes, but it does not change substantially over the course of the experiment.
Figure 8.
Oligomycin treatment causes an increase in ethanol production. Pollen tubes were grown in LPGM for 70 min before the addition of oligomycin to a final concentration of 40 μm (arrow) to half of the cells. Black solid line represents the ethanol concentration in the oligomycin-treated samples. The dashed line represents the control sample. The experiment was performed in quintuplicate, and the error bar represents sd.
DISCUSSION
We set out to test the hypothesis that oscillations in pollen tube growth emerge from oscillations in the mitochondrial ETC. Our data show that instead lily pollen tubes can grow and oscillate in the absence of aerobic respiration. Given previous results showing a strong cross-correlation between NAD(P)H/NAD(P)+ fluorescence and growth and the suggestion that the NAD(P)H signal was coming from mitochondrial NAD(P)H dehydrogenases (Cárdenas et al., 2006), we expected to see a similar correlation between growth and respiration. Instead, we observed that growth is dramatically less sensitive to ETC inhibitors than respiration (Figs. 1 and 2). Previous results showed that the NAD(P)H signal colocalized with mitochondria but did not show that it was involved in the ETC. Examination of the NAD(P)H signal as the pollen tube reacted to respiratory inhibitors showed that the signal does originate in the mitochondria: it reacts as predicted to oligomycin and KCN (Fig. 3; Supplemental Fig. S1), consistent with previous data that NAD(P)H/NAD(P)+ oscillations cross-correlate with growth (Cárdenas et al., 2006). Furthermore, JC-1, a potentiometric ΔΨ indicator, responds similarly to the NAD(P)H signal (Fig. 4). Taken together, our results suggest that the oscillations in growth rate are coupled to oscillations in the ETC during aerobic growth but that they are not essential: in the absence of respiration, the growth rate still oscillates.
We observed that the period of growth rate oscillations changes after respiratory inhibition (Figs. 5 and 6). This suggests that in an uninhibited pollen tube, aerobic respiration does indeed exert an influence on the periodicity of growth oscillations. In the absence of respiration, oscillations continue by using a nonaerobic energy source, suggesting that oscillatory growth itself arises from factors other than oscillations in the ETC. We suggest that the oscillatory phenomenon observed in pollen tubes is an emergent system that develops its frequency and amplitude from a network of limit cycles stabilized by processes such as feedback regulation and substrate limitation.
Many of these limit cycles have received considerable attention in the literature (Moreno et al., 2007). It has already been shown that wall thickness (McKenna et al., 2009), the alkaline band (Lovy-Wheeler et al., 2006), Ca2+ (Pierson et al., 1996), Cl− efflux (Zonia et al., 2002), Rop activation (Gu et al., 2005; Hwang et al., 2005), and the G-/F-actin pool oscillate (Hwang et al., 2005; Cárdenas et al., 2008). We propose that none of these is the unique pacemaker, but rather the totality of these interacting stable limit cycles creates the phenomenon of oscillatory growth. In this scenario, alterations in any given input would shift the emergent growth oscillation. In previous studies, this has been obscured by the fact that most manipulations turn processes off, thus blocking growth completely. Thus, when latrunculin is added at low concentrations, actin dynamics are radically shifted, and oscillations cease (Cárdenas et al., 2008). When Rops and Rics are overexpressed, cells lose polarity (Wu et al., 2001; Gu et al., 2003, 2005, 2006). When the alkaline band is acidified, growth stops (Lovy-Wheeler et al., 2006). The utility of the current manipulation derives from the fact that pollen tubes continue to produce energy without input from the mitochondria. Growth oscillations change character, yet continue to emerge from the system as a whole.
In Figure 7, we demonstrated that mitochondria redistribute after inhibition of the ETC with oligomycin. These data support the hypothesis that in respiring pollen tubes, mitochondria are actively maintained directly behind the apex to provide energy to the growing pollen tube. After inhibition, the mitochondria are no longer maintained at the tip but uniformly distribute along the length of the tube. This observation supports the hypothesis that the mitochondria are critical for growth when they are providing ATP. Upon inhibition, they no longer serve this purpose and their localization changes as they are swept backwards by cytoplasmic streaming.
To offset the loss of energy from mitochondria, the pollen tube begins aerobic fermentation at a rapid rate (Fig. 8). Previous work on growing pollen tubes has demonstrated similar increases in ethanol production though generally over longer time periods in tobacco or petunia (Petunia hybrida) and without inhibition (Bucher et al., 1995; Tadege and Kuhlemeier, 1997; Mellema et al., 2002; Gass et al., 2005). Potentially, the pathway is active in respiring pollen tubes, where it may be used for lipid synthesis and the formation of new membranes (Mellema et al., 2002). After respiratory inhibition, this pathway regenerates NAD+ for glycolysis. Most probably, the pollen tube could not possibly sustain growth based on fermentation for long. Glycolytic production of ATP is much less efficient than through oxidative phosphorylation; it consumes more than 15 times as much Glc to produce the same amount of ATP. Over the short term, it produces enough energy to drive growth at a reduced rate. Gass et al. (2005) demonstrated that oligomycin treatment inhibited growth but not germination. In this experiment, cells were grown for 15 h in oligomycin or were analyzed shortly after germination. No difference was seen in the brief experiment, though there was a significant decrease in growth after 15 h (Gass et al., 2005). This is consistent with the idea that glycolytic energy production is efficient enough for short periods given the pollen tube's high starch reserves but that over long periods, growth cannot be maintained.
One of the most striking observations of this study is the temporary growth halt after respiratory inhibition. The morphology of the pollen tube apex observed by DIC does not appreciably change, though the tip does become a little wider and growth stops (Fig. 3; Supplemental Movies S1 and S2). Cytoplasmic streaming continues unabated, and after approximately 5 min, growth begins anew along with a spike in ethanol production (Figs. 3 and 8). Potentially the pollen tube's metabolism is switching gears during the stoppage. The mitochondria redistribute (Fig. 7), ethanolic fermentation increases (Fig. 8), and growth resumes. It will be interesting to investigate whether other oscillatory processes continue during this stoppage.
CONCLUSION
In conclusion, we suggest that pollen tube growth oscillations are the result of an emergent system arising from interacting stable limit cycles: there is not a single pacemaker. By inhibiting a replaceable contributor to the emergent phenomenon, we have altered the character of the oscillations, after a brief cessation of growth. In future studies, this treatment will allow us to examine changes in the other oscillators. Does the alkaline band or the oscillation of wall thickness change along with the growth oscillations? Perhaps more interestingly, does the Ca2+ flux respond to the change quickly, or is there a lag time? We will also be interested in investigating the mechanisms that concentrate mitochondria in the apical region.
MATERIALS AND METHODS
Cell Growth Conditions
All pollen was from Lilium formosanum and was grown from frozen stocks (−80°C) collected from plants grown under standard greenhouse conditions. Pollen was germinated and cultured on a rotator at room temperature in a growth medium consisting of 7% (w/v) Suc, 1 mm KCl, 1.6 mm H3BO3, 0.1 mm CaCl2, and 15 mm MES buffer adjusted to pH 5.7 with KOH (LPGM; all reagents were from Fisher Scientific unless otherwise noted). For microscopy observations, a pollen suspension was plated on custom-made well slides and immobilized with a growth medium solution containing a final concentration of 0.7% (w/v) low-melting agarose (Sigma-Aldrich). The immobilized pollen was then covered with fresh growth medium for imaging.
Growth Rate and Fluorescence Measurements
Growth rate was measured using the tip-tracking feature of the MetaMorph software package (Molecular Devices). The average fluorescence was measured in a 10-μm2 box centered 5 μm from the pollen tube tip (Cárdenas et al., 2006) using a custom R script (Supplemental Materials S1; Ihaka and Gentleman, 1996).
NAD(P)H and JC-1 Epifluorescence and DIC
DIC, JC-1, and NAD(P)H images were acquired using a CCD camera (Quantix Cool Snap HQ; Roper Scientific) attached to a Nikon TE300 inverted microscope (Nikon Instruments) with a 40×/1.3 numerical aperture oil immersion objective lens. All the equipment was operated with MetaMorph/MetaFluor software. A filter wheel system (Lambda 10-2; Sutter Instruments), mounted immediately before the CCD camera, was used to control the position of emission filters for fluorescence ratio imaging and a polarizing filter for DIC imaging. We used the following filter setup for NAD(P)H imaging: 360 nm (10 nm band-pass) as excitation filter, 380 nm dichroic, and 400-nm long-pass emission filter (all filters were from Chroma). We employed an exposure time of 750 ms and binned the images using ImageJ before analysis. We used the following filter setup for JC-1 imaging: 495 nm (10 nm band-pass) as excitation filter, a triple band (UV/D/F/R) dichroic, and 535- and 580-nm emission filters (all filters were from Chroma). Exposure times were 50 ms for 535 emission and 200 ms for 580 nm. The 580-nm emission was then ratioed to the 535-nm emission and an 8-bit lookup table was applied. We simultaneously collected NAD(P)H using the NAD(P)H excitation and emission filters described above and a 750-ms exposure time. Images were collected at 3-s intervals. The setup allowed fast (<1 s) acquisition of the ratio pair and the corresponding DIC image.
Waveform Analysis
To determine periodicity of both the NAD(P)H and growth rate oscillations, the SSA-MTM toolkit was used (http://www.atmos.ucla.edu/tcd/ssa/). The signal was analyzed with the multitaper method spectrum analysis, and the principle frequency components of the oscillation were recorded (Supplemental Fig. S2). We used the default values for the MTM settings: resolution 2, three tapers, and frequencies from 0 to 0.5.
Clarke Electrode Measurements
Oxygen consumption measurements were performed using a Clarke O2 electrode with a CB1D control Box (Hansatech). The electrode held 2 mL of LPGM with pollen at 1.5 mg mL−1. The temperature of the cell was maintained at 25°C using a recirculating refrigerated water bath. The analog signal from the electrode was converted to digital using a DATAQ DI-158V series data acquisition device (DATAQ Instruments). These data were recorded using WinDaq software (DATAQ Instruments) and then exported to Microsoft Excel for analysis. The raw data were converted to nmol of O2 mg−1 of pollen min−1. Briefly, the slope of the plotted data in mV min−1 was calculated. The saturation and zero point for oxygen were determined for each experiment empirically (fresh buffer versus sodium dithionite). Using the theoretical saturation point, we then calculated the oxygen consumption rate.
Ethanol Determination
Pollen was grown in LPGM in 10 separate 1.5-mL microcentrifuge tubes at 10 mg mL−1. Samples were collected every 30 min until 70 min when oligomycin was added to 40 μM. An aliquot was collected 10 min later and then subsequently every 30 min. Ethanol concentration in the media was determined using an enzymatic assay coupled to the absorbance of NADH. Aldehyde dehydrogenase (100 μL; MP Biomedicals) at 0.8 mg mL−1 and NAD+ at 2.6 mg mL−1 in 50 mm sodium pyrophosphate, pH 9.0, was added to wells in a 96-well plate. To this was added a 1:16 dilution from the growth media. Finally, alcohol dehydrogenase (MP Biomedicals) was added at 1 mg mL−1 in 50 mm sodium pyrophosphate, pH 9.0. Absorbance at 365 nm was collected every min for 15 min until the signal was unchanging on a Spectra Max M5 plate reader (Molecular Devices). A blank, with all enzymes but no sample, was subtracted and the resulting value was compared to standard ethanol concentrations.
Mitochondria Imaging and Analysis
For mitochondrial staining, we used Mitotracker Green FM (Invitrogen), which was dissolved in LPGM at a final concentration of 1 μM. The pollen tubes were stained in the dye medium for 20 min before imaging. Excitation was carried out at 495 nm, with fluorescence emission at 516 nm. A representative frame before and after inhibition was picked for analysis with MetaMorph software (Molecular Devices). A line scan that averaged the values in a 35-pixel-wide band down the center of the pollen tube was recorded. The values for each pollen tube were normalized to the peak value and then the tip of the pollen tube was set to zero. The values for five pollen tubes before and after inhibition were averaged.
Growth Inhibition Titrations
Pollen was germinated and grown in LPGM for 45 min to 1 h and observed to ensure that most cells had germinated. The pollen suspension was then pipetted into duplicate 12-well plates with increasing amounts of inhibitor as well as controls. A concentrated stock of KCN (Fisher Scientific) was made in water and then diluted to the final concentration. Oligomycin (Acros Organics) and antimycin A (MP Biomedicals) were solubilized in ethanol at 10 mm and then diluted to their final working concentrations. The cells were incubated for 15 min and then imaged at 15× zoom with a 1× lens on a stereomicroscope (Leica MZ16FA) using a CCD camera (Leica DF300FX). After a subsequent 20-min incubation, the cells were imaged again. The first incubation ensured that the pollen had been exposed to the inhibitor long enough to be affected. Images were analyzed and cells longer than three pollen grain lengths were measured using ImageJ (sbweb.nih.gove/ij). Statistical analysis was performed using the Xlstat (Addinsoft) plugin for Microsoft Excel.
Growth Inhibition for Individual Cells
Cells were grown as described above. For Figures 3 to 7, a two-tube peristaltic pump was used to add oligomycin solubilized in DMSO and diluted into LPGM (Bio-Rad). One tube was used to remove the growth media from the slide under analysis, while the other added media with inhibitor. The rate was set to between 0.25 mL min−1 for 150 s and then lowered to 0.1 mL min−1 for the duration of the experiment.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. KCN inhibition of growth.
Supplemental Figure S2. Growth rate amplitude and mean frequency.
Supplemental Movie S1. DIC of pollen tube from Figure 3.
Supplemental Movie S2. Epifluorescence image of pollen tube from Figure 3.
Supplemental Material S1. R script for fluorescence and rate analysis.
Supplemental Material S2. Output of SSA-MTM signal analysis.
Supplementary Material
Acknowledgments
We thank members of the Dr. M. Bezanilla lab and Dr. T.I. Baskin lab for helpful discussions and the Dr. Sam Hazen lab for use of the plate reader.
This work was supported by the National Science Foundation (grant nos. MCB–0516852 and MCB–0847876 to P.K.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter K. Hepler (hepler@bio.umass.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ainscow EK, Rutter GA (2002) Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet β-cells: evidence for a Ca2+-dependent mechanism. Diabetes 51 S162–S170 [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marbán E, O'Rourke B (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278 44735–44744 [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, O'Rourke B (2008. a) Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol 641 98–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Roussel MR, Cortassa S, O'Rourke B, Murray DB, Beckmann M, Lloyd D (2008. b) The scale-free dynamics of eukaryotic cells. PLoS One 3 e3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Brander KA, Sbicego S, Mandel T, Kuhlemeier C (1995) Aerobic fermentation in tobacco pollen. Plant Mol Biol 28 739–750 [DOI] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK (2005) Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil Cytoskeleton 61 112–127 [DOI] [PubMed] [Google Scholar]
- Cárdenas L, McKenna ST, Kunkel JG, Hepler PK (2006) NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol 142 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Geitmann A (2007) Mechanical principles governing pollen tube growth. Funct Plant Sci Biotechnol 1 232–245
- Cheung AY, Wu H (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59 547–572 [DOI] [PubMed] [Google Scholar]
- Danø S, Sørensen PG, Hynne F (1999) Sustained oscillations in living cells. Nature 402 320–322 [DOI] [PubMed] [Google Scholar]
- De La Fuente IM, Martínez L, Pérez-Samartín AL, Ormaetxea L, Amezaga C, Vera-López A (2008) Global self-organization of the cellular metabolic structure. PLoS One 3 e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DB (1965) Germination of lily pollen: respiration and tube growth. Science 150 1818–1819 [DOI] [PubMed] [Google Scholar]
- Dickinson DB (1966) Inhibition of pollen respiration by oligomycin. Nature 210 1362–1363 [Google Scholar]
- Feeney CJ, Pennefather PS, Gyulkhandanyan AV (2003) A cuvette-based fluorometric analysis of mitochondrial membrane potential measured in cultured astroycte monolayers. J Neurosci Methods 30 13–25 [DOI] [PubMed] [Google Scholar]
- Fisher AB, Furia L, Chance B (1976) Evaluation of redox state of isolated perfused rat lung. Am J Physiol 230 1198–1204 [DOI] [PubMed] [Google Scholar]
- Fujii S, Komatsu S, Toriyama K (2007) Retrograde regulation of nuclear gene expression in CW-CMS of rice. Plant Mol Biol 63 405–417 [DOI] [PubMed] [Google Scholar]
- Fujii S, Toriyama K (2008) DCW11, down-regulated gene 11 in CW-type cytoplasmic male sterile rice, encoding mitochondrial protein phosphatase 2c is related to cytoplasmic male sterility. Plant Cell Physiol 49 633–640 [DOI] [PubMed] [Google Scholar]
- Gass N, Glagotskaia T, Mellema S, Stuurman J, Barone M, Mandel T, Roessner-Tunali U, Kuhlemeier C (2005) Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 17 2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Chance B (1964) Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun 16 174–181 [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z (2006) Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 18 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Vernoud V, Fu Y, Yang Z (2003) ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot 54 93–101 [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, O'Neill JS (2008) Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol 18 R805–R815 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Lovy-Wheeler A, McKenna S, Kunkel J (2006) Ions and pollen tube growth. In R Malhó, ed, The Pollen Tube. Springer, Heidelberg, Germany, pp 47–69
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17 159–187 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Parris C, Kunkel JG, Hepler PK (2003) Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J Exp Bot 54 65–72 [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM (2005) The axonal transport of mitochondria. J Cell Sci 118 5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Gu Y, Lee Y, Yang Z (2005) Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell 16 5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Statist 5 299–314 [Google Scholar]
- Itoh A, Sekiya J (1994) Tissue specificity of mitochondrial FOF1-ATPase activity of Lilium longiflorum plant. FEBS Lett 356 229–232 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Egli M, Stewart PL (2008) Structural insights into a circadian oscillator. Science 322 697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman S, Fiskum G (2007) Anoxia-induced changes in pyridine nucleotide redox state in cortical neurons and astrocytes. Neurochem Res 32 799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H, Yasuda Y (2007) Effect of low temperature on ethanolic fermentation in rice seedlings. J Plant Physiol 164 1013–1018 [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Kauri LM, Dahlgren GM, Jung S (2002) Metabolic oscillations in beta-cells. Diabetes 51 (Suppl 1): S152–S161 [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Boutin S, Boonstra R, Sinclair ARE, Smith JNM, Dale MRT, Martin K, Turkington R (1995) Impact of food and predation on the snowshoe hare cycle. Science 269 1112–1115 [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB (2006) Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol 66 522–536 [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB (2008) The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell 19 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Warmke HE (1979) Organelle size and number in fertile and T-cytoplasmic male-sterile corn. Am J Bot 66 141–148 [Google Scholar]
- Lovy-Wheeler A, Cárdenas L, Kunkel JG, Hepler PK (2007) Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil Cytoskeleton 64 217–232 [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK (2006) Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell 18 2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221 95–104 [DOI] [PubMed] [Google Scholar]
- McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK (2009) Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21 3026–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellema S, Eichenberger W, Rawyler A, Suter M, Tadege M, Kuhlemeier C (2002) The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J 30 329–336 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Créton R, Jaffe LF, Robinson KR (2000) Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol 222 84–98 [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP (2004) Axonal mitochondrial transport and potential are correlated. J Cell Sci 117 2791–2804 [DOI] [PubMed] [Google Scholar]
- Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52 561–591 [DOI] [PubMed] [Google Scholar]
- Moreno N, Colaço R, Feijó JA (2007) The pollen tube oscillatory: integrating biophysics and biochemistry into cellular growth and morphogenesis. In S Mancuso, S Shabala, eds, Rhythms in Plants. Springer, Heidelberg, Germany, pp 39–62
- Olsen LF, Andersen AZ, Lunding A, Brasen JC, Poulsen AK (2009) Regulation of glycolytic oscillations by mitochondrial and plasma membrane H+-ATPases. Biophys J 96 3850–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke B, Ramza BM, Marban E (1994) Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265 962–966 [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A (1991) Ethanol-induced injuries to carrot cells: the role of acetaldehyde. Plant Physiol 95 748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174 160–173 [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Soole KL, Elthon TE (2004) Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol 55 393–400 [DOI] [PubMed] [Google Scholar]
- Reers M, Smith TW, Chen LB (1991) J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 18 4480–4486 [DOI] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA (2002) Pulsating ion fluxes and growth at the pollen tube tip. Sci STKE 162 PE51. [DOI] [PubMed] [Google Scholar]
- Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD Jr, Chen LB (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA 88 3671–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH (2001) Exploring complex networks. Nature 410 268–276 [DOI] [PubMed] [Google Scholar]
- Tadege M, Kuhlemeier C (1997) Aerobic fermentation during tobacco pollen development. Plant Mol Biol 35 343–354 [DOI] [PubMed] [Google Scholar]
- Tlalka M, Bebber DP, Darrah PR, Watkinson SC, Fricker MD (2007) Emergence of self-organised oscillatory domains in fungal mycelia. Fungal Genet Biol 44 1085–1095 [DOI] [PubMed] [Google Scholar]
- Turner RM (2003) Tales from the tail: what do we really know about sperm motility? J Androl 24 790–803 [DOI] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z (2001) A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13 2841–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Tsurugi K (2007) Role of Gts1p in regulation of energy-metabolism oscillation in continuous cultures of the yeast Saccharomyces cerevisiae. Yeast 24 161–170 [DOI] [PubMed] [Google Scholar]
- Yang J, Yang L, Qu Z, Weiss JN (2008) Glycolytic oscillations in isolated rabbit ventricular myocytes. J Biol Chem 283 36321–36327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe C, Kato Y, Sakamoto W, Noguchi K (2008) Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2. Plant Cell Physiol 49 592–603 [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y, Sakai T, Kamiya N (1981) ATP oscillation in Physarum plasmodium. Protoplasma 109 159–168 [Google Scholar]
- Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmälzlin E, Igal M, Orcaray L, Royuela M, et al (2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol 149 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Cordeiro S, Tupý J, Feijó JA (2002) Oscillatory chloride efflux at the pollen tue apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 14 2233–2249 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.