Abstract
In grapevine (Vitis vinifera), as in many crops, soluble sugar content is a major component of yield and economical value. This paper identifies and characterizes a Glycogen Synthase Kinase3 protein kinase, cloned from a cDNA library of grape Cabernet Sauvignon berries harvested at the ripening stage. This gene, called VvSK1, was mainly expressed in flowers, berries, and roots. In the berries, it was strongly expressed at postvéraison, when the berries accumulate glucose, fructose, and abscisic acid. In grapevine cell suspensions, VvSK1 transcript abundance is increased by sugars and abscisic acid. In transgenic grapevine cells overexpressing VvSK1, the expression of four monosaccharide transporters (VvHT3, VvHT4, VvHT5, and VvHT6) was up-regulated, the rate of glucose uptake was increased 3- to 5-fold, and the amount of glucose and sucrose accumulation was more than doubled, while the starch amount was not affected. This work provides, to our knowledge, the first example of the control of sugar uptake and accumulation by a sugar-inducible protein kinase.
Efficient and coordinated transport of assimilates is essential for plant growth, development, and productivity. Plant growth and plant productivity are not limited by photosynthesis per se but rather by the ability of the plant to export and to partition its assimilates in a proper way. Increased crop yields are paralleled by a better partitioning of assimilates between the harvestable part of the plant and the rest of the plant (Gifford and Evans, 1981; Gifford et al., 1984; Hoffmann-Thoma et al., 1996). Long-distance and intercellular transport of sugars is mediated by proton-coupled Suc transporters (belonging to the disaccharide transporter family) and hexose and polyol transporters (belonging to the monosaccharide transporter family). Many of these transporters have been identified in a wide range of species (Lemoine, 2000; Sauer et al., 2004; Büttner, 2007). Disaccharide transporter genes belong to small multigenic families (e.g. nine members in Arabidopsis [Arabidopsis thaliana] and four in tomato [Solanum lycopersicum]; Sauer et al., 2004; Hackel et al., 2006) and can be clustered into four groups regarding the homologies of the encoded protein sequences. The Arabidopsis genome has 53 homologous sequences encoding putative monosaccharide transporters, which are distributed into seven distinct clusters (Büttner, 2007).
Control of the expression of these transporters in specific cell types at precise stages of development allows fine-tuning in the plant of its carbon distribution. Although many sugar transporters have now been identified and characterized, little is known about their regulation. Up-regulation of a Glc transporter gene has been observed after treatment of Chenopodium rubrum cells with cytokinin (Ehness and Roitsch, 1997). In Arabidopsis, the monosaccharide transporter gene AtSTP4 may be induced by fungal and bacterial elicitors (Truernit et al., 1996; Fotopoulos et al., 2003), whereas the fungal elicitor cryptogein rapidly and completely blocks Glc uptake in tobacco (Nicotiana tabacum) cells (Bourque et al., 2002).
Accumulating evidence indicates that sugars may control the expression and activity of their own transporters. The ability to sense altered sugar concentration is important and allows the plant to regulate its metabolism and compartmentation processes in order to face the demands of sink organs (Roitsch, 1999). There is some discrepancy about the effect of sugars on the control of these transporters. In C. rubrum cell suspensions, monosaccharide transporter genes are constitutively expressed and not regulated by sugar (Roitsch and Tanner, 1994). Suc was reported to repress the sugar beet (Beta vulgaris) Suc transporter BvSUT1 both at the transcriptional and translational levels (Chiou and Bush, 1998; Vaughn et al., 2002; Ransom-Hodgkins et al., 2003), whereas it up-regulates OsSUT1 in rice (Oryza sativa; Matsukura et al., 2000). Down-regulation of monosaccharide transport in response to Glc supply was also observed in suspension-cultured cells of olive (Olea europaea; Oliveira et al., 2002) and in peach (Prunus persica) tree buds (Maurel et al., 2004). By contrast, the expression of VvHT1 in grape (Vitis vinifera) cell suspensions is induced by low concentration of Suc (Atanassova et al., 2003) and repressed at high Suc concentration (Conde et al., 2006), suggesting that the effect of sugars on transporter genes depends on sugar concentration and on the physiological status of the cells.
In contrast to the situation in bacteria, yeast, and mammals, where sugar signaling pathways are extensively studied (Johnston, 1999), signaling cascades involved in response to sugar in plants are still poorly understood. However, the combination of mutant isolation and pharmacological approaches reveals the involvement of protein kinases, protein phosphatases, and other signal transduction mediators such as Ca2+ and calmodulin (Smeekens, 2000). Among the protein kinases identified in sugar sensing and signaling in plants, the importance of sucrose nonfermenting-like protein kinase complexes and interacting proteins has been well established (Rolland et al., 2006). In addition, the involvement of other protein kinases, like calcium-dependent protein kinases (Ohto and Nakamura, 1995) and mitogen-activating protein kinases (Ehness et al., 1997), has also been suggested.
Glycogen Synthase Kinase3 (GSK3) is a constitutively active Ser/Thr kinase involved in diverse physiological activities and functions such as metabolism, cell cycle, gene expression, and development (Rayasam et al., 2009). In mammalian cells, GSK3 is an important signaling player that is ubiquitously expressed and has been implicated in the regulation of Glc transport and metabolism (Grimes and Jope, 2001). The few existing reports about regulation of Glc uptake by GSK3 have produced different findings in different cell systems (Nikoulina et al., 2002; Wieman et al., 2007), implicating GSK3 in either insulin signaling or non-insulin-stimulated conditions in cell types that are not insulin sensitive. GSK3/SHAGGY-like kinases (GSKs) are a large gene family in plants (Jonak and Hirt, 2002). Recent functional studies indicate that plant GSKs are involved in diverse important processes including hormone signaling, development, and stress responses (Dornelas et al., 2000; Jonak et al., 2000; Piao et al., 2001; Choe et al., 2002; Li and Nam, 2002; Perez-Perez et al., 2002; Vert and Chory, 2006; Kempa et al., 2007).
In this work, we report the involvement of VvSK1, a GSK3/SHAGGY-like kinase, in the regulation of sugar accumulation in grape cell suspension in response to sugar. This GSK3-like kinase, whose expression is increased by sugars, probably acts through the regulation of monosaccharide transporter gene expression. The analysis of its gene expression during grape berry development also suggests that it may be involved in the control of sugar accumulation during berry ripening.
RESULTS
Isolation and Sequence Analysis of VvSK1
Total RNA extracted from Cabernet Sauvignon berry cells treated or not with 58 mm Suc was hybridized with 70-mer oligoarrays bearing a set of 14,562 unigenes (Qiagen Operon Array-Ready Oligo Set for the Grape Genome Version 1.0). Among the genes that were significantly up-regulated after 1 h of Suc treatment, a gene corresponding to the EST sequence (TC46745) showed homology to the GSK3 family and was selected for further investigation. This choice was directed by previous data showing that members of the GSK3 family are involved in the regulation of carbohydrate metabolism in eukaryotic cells (Embi et al., 1980; Woodgett and Cohen, 1984; Kempa et al., 2007). The full-length cDNA was amplified by PCR using a cDNA library prepared from postvéraison Cabernet Sauvignon berries and was named VvSK1 (GenBank accession no. XP_002272112; GSVIVT00026235001). VvSK1 encodes a protein of 468 amino acids that contains the 11 highly conserved domains characteristic of kinases, including the Tyr residue in the T-loop. VvSK1 exhibits high amino acid sequence similarities with GSKs from others species: 87% with NtSK91 (tobacco), 87% with LpSK6 (tomato), 87% with PSK6 (Petunia hybrida), and 86% with AtSK8 (Arabidopsis; Fig. 1).
Figure 1.
Sequence alignment of VvSK1 with other GSK3-like protein kinases from various plant species. Identical residues are shown in black, conserved residues in dark gray, and similar residues in light gray. GenBank accession numbers are as follows: VvSK1 (XP_002272112), ASK8 (At4g00720), PSK6 (AJ224164), NSK91 (AJ224163), and LsPK6 (AY575716). The sequence alignment was done by the Mcoffee program at http://tcoffee.vital-it.ch/cgi-bin/Tcoffee/tcoffee_cgi/index.cgi.
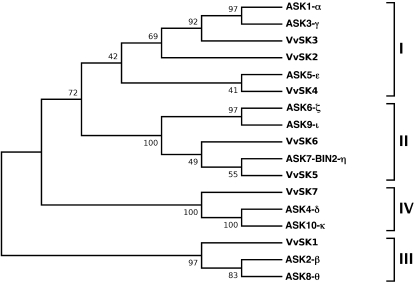
Analysis of the grape genome (Jaillon et al., 2007) allowed the identification of six additional GSK3 members that were numbered from 2 to 7. Interestingly, VvSK1 only shares 44% to 67% identity with other members of the grape GSK family. Based on protein sequence homology, the VvSKs were grouped into four classes (I–IV; Fig. 2). VvSK1 (XP_002272112) belongs to class III. VvSK2 (XP_002279596), VvSK3 (XP_002280673), and VvSK4 (XP_002276754) are related to members of class I. VvSK5 (XP_002270455) and VvSK6 (XP_002281788) are related to class II, and VvSK7 (XP_002263398) is related to class IV.
Figure 2.
Phylogenetic relationship between GSK3 proteins from grape and Arabidopsis. The phylogenetic tree was constructed using MEGA version 4 (Tamura et al., 2007) using the Minimum Evolution method with 2,000 bootstrap replicates. Names and GenBank accession numbers are as follows: VvSK1 (XP_002272112), VvSK2 (XP_002279596), VvSK3 (XP_002280673), VvSK4 (XP_002276754), VvSK5 (XP_002270455), VvSK6 (XP_002281788), VvSK7 (XP_002263398), ASK1-α (At5g26751), ASK2-β (At3g61160), ASK3-γ (At3g05840), ASK4-δ (At1g57870), ASK5-ε (At5g14640), ASK6-ζ (At2g30980), ASK7-BIN2-η (At4g18710), ASK8-θ (At4g00720), ASK9-ι (At1g06390), and ASK10-κ (At1g09840).
Expression of VSK1 in Response to Sugar and ABA Treatments
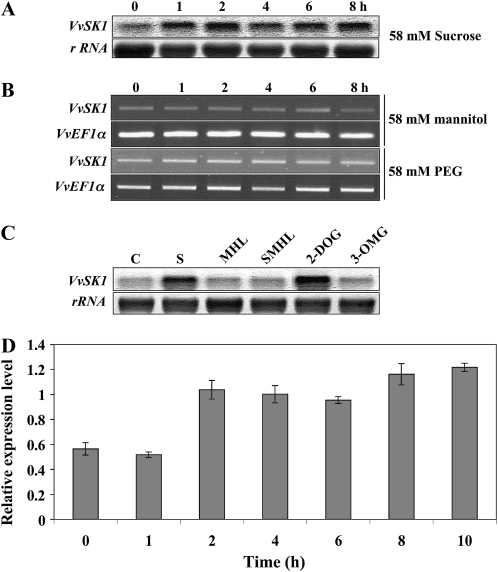
The expression profile of VvSK1 was assessed by RNA blot using RNA samples extracted from Cabernet Sauvignon berry suspension cells collected 0, 1, 2, 4, 6, and 8 h after supply of 58 mm Suc as well as from control cells without sugar supply. VvSK1 transcripts accumulate from 1 h until the end of the experiment (Fig. 3A). To exclude an osmotic effect of Suc, Cabernet Sauvignon berry suspension cells were treated with the same concentration of mannitol or polyethylene glycol 600. None of these chemicals affected VvSK1 expression, indicating that the expression profile previously observed is not due to an osmotic stress but rather to a Suc-specific effect (Fig. 3B).
Figure 3.
Analysis of VvSK1 expression in response to sugar and ABA treatments in Cabernet Sauvignon berry cells. A, RNA gel-blot analysis of VvSK1 accumulation after different times of treatment with 58 mm Suc. B, Semiquantitative RT-PCR analysis of VvSK1 expression in response to 58 mm mannitol and 58 mm polyethylene glycol (PEG) treatment. The elongation factor VvEF1 was used as an internal control. C, Effect of sugar analogs on VvSK1 accumulation determined by RNA gel blot 4 h after treatment. Results are shown for nontreated control cells (C) and cells treated with 58 mm Suc (S), 10 mm MHL (MHL), 58 mm Suc + 10 mm MHL (SMHL), 58 mm 2-DOG (2-DOG), and 58 mm 3-OMG (3-OMG). D, Quantitative RT-PCR of VvSK1 transcript accumulation after treatment with 20 μm ABA. Elongation factor VvEF1 and Actin were used as internal controls. All data are means of three replicates from independent experiments, with error bars indicating se.
In order to elucidate the signaling pathway involved in the transcriptional regulation of this gene, we tested different sugar analogs on Cabernet Sauvignon berry suspension cells (Fig. 3C). Cells were treated with 3-OMG (which is absorbed but poorly phosphorylated) and 2-DOG (which is absorbed and phosphorylated but not further metabolized) or with 58 mm Suc in the presence of the hexokinase (HXK) inhibitor mannoheptulose (MHL; Gibson, 2000). Blockage of HXK activity in cells treated with 10 mm MHL resulted in repressed levels of VvSK1 transcripts. Addition of 58 mm 3-OMG has no effect on VvSK1 expression. By contrast, addition of 58 mm 2-DOG up-regulates VvSK1, as was observed after Suc treatment. Taken together, these results suggest that Suc up-regulates VvSK1 expression through an HXK-dependent pathway.
Hormones play a crucial role during grape berry development. More particularly, endogenous ABA levels increase after véraison, and treatments that delay this increase delay the onset of ripening (Davies et al., 1997; Deluc et al., 2009). In order to investigate a potential effect of ABA on VvSK1 expression, Cabernet Sauvignon berry cell suspensions were treated with 20 μm ABA and collected 0, 2, 4, 6, 8, and 12 h later. VvSK1 expression was monitored by quantitative reverse transcription (RT)-PCR. The results revealed that ABA increased the transcript abundance of VvSK1 by 2-fold within 2 h, and this amount of VvSK1 transcripts was maintained for at least 10 h after ABA supply (Fig. 3D).
Relative Expression Profile of VvSK1 in Grapevine Organs and Berry Tissues
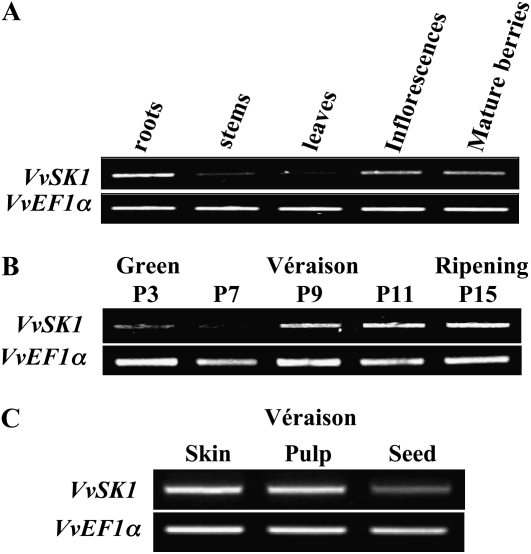
The relative transcript abundance of VvSK1 was determined in different grapevine organs by RT-PCR with RNA extracted from Cabernet Sauvignon roots, stems, leaves, flowers, and mature berries. VvSK1 was predominantly expressed in roots, inflorescences, and mature berries, whereas it was hardly detectable in stems and leaves (Fig. 4A). VvSK1 transcript accumulation was also assessed during berry development. VvSK1 was present in green berries, but the accumulation of the transcript increased mainly after véraison stage and was maintained until the harvesting stage (Fig. 4B). This expression profile is in agreement with microarray data that were previously obtained from Shiraz and Chardonnay developing berries (D. Glissant and S. Delrot, unpublished data).
Figure 4.
Expression of VvSK1 in various tissues from Cabernet Sauvignon plants. Expression of VvSK1 mRNA was determined by semiquantitative RT-PCR, and elongation factor VvEF1 was used as an internal control. A, VvSK1 expression in roots, stems, leaves, inflorescences, and mature berries. B, Expression of VvSK1 in developing berries at 3 weeks (P3), 7 weeks (P7), 9 weeks (P9), 11 weeks (P11), and 15 weeks (P15) post flowering. C, Expression of VvSK1 in berry skin, pulp, and seed at véraison stage. The experiment was repeated three times independently with similar results.
The level of expression of VvSK1 in the different berry compartments at véraison (seeds, pulp, and skin) was also analyzed by RT-PCR. As shown in Figure 3C, VvSK1 showed ubiquitous expression in the berry even though expression levels seemed higher in skin and pulp than in seeds.
Overexpression of VvSK1 in Grapevine Cells Affects Sugar Transport
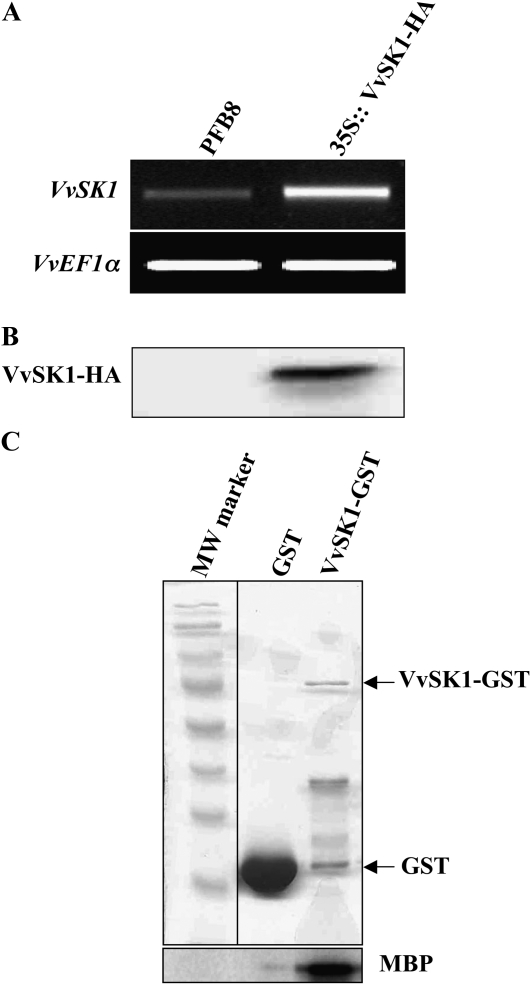
In order to elucidate the function of VvSK1, transgenic grape cells overexpressing hemagglutinin (HA)-tagged VvSK1 under the control of the cauliflower mosaic virus 35S promoter were prepared. After stabilization of the cell suspension by subculture in glycerol-maltose-naphthoxyacetic acid culture medium supplemented with paromomycin and cefotaxime, the expression of VvSK1-HA was tested by PCR and western blotting. A DNA fragment of 1,408 bp corresponding to VvSK1 was strongly amplified by VvSK1-specific primers, in the cells expressing the 35S∷VvSK1-HA construct, in comparison with cells expressing the empty vector (Fig. 5A). In addition, the recombinant protein VvSK1-HA with a mass of 57.2 kD was detected on a western blot with an anti-HA antibody (Fig. 5B). To confirm that VvSK1 is a functional protein kinase, we performed a phosphorylation assay using VvSK1 produced in Escherichia coli as a glutathione S-transferase (GST) fusion protein. VvSK1 phosphorylated myelin basic protein as an artificial substrate (Fig. 5C), showing that in addition to being functional, VvSK1 exhibits a basal constitutive activity.
Figure 5.
Characterization of transgenic cells overexpressing VvSK1. A, RT-PCR of control sample (PFB8) and VvSK1 overexpressors (35S∷VvSK1-HA) detecting VvSK1 transcript. Elongation factor VvEF1 was used as an internal control. B, Protein extracts from cells overexpressing VvSK1-HA were separated and immunodetected using a specific HA antibody. C, GST-VvSK1 phosphorylates myelin basic protein (MBP) in vitro. Top, Coomassie Brilliant Blue staining showing the recombinant protein; bottom, myelin basic protein phosphorylation by VvSK1.
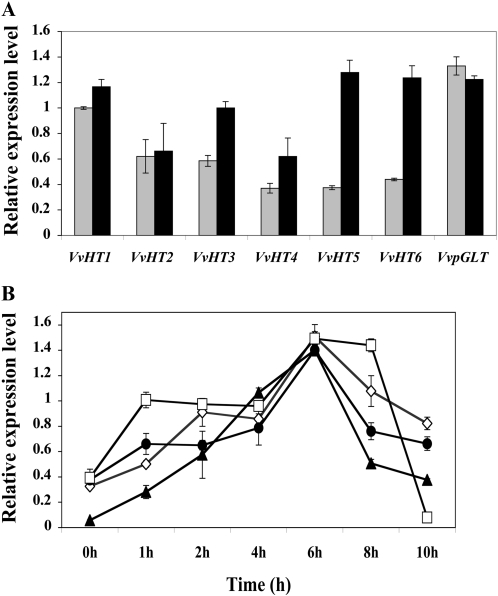
The expression level of different hexose transporter genes in the 35S∷VvSK1-HA grape cells was measured in order to gain greater insights into sugar accumulation during grape berry development. Seven plasma membrane monosaccharide transporter homologs (VvHTs) and a plastidic Glc transporter (pGLT) have been cloned from grape berries (Vignault et al., 2005; Hayes et al., 2007). Specific primers were designed for the sequences of VvHT1 (accession no. AJ001061), VvHT2 (AY663846), VvHT3 (AY538259), VvHT4 (AY538260), VvHT5 (AY538261), VvHT6 (AY861386), and VvpGLT (AY608701) and used to test the corresponding transcript amounts in 41B cells overexpressing VvSK1 (Fig. 6A). Compared with control cells, overexpression of VvSK1 increased the accumulation of VvHT3, VvHT4, VvHT5, and VvHT6 transcripts when compared with control cells, whereas VvHT1, VvHT2, and pGLT transcripts were not significantly affected. Although transcript abundance was increased for all four hexose transporters, they were up-regulated in a slightly different way. Thus, VvHT3 and VvHT4 transcript abundance was increased by 1.7-fold in the VvSK1-overexpressing line, whereas it was increased by 3.4- and 2.8-fold for VvHT5 and VvHT6, respectively. Interestingly, VvHT3, VvHT4, VvHT5, and VvHT6 expression was increased after supply of 58 mm Suc. Their transcripts accumulated within 1 h and peaked 6 h after Suc addition (Fig. 6B). These results suggest that Suc might affect the expression of these four VvHTs through a signaling cascade involving VvSK1.
Figure 6.
Overexpression of VvSK1 modifies the transcription of some grape monosaccharide transporter genes. A, Quantitative RT-PCR analysis of monosaccharide transporters in control (PFB8; gray bars) and 35S∷VvSK1-HA (black bars) grape cells. Expression levels of VvHTs were evaluated in transgenic 41B cells. B, Kinetics of expression of VvHT3 (white diamonds), VvHT4 (black circles), VvHT5 (black triangles), and VvHT6 (white squares) assessed by quantitative RT-PCR using total RNA extracted from Cabernet Sauvignon berry cells treated with 58 mm Suc. Elongation factor VvEF1was used as an internal control. All data are means of three replicates, with error bars indicating se. The primer sequences are given in “Materials and Methods.”
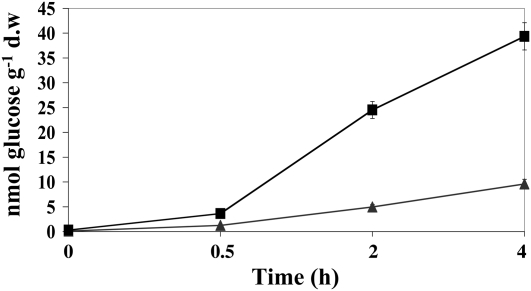
As a consequence, VvSK1 might also affect sugar transport activities in grape cells. To test this hypothesis, uptake of d-[14C]Glc was compared in control cells and cells overexpressing VvSK1. The rate of Glc accumulation by 41B cells overexpressing VvSK1 was 3- to 5-fold higher than in control cells during the time course studied (Fig. 7).
Figure 7.
Activity of the monosaccharide transport system in VvSK1-overexpressing cells. d-[14C]Glc uptake was measured in control cells transformed with PFB8 empty vector (triangles) and 35S∷VvSK1 (squares) cells. Cell aliquots were collected at different time periods (0, 0.5, 2, and 4 h). Errors bars indicate sd. For this experiment, six independent flasks of 41B cell suspension cultures for each condition were used and analyzed. d.w, Dry weight.
Analysis of Sugar Content in Grape Cells Overexpressing VvSK1
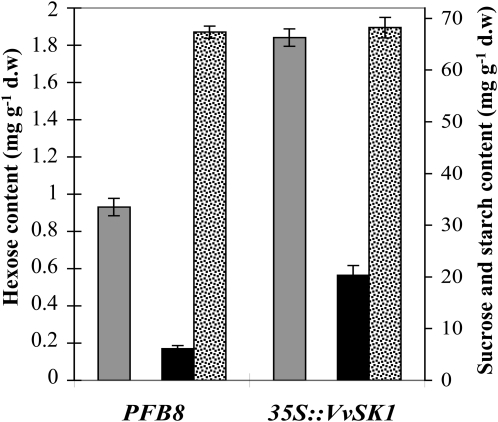
The stimulation of hexose transporter gene expression in 41B cells overexpressing VvSK1 and the enhanced ability of these cells to accumulate Glc indicated that their sugar content might be altered by VvSK1 expression. Soluble sugars (Glc, Fru, and Suc) and starch contents were assayed 7 d after subculture (Fig. 8). Comparison of the sugar profiles showed that 41B cells overexpressing VvSK1 displayed a 2-fold increase in Glc and a 3.3-fold increase in Suc content as compared with PFB8 control cells. By contrast, starch amounts were not significantly different between both cell lines. Fru amounts were too small to be quantified and therefore could not be compared. For this experiment, three independent flasks of 41B cell suspension cultures for each condition were used.
Figure 8.
Sugar content of VvSK1-overexpressing cells. Left y axis shows Glc (dark gray bars) content, and right y axis shows Suc (black bars) and starch (light gray bars) contents. Levels of sugars were analyzed in control cells (PFB8) and 35S∷VvSK1 transgenic cells 7 d after subculture. Errors bars indicate sd. For this experiment, three independent flasks of 41B cell suspension cultures for each condition were used and analyzed. d.w, Dry weight.
DISCUSSION
Sugars provide the reduced carbon needed for the structural materials of the cell, storage compounds, and all major primary and secondary metabolites. They also influence plant growth and development by acting as regulatory signals, which affect the expression of various genes involved in many processes of the plant life cycle (Koch, 1996; Jiang and Carlson, 1997; Smeekens, 1998; Roitsch, 1999; Sheen et al., 1999). Therefore, it is important to identify the molecular players that may directly or indirectly control sugar transport in plants. Although many sugar transporters have now been identified, little is known about sugar transport regulation at the molecular level (Delrot et al., 2000).
Protein kinases are major components of intracellular signal transduction enabling plant cells to rapidly respond to the environment. This paper shows that VvSK1 is a sugar- and abscisic acid (ABA)-induced GSK3 protein kinase that increases the transcript abundance of several monosaccharide transporters and the soluble sugar content of grape cells. MsK4, a GSK3 homolog from group IV, controls carbohydrate metabolism in response to salt stress (Kempa et al., 2007). MsK4 is a starch-associated protein kinase that, when overexpressed, modulates carbohydrate metabolism. Additionally, MsK4 acts as a signaling component in the high-salinity response, linking stress signaling to metabolic adaptation.
The Vitis GSK3 phylogenic tree revealed that VvSK1 was less related to the other VvSKs than to homologs from other species. Indeed, VvSK1 shared highest similarities with GSK3s from Solanaceae proteins NSK91, NSK59, and LpSK6 (87%, 87%, 86.6%), PSK6 and PSK7 (87% and 84.3%), and Arabidopsis ASK2 and ASK8 (78% and 86%). These GSK3s, which belong to the same clade (group III), are preferentially expressed in flowers (Tichtinsky et al., 1998; Charrier et al., 2002; Wilson et al., 2004). Among these, ASK (AtSK3-2) was shown to be involved in the control of cell elongation in flower development and in response to light (Claisse et al., 2007). Our data showed that VvSK1 was not only expressed in flowers but also in two other major sink organs, roots and berries, indicating that its role was not only restricted to flower development in grapevine (Fig. 4A). In berries, VvSK1 transcript amounts transiently decreased after the green stage and increased again after the véraison stage (Fig. 4B), when both sugars and ABA accumulate in this organ (Davies et al., 1997; Pan et al., 2005). Our experiments showed that both sugar and ABA could increase the transcript abundance of VvSK1 in grape cell suspensions (Fig. 3, A and D). It is well known that sugar signaling does not operate alone but rather is integrated in cellular regulatory networks. Most notably, there is a tight interaction between sugar and hormonal signaling, particularly for ABA (Smeekens, 2000; Finkelstein and Gibson, 2002; Léon and Sheen, 2003; Rolland et al., 2006). Also, it is known that ABA enhances sugar accumulation in crop sink organs (Kobashi et al., 2001), and data from the literature show that both sugar and ABA act synergistically (Smeekens, 2000; Léon and Sheen, 2003; Rolland et al., 2006). In peach fruit flesh, sugar accumulation is stimulated by ABA that in turn stimulates uptake of sugars (Kobashi et al., 2001). ABA induces the expression of VvHT1 (Atanassova et al., 2003) and acts synergistically with physiological concentrations of monosaccharides to transiently induce VvHT1 transcription. VvHT1 responsiveness to both signals involves the presence in its promoter of a specific overlapping configuration of the two sugar response cis-elements. This configuration seems a target for the fixation and the action of VvASR (for V. vinifera abscisic acid, stress, ripening-induced) protein (also called VvMSA; Cakir et al., 2003). VvASR expression is strongly up-regulated by the combined effect of sugars and ABA (Cakir et al., 2003). More recently, it was shown that ABA increases invertase activities and amounts during grape development in correlation with soluble sugar concentration (Pan et al., 2005).
VvSK1 was induced by the addition of exogenous Suc, and this induction was not due to an osmotic effect (Fig. 3C). This indicates that VvSK1 was involved downstream of sugar perception. In plants, two signaling pathways relaying sugar sensing have been described. The first one is HXK dependent, and the second is independent of HXK activity (Smeekens, 2000). To investigate the role of HXK in the Suc induction of VvSK1, we tested the effect of Glc analogs and an inhibitor of HXK activity. The results showed that the HXK inhibitor MHL blocked VvSK1 induction by Suc, whereas 3-OMG had no effect and 2-DOG stimulated VvSK1 expression in grape cells (Fig. 3B). This indicates that an HXK-dependent pathway may be involved in the sugar control of VvSK1 expression in grape cells.
VvSK1 overexpression in grape cell suspension affects the level of expression of some sugar transporter genes expressed in grape berries (Fig. 6A). The VvHTs whose expressions were affected by VvSK1 overexpression were also affected at the transcriptional level by sugars and ABA (Fig. 6B; data not shown). This result was in accordance with the observation that VvSK1 overexpression increased by more than 3-fold the uptake rate of radiolabeled Glc in comparison with control cells (Fig. 7). The involvement of VvSK1 in the control of sugar compartmentation was further strengthened by the fact that cells overexpressing VvSK1 accumulate twice more Glc and three times more Suc than the control, whereas the starch content remains unaffected. Sugar levels of suspension cells significantly differ from those in berries, where monosaccharide concentrations can reach 1.5 m, whereas Suc is poorly detectable (Fillion et al., 1999; Liu et al., 2006). In this context, the role of VvSK1 on sugar transport and accumulation in grape berries has to be further investigated by altering VvSK1 expression in berries.
In this work, VvSK1 was identified as a potential regulator of hexose uptake and sugar accumulation in grapevine cells. As a functional protein kinase, VvSK1 can regulate its targets by direct phosphorylation. Protein phosphorylation is one of the most important and best characterized posttranslational protein modifications regulating cellular signaling processes. In plants, previous work suggested that protein phosphorylation plays a direct role in the regulation of sugar transporter transcription and sugar transport activity (Roblin et al., 1998; Ransom- Hodgkins et al., 2003). Key enzymes from carbon metabolism may also be directly regulated by phosphorylation (Sugden et al., 1999; Ikeda et al., 2000). Furthermore, in yeast, the transcription factor RGT1 that functions as a repressor in the absence of Glc becomes a transcriptional activator required for maximal induction of HXT1 gene expression upon phosphorylation in the presence of high concentrations of Glc (Mosley et al., 2003). Therefore, one can hypothesize that VvSK1 can affect sugar uptake and accumulation in grapevine through the regulation of hexose transporter expression. This regulation might be mediated through VvSK1 phosphorylation events affecting the activity and/or stability of specific transcription factors, sugar transporters, and/or enzymes from sugar metabolism. This still has to be demonstrated.
In grapevine, one of the main features of berry development is the accumulation of hexose sugars (i.e. Glc and Fru), which begins at véraison and continues throughout the ripening process. In addition to participating in the regulation of sugar homeostasis in grape cells, VvSK1 may play a role in grape berry development by affecting sugar compartmentation. This hypothesis is further supported by the capacity of VvSK1 to up-regulate the expression of several VvHT genes and more particularly that of VvHT3 and VvHT6. VvHT6 transcript abundance correlates well with hexose accumulation in the berries and was recently proposed to play a significant role in hexose accumulation during berry ripening (Deluc et al., 2007). If VvHT6, which is highly homologous to the tonoplast Glc transporter AtTMT2 (Wormit et al., 2006), acts as a tonoplast sugar transporter, it would only favor indirectly Glc uptake across the plasma membrane by decreasing the cytosolic Glc concentration. VvHT3 transcript accumulates in young berries but also later during the phase of sugar storage (Hayes et al., 2007). VvHT4 expression shows little change throughout berry development, whereas VvHT5 transcript amounts increased by 3-fold at 14 weeks post flowering. As both genes are very weakly expressed in Cabernet Sauvignon berry flesh, these transporters, unlike VvHT3 and VvHT6, may not contribute significantly to sugar import during berry development (Hayes et al., 2007). Nevertheless, the authors did not rule out the possibility that these VvHTs could be better expressed in more specific pulp cell types. Furthermore, as VvSK1 expression in not restricted to the berry, the role of the kinase in the regulation of these two VvHTs in other plant organs is possible if they are expressed in the same organs.
In grape berries, as in other sink organs, sugar accumulation significantly impacts crop yield and economic value. To our knowledge, this work provides the first demonstration that soluble sugar content may be significantly affected by the manipulation of a single gene involved in signaling pathways. VvSK1 transgenic cells may provide a useful model to investigate the metabolic and physiological consequences of a deregulation of sugar uptake and accumulation. Analysis of the effect of Glc analogs and the involvement of ABA place VvSK1 downstream to the HXK-dependent sugar signaling cascade, ABA being involved downstream of HXK1 (Zhou et al., 1998; Arenas-Huertero et al., 2000). The expression profiles of VvSK1 in sink organs (roots and fruits) and during berry development indicate a key role of this protein kinase in the maturation process that still remains to be demonstrated in planta. Overall, our data provide a first trail for the identification of potential signaling components affecting sugar transport and accumulation during grape berry development and ripening.
MATERIALS AND METHODS
Isolation of GSK cDNA
Grapevine (Vitis vinifera) VvSK1 was isolated from an EST (accession no. TC46745 [new TC92799]) collected at the Dana-Farber Cancer Institute Vitis vinifera Gene Index and containing the entire open reading frame. For expression analysis, the entire clone was generated from a cDNA library of grape Cabernet Sauvignon berries (ripening stage) by PCR using synthetic oligonucleotide primers designed to begin and end at the start and stop codons of the open reading frame (forward primer, 5′-TACCCGGGACTCGAGATGAATGTGATGCGTCGTCTCAAGAGCATT-3′; reverse primer, 5′-ATGGATCCTCAGCGGCCGCCTTTCCTTGCATGCTCAGGAATGAGA-3′). The VvSK1 cDNA was cloned into the pGEM-T Easy vector (Promega) for DNA sequencing prior to HA tagging and subcloning into the vector pFB8 downstream of the 35S promoter of cauliflower mosaic virus.
RNA Isolation and cDNA Production
After collection, the cells were immediately frozen in liquid nitrogen and stored at −80°C until RNA extraction. The samples were ground under liquid nitrogen with a mortar and pestle, and RNA was isolated as described previously (Reid et al., 2006). Briefly, the extraction buffer contained 300 mm Tris-HCl (pH 8.0), 25 mm EDTA, 2 m NaCl, 2% cetyltrimethylammonium bromide, 2% polyvinylpolypyrrolidone, 0.05% spermidine trihydrochloride, and 2% β-mercaptoethanol. Fifteen milliliters of prewarmed (65°C) extraction buffer was added to the ground tissue. Samples were incubated at 65°C for 10 min and then extracted twice with chloroform. The final aqueous layer was centrifuged at 30,000g for 20 min at 4°C, then 0.1 volume of 3 m NaOAc (pH 5.2) and 0.6 volume of isopropanol were added to the supernatant and stored overnight at −80°C. Samples were pelleted by centrifugation (3,500g, 30 min) the next day and resuspended in 0.7 mL of Tris-EDTA buffer (pH 7.5). To selectively precipitate the RNA, 0.3 volume of 8 m LiCl was added and the sample was stored overnight at 4°C. RNA was pelleted the next day, washed with ice-cold 70% ethanol, air dried, and dissolved in diethyl pyrocarbonate-treated water. RNA isolation was followed by DNaseI treatment. Total RNA was quantified, and the quality was evaluated on 1% agarose gels.
For RT-PCR analysis, 2 μg of total RNA were reverse transcribed with oligo(dT)12-18 in a 20-μL reaction mixture using the Moloney murine leukemia virus reverse transcriptase (Promega) according to the manufacturer's instructions. After heat inactivation of the reaction mixture, PCR was performed using 2.5 μL of the first-strand cDNA sample with 20 pmol of the primers in a 50-μL reaction.
Real-time PCR expression analysis was performed using a commercial IQ SYBR-Green Supermix kit (Bio-Rad) with the CFX96 Real-Time PCR Detection system (Bio-Rad). Gene transcripts were quantified upon normalization to EF1 and Actin as internal standards by comparing the cycle threshold of the target gene with those of standard genes. All biological samples were tested in triplicate, and se values of means were calculated using standard statistical methods. Specific oligonucleotide primer pairs were designed with Beacon Designer 7 software (Premier Biosoft International). Specific annealing of the oligonucleotides was controlled by dissociation kinetics performed at the end of each PCR run. The efficiency of each primer pair was measured on a PCR product serial dilution. The primers used for amplification were as follows: VvSK1-For, 5′-GGTCGATCAAGAAGCAGAACAG-3′, and VvSK1 -Rev, 5′-GCAGCAGTTTCCTGTGATGTAG-3′; VvHT1-For, 5′-CGTTGTTCACATCGTCGCTTTATC-3′, and VvHT1-Rev, 5′-GAGCAGTCCTCCGAATAGCATTG-3′; VvHT2-For, 5′-GGCATAGGGGTGGTGGTAG-3′, and VvHT2-Rev, 5′-TCGGGCTTTACGGAGAGAG-3′; VvHT3-For, 5′-GCTACTCTCTACTCGTCCGCTTTG-3′, and VvHT3-Rev, 5′-CCCGTCATTGCTTCCGAACTTG-3′; VvHT4-For, 5′-GGGCTGGCGAGTTTCTCTAG-3′, and VvHT4-Rev, 5′-AGTTCTGCTTGGACATCGTTTG-3′; VvHT5-For, 5′-CAGGCTGTTCCACTGTTCTTATCG-3′, and VvHT5-Rev, 5′-AGTTAGGAGGACCGCAGGAATC-3′; VvHT6-For, 5′-TTTATTTGCATGAGGAGGGAGTC-3′, and VvHT 6-Rev, 5′-GAGCAGCAGCCTGGATATAATC-3′; VvpGLT-For, 5′-CAGTGGTTATGGGAGCCGAGTTTG-3′, and VvpGLT-Rev, 5′-TCCACCAGAAGCTCTAGCCTTGAC-3′.
Grapevine Transformation and Culture Conditions
Grapevine transformations were made in 41B rootstock (V. vinifera ‘Chasselas’ × Vitis berlandieri). An embryogenic cell suspension culture was initiated as described previously (Coutos-Thévenot et al., 1992a, 1992b). This cell suspension was subcultured weekly in 25 mL of glycerol-maltose culture medium (Coutos-Thévenot et al., 1992b) supplemented with synthetic auxin (naphthoxy acetic acid at 1 mg L−1) in the dark. Embryogenic cells were transformed using an Agrobacterium tumefaciens cocultivation method (Mauro et al., 1995), and after selection, the transgenic cells were subcultured in the same condition in a medium supplemented with paromomycin at 2 μg mL−1 (final concentration) and cefotaxime at 200 μg mL−1 (Duchefa).
Western-Blot Analysis
Western blotting was performed with 30 μg of protein extracts separated by SDS-PAGE, immunoblotted to poly(vinylidene difluoride) membranes (Millipore), and probed with –HA antibody (Sigma) in Tris-buffered saline, pH 7.4, containing 0.05% Tween 20. Peroxidase-conjugated goat anti-rabbit IgG was used as secondary antibody, and the reaction was revealed by fluorography (ECL; Amersham Biosciences).
In Vitro Kinase Assay
The open reading frame of VvSK1 was cloned into GST fusion protein expression vector pGEX4T-1 (Pharmacia). Expression of the GST fusion protein and affinity purification were performed as described previously (Kiegerl et al., 2000). Kinase reactions were performed in 15 μL of kinase buffer (20 mm HEPES, pH 7.4, 10 mm MgCl2, 5 mm EGTA, and 1 mm dithiothreitol) containing 5 μg of GST fusion protein, 5 μg of myelin basic protein, 0.1 mm ATP, and 2 μCi of [32P]ATP. The protein kinase reactions were performed at room temperature for 30 min, and the reactions were stopped by adding 4× SDS loading buffer and heating for 5 min at 95°C. The phosphorylation of myelin basic protein was analyzed by autoradiography after separation by 12.5% SDS-PAGE.
Glc Uptake Studies
Transgenic cells overexpressing VvSK1 were collected 10 d after subculture and washed three times with culture medium lacking sugar at pH 5.0. Cells were then resuspended in the same fresh medium and equilibrated for 2 h at 120 rpm. Uptake was initiated by the addition of d-[14C]Glc (final concentration, 0.133 MBq g−1 fresh weight). One-milliliter aliquots of the cell suspension were collected after 0, 0.5, 2, and 4 h of incubation with the radioactive sugar. The reaction was stopped by the addition of 2× 5 mL of culture medium without sugar, and the cells were collected by filtration on GF/C Whatman membranes. The filters were dried, and their radioactivity was measured in a Packard Tri-Carb 1900 TR liquid scintillation counter (Packard Instruments).
Analysis of Sugar Content in 41B Cells
41B transgenic cells were collected 7 d after subculture, washed twice with water, and frozen in liquid nitrogen before grinding. Soluble sugars (reducing sugars and Suc) and starch were analyzed enzymatically (kit LISA 200C; CETIM) after sequential extraction with an ethanol:water mixture (80:20, v/v) at 60°C. The NADPH2 released by the enzymatic reactions was measured spectrophotometrically at 340 nm with an absorbance microplate reader (ELx800UV; Bio-Tek Instruments; Grechi et al., 2007).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers XM_002272076 (VvSK1), XM_002279560 (VvSK2), XM_002280637 (VvSK3), XM_002276718 (VvSK4), XM_002270419 (VvSK5), XM_002281752 (VvSK6), XM_002263362 (VvSK7), At5g26751 (ASK1-α), At3g61160 (ASK2-β), At3g05840 (ASK3-γ), At1g57870 (ASK4-δ), At5g14640 (ASK5-ε), At2g30980 (ASK6-ζ), At4g18710 (ASK7-BIN2-η), At4g00720 (ASK8-θ), At1g06390 (ASK9-ι), At1g09840 (ASK10-κ), AJ001061 (VvHT1), AY663846 (VvHT2), AY538259 (VvHT3), AY538260 (VvHT4), AY538261 (VvHT5), AY861386 (VvHT6), AY854146 (VvHT7), and AY608701 (pGLT).
Acknowledgments
We thank Christian Kappel for assistance in bioinformatics, Messaoud Meddar for preparing the cell culture media, Cécile Gaillard for assistance in grape cell transformation, Ghislaine Hilbert and Christelle Renaud for help in sugar content analysis, Prof. Heribert Hirt for critical reading of the manuscript, and Prof. Grant Cramer for improving the style of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Serge Delrot (serge.delrot@bordeaux.inra.fr).
References
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos-Thevenot P, Delrot S (2003) Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiol 131 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque S, Lemoine R, Sequeira-Legrand A, Fayolle L, Delrot S, Pugin A (2002) The elicitor cryptogein blocks glucose transport in tobacco cells. Plant Physiol 130 2177–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner M (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581 2318–2324 [DOI] [PubMed] [Google Scholar]
- Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier B, Champion A, Henry Y, Kreis M (2002) Expression profiling of the whole Arabidopsis shaggy-like kinase multigene family by real-time reverse transcriptase polymerase chain reaction. Plant Physiol 130 577–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Schmitz RJ, Fujioka S, Takatsuto S, Lee MO, Yoshida S, Feldmann KA, Tax FE (2002) Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3β-like kinase. Plant Physiol 130 1506–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claisse G, Charrier B, Kreis M (2007) The Arabidopsis thaliana GSK3/Shaggy like kinase AtSK3-2 modulates floral cell expansion. Plant Mol Biol 64 113–124 [DOI] [PubMed] [Google Scholar]
- Conde C, Agasse A, Glissant D, Tavares R, Gerós H, Delrot S (2006) Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol 141 1563–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutos-Thévenot P, Goebel-Tourand I, Mauro MC, Jouanneau JP, Boulay M, Deloire A, Guern J (1992. a) Somatic embryogenesis from grapevine cells. I. Improvement of embryo development by changes in culture conditions. Plant Cell Tissue Organ Cult 29 125–133 [Google Scholar]
- Coutos-Thévenot P, Maës O, Jouenne T, Mauro MC, Boulay M, Deloire A, Guern J (1992. b) Extracellular protein patterns of grapevine cell suspensions in embryogenic and non-embryogenic situations. Plant Sci 86 137–145 [Google Scholar]
- Davies C, Boss PK, Robinson SP (1997) Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol 115 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S, Atanassova R, Maurousset L (2000) Regulation of sugar, amino acid and peptide plant membrane transporters. Biochim Biophys Acta 1465 281–306 [DOI] [PubMed] [Google Scholar]
- Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8 429–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Quilici DR, Decendit A, Grimplet J, Wheatley MD, Schlauch KA, Mérillon JM, Cushman JC, Cramer GR (2009) Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10 212–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornelas MC, Van Lammeren AA, Kreis M (2000) Arabidopsis thaliana SHAGGY-related protein kinases (AtSK11 and 12) function in perianth and gynoecium development. Plant J 21 419–429 [DOI] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch TH (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehness R, Roitsch T (1997) Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J 11 539–548 [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P (1980) Glycogen synthase kinase-3 from rabbit skeletal muscle: separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107 519–527 [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, Delrot S (1999) Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol 120 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gibson SI (2002) ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Curr Opin Plant Biol 5 26–32 [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI (2000) Plant sugar-response pathways: part of a complex regulatory web. Plant Physiol 124 1532–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RM, Evans LT (1981) Photosynthesis, carbon partitioning, and yield. Annu Rev Plant Physiol 32 485–509 [Google Scholar]
- Gifford RM, Thorne JH, Hitz WD, Giaquinta RT (1984) Crop productivity and photoassimilate partitioning. Science 225 801–808 [DOI] [PubMed] [Google Scholar]
- Grechi I, Vivin P, Hilbert G, Milin S, Robert T, Gaudillère JP (2007) Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ Exp Bot 59 139–149 [Google Scholar]
- Grimes CA, Jope RS (2001) The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65 391–426 [DOI] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45 180–192 [DOI] [PubMed] [Google Scholar]
- Hayes MA, Davies C, Dry IB (2007) Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot 58 1985–1997 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Thoma G, Hinkel K, Nicolay P, Willenbrink J (1996) Sucrose accumulation in sweet sorghum stem internodes in relation to growth. Physiol Plant 97 277–284 [Google Scholar]
- Ikeda Y, Koizumi N, Kusano T, Sano H (2000) Specific binding of a 14-3-3 protein to autophosphorylated WPK4, an SNF1-related wheat protein kinase, and to WPK4-phosphorylated nitrate reductase. J Biol Chem 275 31695–31700 [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 463–467 [DOI] [PubMed] [Google Scholar]
- Jiang R, Carlson M (1997) The Snf1 protein kinase and its activating subunit, Snf4, interact with distinct domains of the Sip1/Sip2/Gal83 component in the kinase complex. Mol Cell Biol 17 2099–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M (1999) Feasting, fasting and fermenting: glucose sensing in yeast and other cells. Trends Genet 15 29–33 [DOI] [PubMed] [Google Scholar]
- Jonak C, Beisteiner D, Beyerly J, Hirt H (2000) Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3. Plant Cell 12 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Hirt H (2002) Glycogen synthase kinase 3/SHAGGY-like kinases in plants: an emerging family with novel functions. Trends Plant Sci 7 457–461 [DOI] [PubMed] [Google Scholar]
- Kempa S, Rozhon W, Samaj J, Erban A, Baluska F, Becker T, Haselmayer J, Schleiff E, Kopka J, Hirt H, et al (2007) A plastid-localized glycogen synthase kinase 3 modulates stress tolerance and carbohydrate metabolism. Plant J 49 1076–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, Eklof S, Till S, Bogre L, Hirt H, et al (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12 2247–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashi K, Sugaya S, Gemma H, Iwahori S (2001) Effect of abscisic acid (ABA) on sugar accumulation in the flesh tissue of peach fruit at the start of the maturation stage. Plant Growth Regul 35 215–223 [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47 509–540 [DOI] [PubMed] [Google Scholar]
- Lemoine R (2000) Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta 1465 246–262 [DOI] [PubMed] [Google Scholar]
- Léon P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8 110–116 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295 1299–1301 [DOI] [PubMed] [Google Scholar]
- Liu HF, Wu BH, Fan PG, Li SH, Li LS (2006) Sugar and acid concentrations in 98 grape cultivars analyzed by principal component analysis. J Sci Food Agric 86 1526–1536 [Google Scholar]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J (2000) Sugar uptake and transport in rice embryo: expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol 124 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel K, Sakr S, Gerbe F, Guilliot A, Bonhomme M, Rageau R, Pétel G (2004) Sorbitol uptake is regulated by glucose through the hexokinase pathway in vegetative peach-tree buds. J Exp Bot 55 879–888 [DOI] [PubMed] [Google Scholar]
- Mauro MC, Toutain S, Walter B, Pinck L, Otten L, Coutos-Thévenot P, Deloire A, Barbier P (1995) High efficiency regeneration of grapevine plants transformed with the GFLV coat protein gene. Plant Sci 112 97–106 [Google Scholar]
- Mosley AL, Lakshmanan J, Aryal BK, Ozcan S (2003) Glucose-mediated phosphorylation converts the transcription factor Rgt1 from a repressor to an activator. J Biol Chem 278 10322–10327 [DOI] [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR (2002) Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes 51 2190–2198 [DOI] [PubMed] [Google Scholar]
- Oliveira J, Tavares RM, Geros H (2002) Utilization and transport of glucose in Olea europaea cell suspensions. Plant Cell Physiol 43 1510–1517 [DOI] [PubMed] [Google Scholar]
- Ohto M, Nakamura K (1995) Sugar-induced increase of calcium-dependent protein kinases associated with the plasma membrane in leaf tissues of tobacco. Plant Physiol 109 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan QH, Li MJ, Peng CC, Zhang N, Zou X, Zou KQ, Wang XL, Yu XC, Wang XF, Zhang DP (2005) Abscisic acid activates acid invertases in developing grape berry. Physiol Plant 125 157–170 [Google Scholar]
- Perez-Perez JM, Ponce MR, Micol JL (2002) The UCU1 Arabidopsis gene encodes a SHAGGY/GSK3-like kinase required for cell expansion along the proximodistal axis. Dev Biol 242 161–173 [DOI] [PubMed] [Google Scholar]
- Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27 305–314 [DOI] [PubMed] [Google Scholar]
- Ransom-Hodgkins R, Vaughn MW, Bush DR (2003) Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 217 483–489 [DOI] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A (2009) Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol 156 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S (1998) Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett 424 165–168 [DOI] [PubMed] [Google Scholar]
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2 198–206 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Tanner W (1994) Expression of a sugar-transporter gene family in a photoautotrophic suspension culture of Chenopodium rubrum L. Planta 193 365–371 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Sauer N, Ludwig A, Knoblauch A, Rothe P, Gahrtz M, Klebl F (2004) AtSUC8 and AtSUC9 encode functional sucrose transporters, but the closely related AtSUC6 and AtSUC7 genes encode aberrant proteins in different Arabidopsis ecotypes. Plant J 40 120–130 [DOI] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2 410–418 [DOI] [PubMed] [Google Scholar]
- Smeekens S (1998) Sugar regulation of gene expression in plants. Curr Opin Plant Biol 1 230–234 [DOI] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Sugden C, Donaghy PG, Halford NG, Hardie DG (1999) Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol 120 257–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tichtinsky G, Tavares R, Takvorian A, Schwebel-Dugué N, Twell D, Kreis M (1998) An evolutionary conserved group of plant GSK-3/shaggy-like protein kinase genes preferentially expressed in developing pollen. Biochim Biophys Acta 1442 261–273 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress- regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Chory J (2006) Downstream nuclear events in brassinosteroid signalling. Nature 441 96–100 [DOI] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dédaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56 1409–1418 [DOI] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC (2007) Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut 1 activity and trafficking. Mol Biol Cell 18 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KS, Stoeva-Popova P, Dimaculangan D (2004) Characterization of LpSK6: a Lycopersicon group three GSK-3/SHAGGY-like protein kinase. Biotechnol Biotechnol Equip 18 20–26 [Google Scholar]
- Woodgett JR, Cohen P (1984) Multisite phosphorylation of glycogen synthase: molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta 788 339–347 [DOI] [PubMed] [Google Scholar]
- Wormit A, Trentmann O, Feifer I, Lohr C, Tjaden J, Meyer S, Schmidt U, Martinoia E, Neuhaus HE (2006) Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18 3476–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]