Abstract
Encapsulated Klebsiella pneumoniae is the predominant causative agent of pyogenic liver abscess, an emerging infectious disease that often complicates metastatic meningitis or endophthalmitis. The capsular polysaccharide on K. pneumoniae surface was determined as the key to virulence. Although the regulation of capsular polysaccharide biosynthesis is largely unclear, it was found that protein-tyrosine kinases and phosphatases are involved. Therefore, the identification and characterization of such kinases, phosphatases, and their substrates would advance our knowledge of the underlying mechanism in capsule formation and could contribute to the development of new therapeutic strategies. Here, we analyzed the phosphoproteome of K. pneumoniae NTUH-K2044 with a shotgun approach and identified 117 unique phosphopeptides along with 93 in vivo phosphorylated sites corresponding to 81 proteins. Interestingly, three of the identified tyrosine phosphorylated proteins, namely protein-tyrosine kinase (Wzc), phosphomannomutase (ManB), and undecaprenyl-phosphate glycosyltransferase (WcaJ), were found to be distributed in the cps locus and thus were speculated to be involved in the converging signal transduction of capsule biosynthesis. Consequently, we decided to focus on the lesser studied ManB and WcaJ for mutation analysis. The capsular polysaccharides of WcaJ mutant (WcaJY5F) were dramatically reduced quantitatively, and the LD50 increased by 200-fold in a mouse peritonitis model compared with the wild-type strain. However, the capsular polysaccharides of ManB mutant (ManBY26F) showed no difference in quantity, and the LD50 increased by merely 6-fold in mice test. Our study provided a clear trend that WcaJ tyrosine phosphorylation can regulate the biosynthesis of capsular polysaccharides and result in the pathogenicity of K. pneumoniae NTUH-K2044.
Protein phosphorylation is one of the most biologically relevant and ubiquitous post-translational modifications in both eukaryotic and prokaryotic organisms. It is best known that protein phosphorylation is a reversible enzyme-catalyzed process that is controlled by various kinases and phosphatases. The aberrant functions often result in irregular protein phosphorylation and ultimately lead to serious disease states such as malignant transformation, immune disorders, and pathogenic infections in mammals (1, 2). Recently, accumulating evidences suggest that Ser/Thr/Tyr phosphorylations also contribute to regulate a diverse range of cellular responses and physiological processes in prokaryotes (1). Among them, tyrosine phosphorylation in encapsulated bacteria has been discovered to play key roles in capsular polysaccharide (CPS1; K antigen) biosynthesis, which leads to virulence (3, 4). This thick layer of exopolysaccharide on many pathogenic bacteria can act as a physical boundary to evade phagocytosis and complement-mediated killing and further inhibit complement activation of the host (1, 5, 6).
In 1996, Acinetobacter johnsonii protein-tyrosine kinase (Ptk) was first discovered and categorized under the bacterial protein-tyrosine kinase (BY-kinase) family (1, 7, 8). Shortly after, its function in bacterial exopolysaccharide production and transport was characterized (1, 7, 8). From then on, many more bacterial tyrosine kinases such as Wzc of Escherichia coli (1, 9) and EpsB of Pseudomonas solanacearum (10, 11) were found to possess this conserved property; deletion of such tyrosine kinases will result in the loss of exopolysaccharide production (12). Therefore, several experiments were conducted to investigate the role of the downstream substrates of the tyrosine kinases in different strains of bacteria, and some targeted proteins were found to participate in the exopolysaccharide anabolism (13, 14). These findings demonstrated a direct relationship between bacterial tyrosine phosphorylation and exopolysaccharide biosynthesis that was directly reflected in the strain virulence.
In the past, the functional roles of the critical components involved in protein phosphorylation were defined by basic biochemical and genetic approaches (1). However, there exists a salient gap between the growing number of identified protein-tyrosine kinases/phosphatases and the relative paucity of protein substrates characterized to date. Genomic sequence analyses and advanced high resolution/high accuracy MS systems with vastly improved phosphopeptide enrichment strategies are among the two key enabling technologies that allow a high efficiency identification of the scarcely detectable site-specific phosphorylations in bacterial systems (15). Mann et al. (16) were the first to initiate a systematic study of the phosphoproteome of B. subtilis in 2007 followed by similar site-specific phosphoproteomics analyses of E. coli (17), Lactococcus lactis (18), and Halobacterium salinarum (19). These pioneering works have since set the foundation in bacterial phosphoproteomics but have not been specifically carried out to address a particular biological issue of causal relevance to virulence or pathogenesis.
Klebsiella pneumoniae is a Gram-negative, non-motile, facultative anaerobic, and rod-shaped bacterium. It is commonly found in water and soil (20) as well as on plants (21) and mucosal surfaces of mammals, such as human, horse, and swine (22, 23). It was demonstrated that CPS on the surface of K. pneumoniae is the prime factor of virulence and toxicity in causing pyogenic liver abscess (PLA), a common intra-abdominal infection with a high 10–30% mortality rate worldwide (24–29). There are also variations in virulence in regard to different capsular serotypes; K1 and K2 were found to be especially pathogenic in causing PLA in a mouse model (30) compared with other serotypes, which show little or no effect (31–34). The K. pneumoniae NTUH-K2044 (K2044) strain, encapsulated with K1 antigen (35), was isolated from clinical K. pneumoniae liver abscess patients. It has become an important emerging pathogen (36) because it usually complicates metastatic septic endophthalmitis and irreversible central nervous system infections independent of host underlying diseases (30, 34). The transmission rate is high (37), and it often rapidly leads to outbreaks of community-acquired infections, such as bacteremia, nosocomial pneumonia, and sepsis, common in immunocompromised individuals (38).
In this study, we wanted to prove that the biosynthesis of CPS is mediated through tyrosine phosphorylation of a subset of proteins. An MS-based systematic phosphoproteomics analysis was conducted on K2044 to identify tyrosine phosphorylated proteins that are also associated with CPS biosynthesis. We further validated the relationship between tyrosine phosphorylation on those proteins and virulence of K2044 by site-directed mutagenesis, CPS quantification, serum killing, and mouse lethality assay.
EXPERIMENTAL PROCEDURES
Cell Culture and Lysate Preparation
K. pneumoniae NTUH-K2044 were grown in LB medium with vigorous shaking. The cells were collected by centrifugation (30 min at 30,000 rpm) at an A600 of 0.6. The cell pellets were resuspended in ice-cold lysis buffer containing 10 mm Tris-Cl (pH 7.4), 1% N-octyl glucoside (Sigma), protease mixture (Roche Applied Science), and a 5 mm concentration of each of the following phosphatase inhibitors: sodium fluoride, 2-glycerol phosphate, sodium vanadate, and sodium pyrophosphate (Sigma). The cell wall and cell membrane were broken by sonication. The cellular debris were removed by centrifugation (30 min at 20,000 rpm). The crude protein extract was extensively dialyzed against deionized water containing phosphatase inhibitors and finally lyophilized.
Protein Digestion
About 30 mg of the lyophilized crude protein extract were dissolved and denatured in 6 m urea and 2 m thiourea. The proteins were reduced with 12.5 mm DTT at 37 °C for 45 min and carbamidomethylated with 40 mm iodoacetamide at room temperature in the dark for 45 min. Alkylated proteins were diluted 8 times with 25 mm ammonium bicarbonate and then digested first with endopeptidase Lys-C (1:100, w/w) (Waco) for 2 h and then further digested with sequencing grade modified trypsin (1:100, w/w) (Promega) overnight at 37 °C. The resulting peptide mixture was acidified with TFA to pH < 3 and divided into two SCX technical replicates.
Phosphopeptide Enrichment
The first stage of phosphopeptide enrichment was performed on a Resource S (GE Healthcare) SCX column as described previously (16–18). Fifteen fractions were collected in each run and subjected to the second stage of phosphopeptide enrichment using titanium oxide as described previously (39, 40). Briefly, the TiO2 beads and all 15 SCX fractions were preincubated with 1 m glycolic acid (Fluka) in 80% acetonitrile, 5% TFA. About 3 mg of TiO2 beads were added to each fraction and then incubated batchwise for 1 h at room temperature. After incubation, the beads were washed three times with 80% acetonitrile, 5% TFA. The bound peptides were eluted by 0.3 n ammonium solution (pH 10.5) in 40% acetonitrile. The eluents were dried almost to completeness and resuspended in 10 μl of 0.1% formic acid for C18 desalting and further for analysis by LC-MS.
Affinity Purification of Tyr(P) Proteome Subset
A sample of crude protein extracts (250 mg of cellular proteins in 50 ml of lysis buffer) was incubated with 1.0 ml of agarose beads (Sigma) at 4 °C for 1 h to preclean the proteins that are nonspecifically associated with beads. The agarose beads and bound proteins were discarded, and the supernatant was then collected by centrifugation for immunoprecipitation as described previously (2). The eluent was digested by trypsin (1:100, w/w), and the phosphopeptides were enriched by TiO2 beads.
LC-MS/MS Analysis
LC was performed on an Agilent 1100 series HPLC system (Agilent Technologies) with a micro-T for flow splitting coupled to an LTQ-Orbitrap XL hybrid mass spectrometer (Thermo Electron) equipped with a PicoView nanospray interface (New Objective). Peptide mixtures were loaded onto a 75-μm × 250-mm fused silica capillary column packed in house with C18 resin (5 μm, Nucleosil 120-5 C18, Macherey-Nagel, GmbH and Co. KG) and were separated using a segmented gradient in 110 min from 2 to 50% solvent B (95% acetonitrile with 0.1% formic acid) at a flow rate of 300 nl/min. Solvent A was 0.1% formic acid in water. The LTQ-Orbitrap was operated in the positive ion mode with the following acquisition cycle: a full scan (m/z 350–1600) recorded in the orbitrap analyzer at resolution R = 30,000 was followed by MS/MS of the 10 most intense peptide ions in the LTQ analyzer. To improve the fragmentation spectra of the phosphopeptides, “multistage activation” at 97.97, 48.99, and 32.66 thompsons relative to the precursor ion was enabled in all MS/MS events. All the measurements in the orbitrap were performed with the lock mass option to improve mass accuracy of precursor ions.
Data Analysis
All MS and MS/MS raw data were processed by Bioworks 3.3.1 and searched against a composite target-decoy protein sequence database downloaded from NCBI (41) containing 5684 K2044 protein sequences and 26 of the most commonly observed contaminants using the Mascot Daemon 2.2 server (16, 18). Search criteria used were as follows: trypsin digestion; variable modifications set as carbamidomethyl (Cys), oxidation (Met), acetylated protein N terminus, and phosphorylation (Ser/Thr, Tyr, Asp, and His); up to two missed cleavages allowed; and mass accuracy of 10 ppm for the parent ion and 0.60 Da for the fragment ions. Samples from different preparations were analyzed and searched individually with each result set independently filtered by eliminating all putative hits with Mascot score <25 at significant threshold <0.05, thus giving a peptide identification false discovery rate of ∼1% as evaluated using the target-decoy strategy (42, 43). For those phosphopeptides identified in more than one run, the scores reported in supplemental Data 1 refer only to the highest score. The reliability of phosphorylation site assignments were assessed by calculating and reporting a normalized delta ion score with only those of ≧0.4 accepted automatically as unambiguous (2, 44), whereas the phosphopeptides either having a normalized delta ion score less than 0.4 or containing more than one aspartate, histidine, serine, threonine, or tyrosine residue were manually inspected to assign the precise phosphorylation sites based on clearly observed site-determined ions. Annotated MS/MS spectra of all identified phosphopeptides are presented in supplemental Data 2, and the ambiguous phosphorylation sites are bracketed in the report.
Bioinformatics Analysis
For functional annotation, the identified phosphoproteins were categorized into biological function and cellular component classes according to Gene Ontology terms by Blast2GO. To gain information on the over-representation of certain protein classes among the identified phosphoproteins, the enrichment analysis was performed with Fisher's test of false discovery rate <0.05 by using Blast2GO.
Generation of Site-specific Mutants
pGEM-T easy-manB or pGEM-T easy-wcaJ was used as template, replacing each indicated amino acid (tyrosine) with phenylalanine coding sequences, for PCR (45). Primers used for site-directed mutagenesis were manB-Y26F (5′-TGAACGAGGACATCGCCTTCCGTATCGGC-3′) and wcaJ-Y5F (5′-GGAAATCTTCATGGCAACCTTGTTTCATCGGACTCG-3′) (see supplemental Data 3). The site-directed mutants were generated using a modified pKO3 vector, pKO3-Km plasmid (46, 47). In brief, a kanamycin resistance gene from a pUC4K plasmid was digested by AccI and ligated into the AccI site of the pKO3 plasmid. The manB and wcaJ site-directed fragments described above were cloned into the pKO3-Km plasmid separately. The resulting constructs were transformed by electroporation into the K2044 strain and cultured at 43 °C. Several colonies were picked in 1 ml of LB broth followed by serial dilution and plating onto LB plates containing 5% sucrose cultured at 30 °C. Colonies sensitive to kanamycin were screened, and PCR fragments were obtained using appropriate primers for further sequencing.
Sugar Composition Analysis
CPS was treated with methanol-HCl for 14 h at 80 °C and then dried down. The released methyl glycosides were N-acetylated by using methanol, acetic anhydride, and pyridine (50:10:1,v/v/v) at room temperature for 20 min. The acetylated samples were trimethylsilylated with trimethylsilyl reagent (3:1:9 hexamethyldisilazane:trimethylchlorosilane:pyridine) at room temperature for 1 h and dried under a stream of N2. The products were dissolved in n-hexane and centrifuged to remove insoluble materials. The clear supernatants were analyzed and detected by the Hewlett Packard HP6890 gas chromatography system and HP5973 mass selective detector.
Resistance to Serum Killing
The serum resistance of K. pneumoniae was determined by an established method (6, 48). 104 CFU of mid-log phase bacteria were mixed with nonimmune human serum donated by health volunteers at a 1:3 (v/v) ratio. The mixture was incubated at 37 °C for 3 h. The colony of viable bacteria was determined by plating, and the average survival rate was characterized as the serum susceptibility.
Extraction and Quantification of CPS
The method of capsular polysaccharide extraction was described previously (49, 50). Briefly, 500 μl of bacteria cultures were mixed with 100 μl of 1% zwitterionic 3–14 detergent in 100 mm citric acid (pH 2.0), and then the mixtures were incubated at 50 °C for 20 min. After centrifugation, 250 μl of supernatants were transferred to new tubes, and 1 ml of absolute ethanol was added to precipitate the CPS. The pellets were dissolved in 200 μl of distilled water, and then 1200 μl of 12.5 mm borax in concentrated H2SO4 were added. The mixtures were vigorously vortexed, boiled for 5 min, and then cooled. 20 μl of 0.15% 3-hydroxydiphenol in 0.5% NaOH were added to the mixture, and the absorbance was measured at 520 nm. The uronic acid concentration in each sample was determined from a standard curve of glucuronic acid and expressed in micrograms per 109 CFU.
Mouse Lethality Assay
Female BALB/cByl 6-week-old mice were obtained from the animal center of National Taiwan University and were acclimatized in an animal house for 3 days. Five mice of a group were injected intraperitoneally with bacteria resuspended in 0.1 ml of saline consisting of 102–106 mid-logarithmic growth phase CFU in 10-fold step-graded doses (51). The LD50, based on the number of survivors after 1 week, was calculated by the method of Reed and Muench (52) and expressed as CFU. The animal experiments were repeated by different investigators, and the results were reproducible.
RESULTS
Phosphoproteomics analysis of K2044 extract was based on a classical shotgun LC-MS/MS approach (16–19). Due to the lesser abundance of the tyrosine phosphoproteome, anti-phosphotyrosine (Tyr(P)) antibody was used to enrich the Tyr(P) population. First of all, K2044 were grown to mid-log phase at 37 °C before harvesting. Both the total proteome extraction and the anti-Tyr(P) antibody enriched subset were digested separately. The non-Tyr(P) enriched sample was then subjected to SCX chromatography fractionation before phosphopeptide enrichment of both subsets at the peptide level with TiO2. The captured and eluted peptides were analyzed by nanoscale LC coupled to the LTQ-Orbitrap individually from the two preparations to increase the coverage and confidence of the positive hits (52). The resulting MS/MS data were searched against a composite target-decoy protein database constructed from NCBI (accession numbers AP006725 and AP006726), and all confident hits were then compiled into a data set reported in Table I and supplemental Data 1, and the manually annotated MS/MS spectra are presented as supplemental Data 2.
Table I. The phosphoproteins, phosphopeptides, and phosphorylated sites identified in this study.
All listed phosphopeptides were derived from both anti-Tyr(P) antibody enriched and total extraction subsets. Rather than protein names, non-redundant gene names are used for clarity. U/N represents an unknown gene name. Ambiguous phosphorylated sites are presented in brackets. Detailed information can be found in supplemental Data 1 and 2.
| Gene name | Phosphopeptide sequence | Gene name | Phosphopeptide sequence |
|---|---|---|---|
| acnB | IpDEVFIGSCMTNIGHFR | gldA | p[SLAD]AGLAAEIAPFGGECSHNEINR |
| ahpC | AAQpYVASHPGEVCPAK | glpK | AVTpSTHGLLTTIACGPR |
| argD | QLCpDQHNALLIFDEVQTGVGR | AVpTSTHGLLTTIACGPR | |
| atpA | IpHGLAECMQGEMISLPGNR | AVp[TSTH]GLLTTIACGPR | |
| atpD | AAPp[SY]EELSSSQELLETGIK | gltA | p[DSH]PMAVMCGITGALAAFYHDSLDVNNPR |
| csrA | IQAEKSQQSSpY | DSHPMAVMCGIpTGALAAFYHDSLDVNNPR | |
| IQAEKSQQSp[SY] | gpmA | pHGESQWNNENR | |
| deoB | FGpDVGADTLGHIAEACAK | groEL | QIVSNAGEEPpSVVANNVK |
| deoC | AAIApYGADEVDVVFPYR | hflB | REVRPPAGWEEPGp[SSNNS]DNNGTPR |
| efp | ATpYYSNDFR | hisS | ApHFAGLCALLDDAGIR |
| fabF | GPpSISIATACTSGVHNIGQAAR | hutH | pHLLTDDSAISQSHHNCSK |
| fbaA | HNLPHNSLNFVFHGGp[SGSS]AQEIK | infB | NWpSETSDSPEDSSDYHVTTSQHAR |
| feaB | QpHGLYIDGAPCAAQSENR | kbl | GpSHEYCDVMGR |
| fimA | GEVVNAACAVpDAGSIDQTVQLGQVR | kdsA | ApSNNSPVIFDVTHALQCR |
| LATAGSTSpSAVGFNIQLDDCDTTVATK | manB | GELGEELNEDIApYR | |
| LApTAGSTSSAVGFNIQLDDCDTTVATK | mlc | DALpYNGSLLIR | |
| YpYATGAATAGIANADATFK | msrA | EVCpSGQTGHAEAVR | |
| LATAGSp[TS]SAVGFNIQLDDCDTTVATK | nagA | KPpDAALVDFLCDNADVITK | |
| LAp[TAGS]TSSAVGFNIQLDDCDTTVATK | narG | FpSEVCVGHLGK | |
| fimD | DVpTFQADAQGHGLSPCLTR | nuoF | pTFCAHAPGAVEPLQSAIK |
| frdA | EPIPVRPp[TAH]YTMGGIETDQQCETR | ompA | GMGEpSNPVTGNTCDNVK |
| ftsA | pHGCALGSIVGKDENVEVPSVGGRPPR | ompC | NGpSVSGEGATNNGR |
| HISCQNEIGMVPIpSEEEVTQDDVENVVHTAK | pckA | GIApSMHCSANVGEK | |
| pHISCQNEIGMVPISEEEVTQDDVENVVHTAK | pepD | pTPNIQIIHAGLECGLFK | |
| gabT | ALCpDEHGIMLIADEVQSGAGR | pepN | pHDGSAATCDDFVQAMEDASNVDLSHFR |
| galU | GLGHAVLCAHPVVGDEPVAVILPDVILDEpYESDLSR | HDGSAATCDDFVQAMEDApSNVDLSHFR | |
| gcvT | AQQpTPLYEQHTLCGAR | pgk | pYAALCDVFVMDAFGTAHR |
| FApDVACAGPLLAAELDALGK | |||
| pgm | KGGPLADGIVITPpSHNPPEDGGIK | wzc | YGEYAYpYEpYEpYKSKE |
| KGGPLADGIVIpTPSHNPPEDGGIK | YGEYAYYEYEpYK | ||
| phoC | IRPFAFYGVSTCNTTEQpDK | pYGEYAYYEpYEYK | |
| ppsA | pTCHAAIIAR | YGEYApYYEYEpYK | |
| GGRpTCHAAIIAR | pYGEYAYYEYEpYK | ||
| LpYTSLGDAAVGR | YGEYApYYEpYEYKSKE | ||
| purL | pDNPACADQEHQAK | YGEYAYpYEYEpYKSKE | |
| pyrE | pDYGCQVISIITLK | p[YGEY]AYYEpYEpYKSKE | |
| rncS | GEAHDQEFp[TIH]CQVSGLSEPVVGTGSSR | YGEYApYpYEpYEpYKSKE | |
| rplB | DGAYVpTLR | pYGEYApYYEpYEpYKSKE | |
| rplJ | AAAFEGELIPApSQIDR | YGEYApYYEpYEp[YKS]KE | |
| rplN | FpDGNACVILNNNSEQPIGTR | ybbN | pSQHCEQLTPVLER |
| rplU | MpYAVFQSGGK | yceF | FPNp[HLIIGSD]QVCVLDGEITGKPHTEENAR |
| rpmE | pSTVGHDLNLDVCGK | ygaG | p[DH]LNGNGVEIIDISPMGCR |
| GIpHPNYDEITATCSCGNVMK | yhaH | FGPDPKPFSpY | |
| rpoB | p[STGSY]SLVTQQPLGGK | U/N | KPIPLGVpSESSVPAPK |
| rpoC | NMpDLEQECEQLREELNETNSETK | U/N | FNALpYEAIHVQGAK |
| rpsA | pSNPWQQFAETHNK | SSNNpYLGAAR | |
| rpsB | pDAALSCDQFFVNHR | U/N | pHASIVPHCAETFAHLVR |
| rpsI | AENQpYYGTGR | U/N | ALpTICTVSDHIR |
| rpsL | pSNVPALEACPQKR | U/N | ISpSTAVR |
| rpsQ | LpHVHDENNECGIGDK | U/N | AAHDFpY |
| terZ | SpSCGSVVHSGDNLTGEGDGDDEIIHVNLLK | U/N | LLNNpSELTHAPCAPGTLETLAR |
| tufB | EGGRpTVGAGVVAK | GpYLIPK | |
| pHYAHVDCPGHADYVK | pSPVNDHPLCLFNPQEDAQILQK | ||
| uxaC | pDQPIFDYHCHLPPQQVAENYR | U/N | VCVpSPGIGFGDYGDTHVR |
| wcaJ | ATLpYHR | U/N | EpTGACNVQVIGK |
| wzc | QQNSpSVDLTMEAK | U/N | IApYNVEAAR |
| INNLPEpTQQEVLR | SGIGPVTAADIp[THD]GDVEIVKPQHVICHLTDENAAISMR | ||
| NNILMISGPpSPEIGK | p[SGIGPVTAADIT]HDGDVEIVKPQHVICHLTDENAAISMR | ||
| TFVp[ST]NLAGVVAQAGQK | U/N | VRHDVFEpSYPEELHGK |
Phosphoproteome of Mid-log Phase K. pneumoniae
A total of 117 phosphopeptides were identified from 81 K. pneumoniae proteins in the combination of both K2044 preparations with a false discovery rate estimated at less than 1%. Among those, there were 93 distinct phosphorylation sites (Table I): a total of 29 Ser (31.2%), 14 Thr (15.1%), and 24 Tyr (25.8%). This result indicates that the Tyr(P) proportion in K2044 is significantly higher, especially in the Tyr(P) enriched subset compared with the phosphoproteomic composition in other bacteria (16–19). There were 17 unique Tyr(P) sites from 13 different proteins found selectively in the anti-Tyr(P) antibody enriched subset. Consistent with the previous phosphoproteomics study on L. lactis (18), high numbers for His and Asp phosphorylation were identified by Mascot searches, 12 on His (12.9%) and 14 on Asp (15.1%), and the phosphorylation of His11 of phosphoglycerate mutase I (gpmA), an E. coli homologue (53), was also observed. Manual inspection of the MS/MS data (supplemental Data 2) revealed that the y ions were consistent overall with the assigned peptide sequence but mostly fell short of unambiguously identifying the inferred Asp/His phosphorylation sites. Therefore, other unusual forms of modifications with the same residual mass increments cannot be ruled out and should be considered with caution.
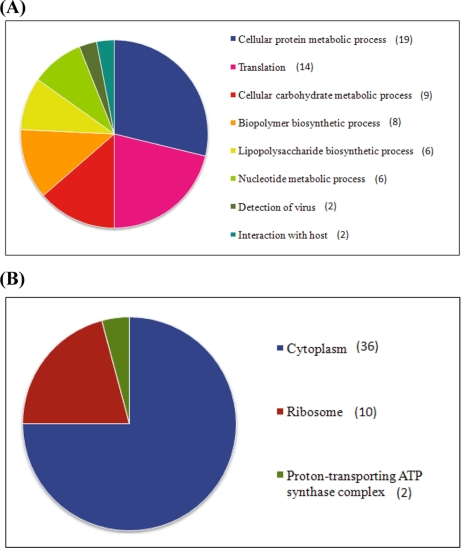
Phosphoproteins derived from the identified phosphopeptides (Table I) were categorized according to biological process and cellular components by Blast2GO (shown in Fig. 1). The 75 (excluding six hypothetical proteins that were not annotated) identified phosphorylated proteins were analyzed against 3512 of 5107 K2044 genes, selected by putative gene ontology annotations, as reference. Those 75 proteins were largely distributed in the housekeeping pathways with significant over-representation (Fig. 1A) and found mostly to be involved in translation and protein/carbohydrate metabolism. Our analysis showed that enzymes such as fructose-1,6-bisphosphate aldolase (fbaA), 2-deoxyribose-5-phosphate aldolase (deoC), phosphoglycerate mutase I (gpmA), phosphoglycerate kinase (pgk), phosphoglucomutase (pgm), and phosphoenolpyruvate synthase (ppsA) of the glycolysis pathway are phosphorylated, in accord with previous studies (16–18). From the cellular component perspective, most of the over-represented phosphoproteins were found to be located in cytoplasm or constituted the ribosome as shown in Fig. 1B.
Fig. 1.
Classification of the identified phosphoproteins in K. pneumoniae NTUH-K2044. The annotations of biological process (A) and cellular component (B) were categorized by GO analysis according to the description of the European Bioinformatics Institute's GO Annotation database with Gossip Fisher's exact test false discovery rate <0.05. Some proteins are present in multiple categories.
Phosphotyrosine Proteome Subset of K. pneumoniae
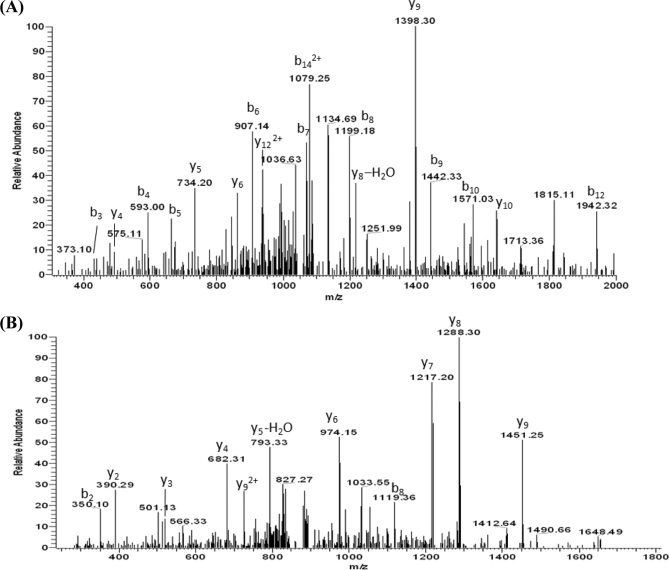
GO enrichment analysis of the identified phosphotyrosine proteins against whole K2044 proteome indicated that only the fraction sorted in the biopolymer metabolic process was statistically over-represented. And among the identified tyrosine phosphorylated proteins, we further identified tyrosine kinase (Wzc), phosphomannomutase (ManB), and undecaprenyl-phosphate glycosyltransferase (WcaJ) located in the cps locus. Along with previously published evidence (1, 54), our findings imply a strong correlation between protein tyrosine phosphorylation and CPS production in K. pneumoniae. It has been reported that Wzc, a BY-kinase involved in the biosynthesis and transport of exopolysaccharides, can undergo autophosphorylation at tyrosine residues clustered on the C terminus; however, there is no fixed number or position (54, 55). In support of that finding, as shown in Table I, 11 phosphopeptides identified in the Wzc C terminus tyrosine-rich cluster displayed different phosphorylation states. MS/MS spectra of two representative phosphopeptides, pYGEYApYYEpYEpYKSKE717 and YGEYApYYEYEpYK714 (where pY is phosphotyrosine), reconstructed the exact position of phosphorylation as shown in Fig. 2. Different phosphorylation states are supposed to modulate the enzyme activity and protein-protein interaction in vivo (54, 56). We also found phosphorylation on Ser532 located in the Walker A motif as well as on Ser299 and Thr368 located in the periplasmic loop of Wzc; both were never observed previously. Again, it cannot be overemphasized that ManB and WcaJ also reside in the cps locus (33, 41) and were first discovered to be tyrosine phosphorylated.
Fig. 2.
MS/MS spectra of the phosphopeptide pYGEYApYYEpYEpYKSKE and YGEYApYYEYEpYK from Wzc tyrosine kinase. The precursor ion mass was measured in the orbitrap mass analyzer with deviations of 1.4 and 1 ppm, respectively. The MS/MS spectra were acquired by the LTQ mass spectrometer and showed rich backbone fragmentation in which phosphorylation sites were detected on Tyr703, Tyr708, Tyr711, and Tyr713 (A) and Tyr708 and Tyr713 (B).
Generation and Characterization of Site-directed Mutants
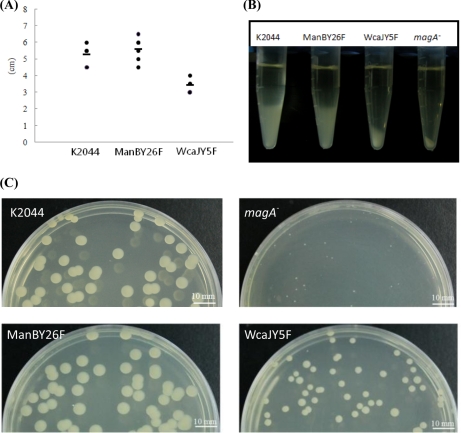
WcaJ and ManB phenylalanine-substituted mutants, WcaJY5F and ManBY26F, were generated to investigate the role of tyrosine phosphorylation in CPS biosynthesis. The WcaJY5F mutant exhibited a reduction in colony size with decreased mucosity by string test (Fig. 3A) and by centrifugation (Fig. 3B), whereas the ManBY26F mutant was indistinguishable from wild type (Fig. 3). In terms of sugar composition, there was no difference between mutants and wild type in gas chromatography-MS analysis. However, by measuring the glucuronic acid content of each strain (57), we demonstrated that WcaJY5F mutant synthesized less CPS compared with ManBY26F mutant or wild type (Table II). Nevertheless, both WcaJY5F and ManBY26F mutants showed no difference in resistance to the human serum killing effect compare with wild type (Table III). The bacterial toxicity was determined by mouse lethality assay (Table III). Intraperitoneal inoculation of 104–106 CFU of either wild type or ManBY26F mutant in BALB/cByl mice resulted in fatality within 2 days. In comparison, mice inoculated with 104 CFU of WcaJY5F mutant remained alive after 1 week. Similar to the previous studies, our negative control of non-encapsulated magA knock-out strain (magA−) injection did not affect all subjected BALB/cByl mice 1 week after inoculation of 102–106 CFU. According to the method established by Reed and Muench (52), the LD50 of ManBY26F mutant was 1.6 × 103, which is about 6–7-fold higher than that of wild type (2.5 × 102 CFU), whereas the LD50 of WcaJY5F mutant containing less CPS showed a significant 200-fold higher LD50 compared with the wild-type positive control. In summary, our results provide prime evidence that tyrosine phosphorylation of WcaJ can affect the regulation of capsule biosynthesis, the key factor in K2044 virulence. This is the first evidence that the downstream tyrosine phosphorylated substrates within the cps locus can contribute to the toxicity of bacterial virulence in addition to upstream kinases and phosphatases.
Fig. 3.
Hypermucosity string test, precipitation speeds, and colony morphology comparison on K2044, ManBY26F, WcaJY5F, and magA−. A, the hypermucosity string test was performed by formation of a viscous string stretched between the bacteria colony and the inoculation loop. The average lengths of K2044, ManBY26F, and WcaJY5F were measured as 5.25, 5.58, and 3.41 cm, respectively. The string lengths of the wild-type and ManBY26F strain were generally longer than that of the WcaJY5F strain. The length of magA− was immeasurable. B, the precipitation test was carried out on the overnight cultured strains in LB broth at 37 °C, and each pellet was evaluated after centrifugation at 10,000 × g for 5 min. The non-capsulated strain magA− served as a negative control with the most compact pellet. The capsule-deficient WcaJY5F mutant showed a pellet that was more compact than those of K2044 and ManBY26F. C, each strain were cultured overnight on agar plates at 37 °C for colony formation. The colony diameters of K2044 and ManBY26F were measured to be 4.5–5 mm on average and averaged 3 mm for WcaJ. The colonies of K2044 and ManBY26F were physically larger than WcaJ and magA− strains; the magA− strain had the smallest average colony diameter, less than 1.5 mm.
Table II. The capsule quantification and mucoid phenotype of K. pneumoniae strain NTUH-K2044, ManBY26F, WcaJY5F, and magA−.
The capsular polysaccharides of 0.5-ml bacteria cultures were extracted. The uronic acid concentration in each sample was determined from a standard curve of glucuronic acid.
| Strain | Quantity (mean ± S.D.)a | -Foldb | Mucoid phenotypec |
|---|---|---|---|
| K2044 | 16.95 ± 1.07 | 4.24 | ++ |
| WcaJY5F | 7.08 ± 0.55 | 1.77 | + |
| ManBY26F | 15.80 ± 0.49 | 3.95 | ++ |
| magA− | 4.00 ± 0.50 | 1.00 | − |
a Values are the averages of triplicate samples and are given as micrograms of uronic acid/109 CFU.
b Comparison based on the non-capsulated strain magA−.
c Assessed by string formation test after 24 h of growth on LB medium. −, negative; +, positive; ++, very strong.
Table III. Virulence properties of K. pneumoniae NUTH-K2044, magA−, and mutants.
In the serum killing assay, 104 CFU of mid-log phase bacteria were mixed with non-immune human serum at a 1:3 (v/v) ratio. The colony of viable bacteria was determined by plating, and the average survival rate was characterized as the serum susceptibility. Five BALB/cByl 6-week-old mice were injected intraperitoneally with 102–106 CFU of mid-log bacteria resuspended in 0.1 ml of normal saline. The LD50, based on the number of survivors after 1 week, is expressed as CFU.
| Strain | LD50 | Survival rate in human seruma |
|---|---|---|
| CFU | ||
| K2044 | 250 | Proliferative |
| WcaJY5F | 5 × 104 | Proliferative |
| ManBY26F | 1.6 × 103 | Proliferative |
| magA− | >106 | 0% |
a Percent survival rate in human serum is expressed as 100 × (the number of viable bacteria after treatment/the number of viable bacteria before treatment).
DISCUSSION
Phosphoproteomics research on multiple bacterial species has been well developed in recent years. We found that the number and functional distribution of identified phosphoproteins in K. pneumoniae are similar to those of other bacteria (16–19). As for Wzc phosphorylation, many phosphorylation sites apart from the tyrosine cluster have received little attention so far because there were no proper tools to investigate those undiscovered locations in the past. In this study, several phosphorylated residues (except for tyrosine) were identified either in the Walker A motif or in the periplasmic loop region of Wzc. Because the Walker A motif is an ATP binding site (58), the Ser(P)532 in this motif is thought to affect ATP binding and Wzc kinase activity. The C terminus of Wzc is believed to account for oligomerization and autophosphorylation, which is important in polysaccharide transportation (59). The periplasmic loop and transmembrane regions of Wzc are topologically similar to Wzz, a regulator of O antigen chain length and in which the periplasmic loop was hypothesized to control the chain length of CPS (59, 60). Thus, phosphorylation on Ser299 and Thr368 residues of the periplasmic domain may influence the K antigen chain length. The periplasmic loop was also proposed to receive environmental stimuli (1); thereby, the succeeding signal transduction might be triggered through dynamic of phosphorylation on the identified sites. This could be the first bacterial one-component system in the form of a single protein serving both as a signal acceptor and effector. Such a one-component system could cross-talk with the already existing two-component system and contribute to optimize a responsive signal transduction network against environmental stimulations.
CPS of K2044 has been demonstrated as a bacterial pathogenic factor and thus motivated the studies of CPS biosynthesis (6, 30, 61). CPS is synthesized through a series of catalytically enzymatic processes in which four groups of enzymes are involved (supplemental Data 4), and all the related genes are clustered in the cps locus (33). The first group of intracellular enzymes activate monosaccharides by adding a phosphate group to form phosphosugars such as glucose 1-phosphate and glucose 6-phosphate. The subsequent nucleotidylation is performed by the second group of enzymes in the cytoplasm. In this step, phosphosugars are converted into sugar nucleotides such as UDP-glucose. The third group of enzymes is located in the inner membrane and is termed as undecaprenyl-phosphate glycosyltransferases that initiate K antigen synthesis by transferring the glucose 1-phosphate from UDP-glucose to a lipid carrier, undecaprenyl phosphate. Successively, the inner membrane peripheral glycosyltransferases add the remaining sugars to form the trisaccharide repeat unit that could be assembled into the nascent polysaccharide. Finally, a set of enzymatic system including Wzx, Wzc, Wza, and Wzy spanning the inner and outer membrane takes over the higher polymerization and transportation of polysaccharides (62). Moreover, the extent of Wzc autophosphorylation regulated by the cognate low molecular weight protein-tyrosine phosphatases (Wzb) or protein-histidine phosphatases can influence the amount, length, and properties of CPS production (54, 63). The most striking finding so far is that protein tyrosine phosphorylation correlates with the production and transport of CPS (56, 60). In earlier studies, considerable efforts were put into identifying tyrosine kinases and phosphatases (1, 54). However, the natural substrates of such enzymes and their connection with CPS biosynthesis remain unclear. To answer those questions, we completed a series of phosphoprotein screens by MS, and the number of identified Tyr(P) proteins, including ManB and WcaJ, were further enriched by immunoprecipitation. The GO enrichment analysis indicated that most identified Tyr(P) proteins were categorized under the biopolymer metabolic process. In addition, the tyrosine kinase (wzc) or phosphatase (wzb) null mutant K2044 strains showed a capsule-deficient phenotype (supplemental Data 5), reinforcing that tyrosine phosphorylation might directly or indirectly take part in the regulation of CPS biosynthesis.
Furthermore, we demonstrated that phosphorylation on the Tyr5 residue of WcaJ can influence polysaccharide production. The phenomenon was observed in the WcaJY5F mutant with a lesser amount of CPS production than in the wild type under the same incubation condition. We also found that CPS can effectively protect bacteria from serum killing, even in the WcaJY5F mutant, which contains about 50% less CPS. Because WcaJ cooperates with the other cluster members in a stepwise manner, the disruption in tyrosine phosphorylation of WcaJ may abolish not only the initiation synthesis sequence but also the enzyme complex stability in CPS assembly because of alternated enzyme activity or protein-protein interaction. The results from the mouse peritonitis model together with the observation of colony morphology indicate that the tyrosine phosphorylation of WcaJ has a more prominent role than ManB in regulating the CPS production.
ManB is a phosphomannomutase that catalyzes the conversion between mannose 6-phosphate and mannose 1-phosphate. Mannose 1-phosphate is required for the synthesis of GDP-mannose, which is converted to GDP-fucose as the trisaccharide repeat unit precursor in CPS assembly. It has been reported that the Ser98 residue in the active site of ManB could be phosphorylated to facilitate the ManB activation (64). Because the identified Tyr26 is outside the enzyme active site, it is postulated that it may not be important like Ser98 in enzyme activity regulation; and indeed, there was no significant difference in CPS production of ManBY26F mutant. However, a slight change in the virulence of ManBY26F mutant as compared with wild type was observed. One can speculate that phosphorylation of ManB on Tyr26 does not result in a change in quantity of CPS but affects other undefined biological processes instead. Notably, it has been reported that bacterial phosphorylation could increase the host viability, for example by increasing resistance to cationic peptides and polymyxin-type antibiotics (65, 66).
The concern for bacterial pathogenesis is rising because of the increasing resistance of pathogens to conventional antibiotics, the emergence of new microbial diseases, the resurgence of others that were thought to be eradicated, and the current paucity of effective therapeutics. Over the last two decades, the etiological agent of PLA has shifted to a single microorganism, K. pneumoniae, from polymicrobial infection such as E. coli, streptococci, and anaerobic bacteria with the prevalence as high as 78% in Taiwan and 41% in the United States (24, 30, 67, 68). It is necessary to expend our knowledge of the mechanism of bacterial pathogenesis to explore new molecular targets for prevention and treatment purposes. The bacterial exclusive BY-kinases were previously shown to be an attractive target; however, normal flora could be eliminated due to gene conservation in different microorganisms. In this report, the vital link between tyrosine phosphorylation and bacterial virulence was again demonstrated. Furthermore, the identified WcaJ can serve as a very specific molecular target because there are no homologues in eukaryotic cells, and the phosphorylation on Tyr5 is unique to K. pneumoniae (supplemental Data 6). Further investigation of the molecular and cellular bases of global protein phosphorylation is urgently needed, and based on the discoveries, we might be able to develop appropriate therapeutic strategies for clinical application.
Supplementary Material
Acknowledgments
We express our gratitude to Dr. Shih-Feng Tsai of the Division of Molecular and Genomic Medicine of the National Health Research Institutes, National Yang-Ming University Veterans General Hospital Genome Research Center, and Yang-Ming for providing protein sequence of K2044. We also thank the National Research Program for Genomic Medicine Core Facilities for Proteomics Research for bioinformatic assistants.
Footnotes
* This work was supported by the National Science Council, Taiwan (Grants NSC 96-3112-B-001 and NSC 97-3112-B-001).
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental Data 1–6.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental Data 1–6.
1 The abbreviations used are:
- CPS
- capsular polysaccharide
- K2044
- K. pneumoniae NTUH-K2044
- GO
- Gene Ontology
- PLA
- pyogenic liver abscess
- cps
- capsular biosynthetic
- ManB
- phosphomannomutase
- WcaJ
- undecaprenyl-phosphate glycosyltransferase
- BY-kinase
- bacterial protein-tyrosine kinase
- SCX
- strong cation exchange
- LTQ
- linear trap quadrupole
- CFU
- colony-forming units.
REFERENCES
- 1.Cozzone A. J. ( 2005) Role of protein phosphorylation on serine/threonine and tyrosine in the virulence of bacterial pathogens. J. Mol. Microbiol. Biotechnol 9, 198– 213 [DOI] [PubMed] [Google Scholar]
- 2.Chang Y. C., Lin S. Y., Liang S. Y., Pan K. T., Chou C. C., Chen C. H., Liao C. L., Khoo K. H., Meng T. C. ( 2008) Tyrosine phosphoproteomics and identification of substrates of protein tyrosine phosphatase dPTP61F in Drosophila S2 cells by mass spectrometry-based substrate trapping strategy. J. Proteome Res 7, 1055– 1066 [DOI] [PubMed] [Google Scholar]
- 3.Cozzone A. J., Grangeasse C., Doublet P., Duclos B. ( 2004) Protein phosphorylation on tyrosine in bacteria. Arch. Microbiol 181, 171– 181 [DOI] [PubMed] [Google Scholar]
- 4.Backert S., Selbach M. ( 2005) Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol 13, 476– 484 [DOI] [PubMed] [Google Scholar]
- 5.Lin W. S., Cunneen T., Lee C. Y. ( 1994) Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J. Bacteriol 176, 7005– 7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang C. T., Chuang Y. P., Shun C. T., Chang S. C., Wang J. T. ( 2004) A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med 199, 697– 705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duclos B., Grangeasse C., Vaganay E., Riberty M., Cozzone A. J. ( 1996) Autophosphorylation of a bacterial protein at tyrosine. J. Mol. Biol 259, 891– 895 [DOI] [PubMed] [Google Scholar]
- 8.Grangeasse C., Doublet P., Vaganay E., Vincent C., Deléage G., Duclos B., Cozzone A. J. ( 1997) Characterization of a bacterial gene encoding an autophosphorylating protein tyrosine kinase. Gene 204, 259– 265 [DOI] [PubMed] [Google Scholar]
- 9.Vincent C., Doublet P., Grangeasse C., Vaganay E., Cozzone A. J., Duclos B. ( 1999) Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol 181, 3472– 3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Carney B. F., Denny T. P., Weissinger A. K., Schell M. A. ( 1995) A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum. J. Bacteriol 177, 1259– 1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J., Schell M. ( 1995) Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol. Microbiol 16, 977– 989 [DOI] [PubMed] [Google Scholar]
- 12.Vincent C., Duclos B., Grangeasse C., Vaganay E., Riberty M., Cozzone A. J., Doublet P. ( 2000) Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J. Mol. Biol 304, 311– 321 [DOI] [PubMed] [Google Scholar]
- 13.Grangeasse C., Obadia B., Mijakovic I., Deutscher J., Cozzone A. J., Doublet P. ( 2003) Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J. Biol. Chem 278, 39323– 39329 [DOI] [PubMed] [Google Scholar]
- 14.Bugert P., Geider K. ( 1995) Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol. Microbiol 15, 917– 933 [DOI] [PubMed] [Google Scholar]
- 15.Soufi B., Jers C., Hansen M. E., Petranovic D., Mijakovic I. ( 2008) Insights from site-specific phosphoproteomics in bacteria. Biochim. Biophys. Acta 1784, 186– 192 [DOI] [PubMed] [Google Scholar]
- 16.Macek B., Mijakovic I., Olsen J. V., Gnad F., Kumar C., Jensen P. R., Mann M. ( 2007) The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6, 697– 707 [DOI] [PubMed] [Google Scholar]
- 17.Macek B., Gnad F., Soufi B., Kumar C., Olsen J. V., Mijakovic I., Mann M. ( 2008) Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol. Cell. Proteomics 7, 299– 307 [DOI] [PubMed] [Google Scholar]
- 18.Soufi B., Gnad F., Jensen P. R., Petranovic D., Mann M., Mijakovic I., Macek B. ( 2008) The Ser/Thr/Tyr phosphoproteome of Lactococcus lactis IL1403 reveals multiply phosphorylated proteins. Proteomics 8, 3486– 3493 [DOI] [PubMed] [Google Scholar]
- 19.Aivaliotis M., Macek B., Gnad F., Reichelt P., Mann M., Oesterhelt D. ( 2009) Ser/Thr/Tyr protein phosphorylation in the archaeon Halobacterium salinarum—a representative of the third domain of life. PLoS ONE 4, e4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown C., Seidler R. J. ( 1973) Potential pathogens in the environment: Klebsiella pneumoniae, a taxonomic and ecological enigma. Appl. Microbiol 25, 900– 904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagley S. T., Seidler R. J., Talbot H. W., Jr., Morrow J. E. ( 1978) Isolation of Klebsielleae from within living wood. Appl. Environ. Microbiol 36, 178– 185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsen J. M., Spindler J. A., Blosser R. O. ( 1974) Characterization of Klebsiella isolates from natural receiving waters and comparison with human isolates. Appl. Microbiol 28, 672– 678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidler R. J., Knittel M. D., Brown C. ( 1975) Potential pathogens in the environment: cultural reactions and nucleic acid studies on Klebsiella pneumoniae from clinical and environmental sources. Appl. Microbiol 29, 819– 825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahimian J., Wilson T., Oram V., Holzman R. S. ( 2004) Pyogenic liver abscess: recent trends in etiology and mortality. Clin. Infect. Dis 39, 1654– 1659 [DOI] [PubMed] [Google Scholar]
- 25.Hibberd P. L., Patel A. ( 2004) Challenges in the design of antibiotic equivalency studies: the multicenter equivalency study of oral amoxicillin versus injectable penicillin in children aged 3–59 months with severe pneumonia. Clin. Infect. Dis 39, 526– 531 [DOI] [PubMed] [Google Scholar]
- 26.Chang S. C., Fang C. T., Hsueh P. R., Chen Y. C., Luh K. T. ( 2000) Klebsiella pneumoniae isolates causing liver abscess in Taiwan. Diagn. Microbiol. Infect. Dis 37, 279– 284 [DOI] [PubMed] [Google Scholar]
- 27.Lederman E. R., Crum N. F. ( 2005) Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol 100, 322– 331 [DOI] [PubMed] [Google Scholar]
- 28.Chang F. Y., Chou M. Y. ( 1995) Comparison of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J. Formos. Med. Assoc 94, 232– 237 [PubMed] [Google Scholar]
- 29.Chan K. S., Yu W. L., Tsai C. L., Cheng K. C., Hou C. C., Lee M. C., Tan C. K. ( 2007) Pyogenic liver abscess caused by Klebsiella pneumoniae: analysis of the clinical characteristics and outcomes of 84 patients. Chin. Med. J 120, 136– 139 [PubMed] [Google Scholar]
- 30.Fung C. P., Chang F. Y., Lee S. C., Hu B. S., Kuo B. I., Liu C. Y., Ho M., Siu L. K. ( 2002) A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50, 420– 424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higaki M., Chida T., Takano H., Nakaya R. ( 1990) Cytotoxic component (s) of Klebsiella oxytoca on HEp-2 cells. Microbiol. Immunol 34, 147– 151 [DOI] [PubMed] [Google Scholar]
- 32.Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. ( 1983) Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect. Immun 40, 56– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang Y. P., Fang C. T., Lai S. Y., Chang S. C., Wang J. T. ( 2006) Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis 193, 645– 654 [DOI] [PubMed] [Google Scholar]
- 34.Fang C. T., Lai S. Y., Yi W. C., Hsueh P. R., Liu K. L., Chang S. C. ( 2007) Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis 45, 284– 293 [DOI] [PubMed] [Google Scholar]
- 35.Struve C., Bojer M., Nielsen E. M., Hansen D. S., Krogfelt K. A. ( 2005) Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J. Med. Microbiol 54, 1111– 1113 [DOI] [PubMed] [Google Scholar]
- 36.Ko W. C., Paterson D. L., Sagnimeni A. J., Hansen D. S., Von Gottberg A., Mohapatra S., Casellas J. M., Goossens H., Mulazimoglu L., Trenholme G., Klugman K. P., McCormack J. G., Yu V. L. ( 2002) Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis 8, 160– 166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kühn I., Ayling-Smith B., Tullus K., Burman L. G. ( 1993) The use of colonization rate and epidemic index as tools to illustrate the epidemiology of faecal Enterobacteriaceae strains in Swedish neonatal wards. J. Hosp. Infect 23, 287– 297 [DOI] [PubMed] [Google Scholar]
- 38.Sahly H., Podschun R. ( 1997) Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin. Diagn Lab. Immunol 4, 393– 399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thingholm T. E., Jørgensen T. J., Jensen O. N., Larsen M. R. ( 2006) Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc 1, 1929– 1935 [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama N., Masuda T., Shinoda K., Nakamura A., Tomita M., Ishihama Y. ( 2007) Phosphopeptide enrichment by aliphatic hydroxy acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Mol. Cell. Proteomics 6, 1103– 1109 [DOI] [PubMed] [Google Scholar]
- 41.Wu K. M., Li L. H., Yan J. J., Tsao N., Liao T. L., Tsai H. C., Fung C. P., Chen H. J., Liu Y. M., Wang J. T., Fang C. T., Chang S. C., Shu H. Y., Liu T. T., Chen Y. T., Shiau Y. R., Lauderdale T. L., Su I. J., Kirby R., Tsai S. F. ( 2009) Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J. Bacteriol 191, 4492– 4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munton R. P., Tweedie-Cullen R., Livingstone-Zatchej M., Weinandy F., Waidelich M., Longo D., Gehrig P., Potthast F., Rutishauser D., Gerrits B., Panse C., Schlapbach R., Mansuy I. M. ( 2007) Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol. Cell. Proteomics 6, 283– 293 [DOI] [PubMed] [Google Scholar]
- 43.Endler A., Reiland S., Gerrits B., Schmidt U. G., Baginsky S., Martinoia E. ( 2009) In vivo phosphorylation sites of barley tonoplast proteins identified by a phosphoproteomic approach. Proteomics 9, 310– 321 [DOI] [PubMed] [Google Scholar]
- 44.Beausoleil S. A., Villén J., Gerber S. A., Rush J., Gygi S. P. ( 2006) A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol 24, 1285– 1292 [DOI] [PubMed] [Google Scholar]
- 45.Makarova O., Kamberov E., Margolis B. ( 2000) Generation of deletion and point mutations with one primer in a single cloning step. BioTechniques 29, 970– 972 [DOI] [PubMed] [Google Scholar]
- 46.Link A. J., Phillips D., Church G. M. ( 1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol 179, 6228– 6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor L. A., Rose R. E. ( 1988) A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res 16, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podschun R., Sievers D., Fischer A., Ullmann U. ( 1993) Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J. Infect. Dis 168, 1415– 1421 [DOI] [PubMed] [Google Scholar]
- 49.Domenico P., Schwartz S., Cunha B. A. ( 1989) Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect. Immun 57, 3778– 3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favre-Bonte S., Joly B., Forestier C. ( 1999) Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect. Immun 67, 554– 561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai Y. C., Peng H. L., Chang H. Y. ( 2003) RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J. Bacteriol 185, 788– 800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed L. J., Muench H. ( 1938) A simple method of estimating the fifty percent endpoints. Am. J. Hyg 27, 493– 497 [Google Scholar]
- 53.Puttick J., Baker E. N., Delbaere L. T. ( 2008) Histidine phosphorylation in biological systems. Biochim. Biophys. Acta 1784, 100– 105 [DOI] [PubMed] [Google Scholar]
- 54.Grangeasse C., Cozzone A. J., Deutscher J., Mijakovic I. ( 2007) Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem. Sci 32, 86– 94 [DOI] [PubMed] [Google Scholar]
- 55.Paiment A., Hocking J., Whitfield C. ( 2002) Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J. Bacteriol 184, 6437– 6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee D. C., Zheng J., She Y. M., Jia Z. ( 2008) Structure of Escherichia coli tyrosine kinase Etk reveals a novel activation mechanism. EMBO J 27, 1758– 1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamze S., Martinez-Pomares L., Jones H., Taylor P. R., Stillion R. J., Gordon S., Wong S. Y. ( 2002) Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J. Biol. Chem 277, 41613– 41623 [DOI] [PubMed] [Google Scholar]
- 58.Walker J. E., Saraste M., Runswick M. J., Gay N. J. ( 1982) Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1, 945– 951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coxon J. P., Stinear C. M., Byblow W. D. ( 2009) Stop and go: the neural basis of selective movement prevention. J. Cogn. Neurosci 21, 1193– 1203 [DOI] [PubMed] [Google Scholar]
- 60.Whitfield C. ( 2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem 75, 39– 68 [DOI] [PubMed] [Google Scholar]
- 61.Keynan Y., Karlowsky J. A., Walus T., Rubinstein E. ( 2007) Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand. J. Infect. Dis 39, 828– 830 [DOI] [PubMed] [Google Scholar]
- 62.Margaritis A., Pace G. W. ( 1985) Microbial polysaccharides, in Comprehensive Biotechnology: the Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine ( Blanch H. W., Drew S., Wang D. I. C., eds) Vol. 3, p. 41, Pergamon Press, New York [Google Scholar]
- 63.Jers C., Soufi B., Grangeasse C., Deutscher J., Mijakovic I. ( 2008) Phosphoproteomics in bacteria: towards a systemic understanding of bacterial phosphorylation networks. Expert Rev. Proteomics 5, 619– 627 [DOI] [PubMed] [Google Scholar]
- 64.Shackelford G. S., Regni C. A., Beamer L. J. ( 2004) Evolutionary trace analysis of the alpha-D-phosphohexomutase superfamily. Protein Sci 13, 2130– 2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breazeale S. D., Ribeiro A. A., Raetz C. R. ( 2003) Origin of lipid A species modified with 4-amino-4-deoxy-L-arabinose in polymyxin-resistant mutants of Escherichia coli. An aminotransferase (ArnB) that generates UDP-4-deoxyl-L-arabinose. J. Biol. Chem 278, 24731– 24739 [DOI] [PubMed] [Google Scholar]
- 66.Klein G., Dartigalongue C., Raina S. ( 2003) Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Mol. Microbiol 48, 269– 285 [DOI] [PubMed] [Google Scholar]
- 67.Casella F., Manenti M. G., Conca C., Repetti V., Longhi P., Lazzaroni S., Mercieri A., Furlan R. ( 2008) Liver abscess caused by Klebsiella pneumoniae. Dig. Liver Dis 10.1016/j.dld.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 68.Tsay R. W., Siu L. K., Fung C. P., Chang F. Y. ( 2002) Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch. Intern. Med 162, 1021– 1027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.