Abstract
The minor-vein phloem of mature leaves is developmentally and physiologically distinct from the phloem in the rest of the vascular system. Phloem loading of transport sugars occurs in the minor veins, and consistent with this, galactinol synthase is expressed in the minor veins of melon (Cucumis melo) as part of the symplastic-loading mechanism that operates in this species. A galactinol synthase promoter from melon drives gene expression in the minor-vein companion cells of both transgenic tobacco (Nicotiana tabacum) and Arabidopsis. Neither of these plants use galactinol in the phloem-loading process, implying that the promoter responds to a minor-vein-specific regulatory cascade that is highly conserved across a broad range of eudicotyledons. Detailed analysis of this promoter by truncation and mutagenesis identified three closely coupled sequences that unambiguously modulate tissue specificity. These sequences cooperate in a combinatorial fashion: two promote expression throughout the vascular system of the plant, whereas the third functions to repress expression in the larger bundles. In a complementary approach, phylogenetic footprinting was used to obtain single-nucleotide resolution of conserved sites in orthologous promoters from diverse members of the Cucurbitaceae. This comparative analysis confirmed the importance of the closely coupled sites but also revealed other highly conserved sequences that may modulate promoter strength or contribute to expression patterns outside of the phloem. The conservation of this regulatory design among species that phloem load by different mechanisms supports a model for organismal development in which tissues and cell types are controlled by relatively ancient and conserved paradigms but expression of genes influencing final form and function are relatively plastic.
Different vein classes in a leaf occupy distinct developmental and physiological niches. The leaf veins of tobacco (Nicotiana tabacum), for example, are subdivided into as many as six classes, with Class I consisting of the primary vein (mid-rib), and Classes V and VI representing the finest veins—referred to as minor veins—that enclose the areoles or terminate as blind endings in the areoles (Haritatos et al., 2000a). The smallest veins are not merely diminutive extensions of larger ones but are developmentally and functionally distinct. In growing leaves, the companion cells and sieve elements of the phloem are not fully developed in minor veins, and do not participate in nutrient transport. Rather, they mature during the sink-to-source transition when the productive capacity of the leaf exceeds its metabolic requirements and begins exporting photoassimilate to regions of net demand (Turgeon, 1989). Consistent with this, phloem loading, the energy-requiring process of accumulating photoassimilate in the phloem in preparation for export, occurs primarily, if not exclusively, in the minor veins.
Despite long-term interest in minor-vein structure and function, little is known at the molecular level. This is in part due to technical challenges. The minor veins are relatively inaccessible due to their position in the middle of the lamina. They are exceptionally small with an entire bundle being smaller in diameter than a mesophyll cell, and they are comprised of several distinct cell types (i.e. Haritatos et al., 2000b). As a result, genetic screens for mutants with visible phenotypes and technologies that rely on relatively pure tissue preparations are not ideal for cataloging genes expressed in minor veins.
An alternative approach to identifying minor-vein-specific genes may be through the presence of common sequence motifs in their promoters. Genes that are coordinately expressed, either during a developmental or an environmental cue, tend to respond to the same regulatory cascades and thus tend to share regulatory motifs in their promoters. This tendency has been exploited to identify unknown genes involved in a specific response by searching for the presence of regulatory elements similar to those found in well-characterized genes (Iyer et al., 2001; Berman et al., 2002). Accordingly, identifying components of the regulatory cascades that govern gene expression in the phloem cells of mature minor veins may provide valuable insight into how those cascades control other genes contributing to minor-vein phloem structure and function.
Because phloem loading is a prominent activity of minor veins, characterizing the regulation of a gene involved in this process is a reasonable starting point for identifying other minor-vein genes. Phloem loading occurs by one of two mechanisms: from the apoplast by cotransport with protons or from the symplast by a polymer-trapping mechanism (Grusak et al., 1996; Turgeon, 1996). In apoplastic phloem loading, Suc is pumped into minor veins from the apoplast by the action of Suc transporters. However, Suc transport is common to many cell types and is not specific to the minor veins. Suc transporters involved in phloem loading are also expressed along the path phloem (i.e. transport phloem in between loading and unloading zones) for retrieval of leaked Suc (Williams et al., 2000). Therefore, analyzing the regulation of Suc transporters would not necessarily illuminate regulatory cascades that are specific to the minor veins.
However, the polymer trap mechanism of phloem loading is restricted to minor veins (Turgeon 1996), and genes involved in this process therefore constitute preferable starting points for understanding minor-vein-specific gene expression. In cucurbits and a number of other plants that load through the symplast by polymer trapping, Suc from the mesophyll is converted to raffinose family oligosaccharides (RFOs)—primarily the tetrasaccharide stachyose—in specialized companion cells called intermediary cells, which are only present in the minor veins. These higher order oligosaccharides are thought to be too large to diffuse back into the mesophyll and therefore accumulate to high concentration in the minor-vein phloem. Conversion of Suc to RFOs is therefore an integral aspect of phloem loading in these species, and compartmentalization of the biosynthetic enzymes to the minor-vein phloem is vital for proper function (Turgeon, 1996). The first committed enzyme in this pathway is galactinol synthase, which catalyzes the synthesis of galactinol (O-α-d galactopyranosyl-(1→1)-myo-inositol). Galactinol is the galactosyl donor for the synthesis of RFOs (Keller and Pharr, 1996).
Consistent with its role in phloem loading, galactinol synthase immunolocalizes to the intermediary cells of minor veins in cucurbits (Beebe and Turgeon, 1992). Furthermore, the promoter from a melon (Cucumis melo) galactinol synthase gene (CmGAS1) drives reporter gene expression specifically in the minor-vein companion cells of transgenic tobacco and Arabidopsis (Haritatos et al., 2000a). Both of these species load Suc from the apoplast by cotransport with protons (Burkle et al., 1998; Gottwald et al., 2000). Although Arabidopsis synthesizes a small amount of galactinol in mature leaves and transports a minor amount of raffinose, tobacco does not, and in neither species is galactinol or galactinol synthase associated with phloem loading (Haritatos et al., 2000a, 2000b).
This finding that the expression pattern conferred by the CmGAS1 promoter is conserved among species regardless of the requirement for galactinol indicates that the regulatory mechanisms conferring minor-vein expression are highly conserved irrespective of the loading mechanism employed. Such conservation in turn implies that the same regulatory system controls numerous other genes with disparate roles in the structure and function of minor-vein companion cells (Doebley and Lukens, 1998). Conversely, if the regulatory cascade were specific to genes directly involved in phloem loading, one would expect the cascade to have diverged along with the loading mechanisms and be inoperable in distantly related species.
To gain insight into the regulatory mechanisms that govern gene expression in the minor-vein phloem, functional domains of the CmGAS1 promoter were mapped by two complementary approaches: truncation/mutagenesis and comparative sequence analysis. The latter approach, referred to as phylogenetic footprinting, is based on the supposition that cis-acting sequences that interact with sequence-specific trans-acting factors are evolutionarily conserved, whereas neutral sequences undergo genetic drift (Fickett and Wasserman, 2000). Phylogenetic footprinting confirmed the importance of sequences identified by promoter truncations and mutagenesis but also revealed additional sequences that are highly conserved but undetected in our functional analysis. Phylogenetic footprinting also provided single-nucleotide resolution of conserved sequences, but species selection was imperative: Sequence similarity within the genus Cucumis was too great to identify functionally conserved elements, whereas comparisons at the family level (Cucurbitaceae) were highly informative. Our results support the hypothesis that a highly conserved regulatory cascade operates in the minor veins of higher plants and identifies the cis-acting sequences involved. These sequences operate in a combinatorial fashion to enhance or silence gene expression in specific vascular tissues and together limit expression to the companion cells of minor veins.
RESULTS
Promoter Truncations
The CmGAS1 promoter (National Center for Biotechnology Information [NCBI] accession no. AF249912), isolated as a 3,081-bp fragment and fused to the uidA (β-glucuronidase [GUS]) reporter gene, confers prominent gene expression to the minor veins of both tobacco and Arabidopsis (Haritatos et al., 2000a). Expression is also evident in leaf hydathodes, the smallest veins of sepals, petals, and stamens, and in root apical meristems. Notably, staining is not observed in larger leaf veins and stops abruptly where minor veins join larger ones (Haritatos et al., 2000a). To identify the cis-acting elements that are necessary and sufficient for minor-vein expression, 5′ to 3′ promoter truncations were created with conveniently located restriction sites (Fig. 1A). The expression pattern conferred by the 3,081-bp promoter element was retained in both tobacco and Arabidopsis by a promoter truncated to a XhoI site located 1,761 bp upstream of the CmGAS1 open reading frame (ORF), however, a reduction in staining intensity was noted in histochemical assays with the GUS substrate 5-bromo-4-chloro-3-indolyl-β-d-GlcUA (X-GlcA; not shown). Further truncation to a BamHI site located 1,077 bp upstream of the ORF abolished activity in the minor veins, but activity was retained in hydathodes. These results demonstrate that sequence elements upstream of –1,077 are essential for minorvein expression.
Figure 1.
CmGAS1 promoter truncations, and resulting GUS activity. A, Promoter truncations (5′) created with convenient restriction endonuclease recognition sites. B, Promoter truncations (5′ and 3′) created by PCR or site-directed mutagenesis and possessing a synthetic, basal promoter consisting of the first 60 bp of the 35s promoter fused to a translational enhancer from TEV (represented by hatched boxes; TT, transcription initiation/translational enhancer). Promoter fragments that confer minor-vein uidA expression, determined in histochemical assays with X-GlcA, are indicated as gray bars, and white bars indicate those that do not. Sequence coordinates are indicated along the top, relative to the CmGAS1 start codon (+1). The uidA reporter gene is indicated in black and is not drawn to scale. Plasmids are labeled to the right of each schematic (pGPTV designation omitted). Relative GUS activities measured in fluorescent assays with 4-methylumbelliferyl β-d-glucuronide hydrate (MUG) as substrate, in arbitrary units, are indicated on the right by black circles; ×15 indicates 15-fold GUS activity relative to a single black circle; ND, not determined; hollow circles, no activity; n > 12 pooled seedlings. The black circle(s) representing GUS activity in plants harboring pGPTVBam101 and pGPTV-TT(–1,333–1025) result from GUS activity in hydathodes, because minor-vein X-GlcA staining was not observed.
To further delimit the region of the promoter essential for the minor-vein expression pattern, additional truncations were made from both the 5′ and 3′ directions (Fig. 1B). The –60 region of the cauliflower mosaic virus (CaMV) 35S promoter (35S-60) was used to compensate for deletion of the CmGAS1 TATA box and transcriptional start site. The 35S-60 basal promoter provides sequences sufficient for transcription initiation, but does not itself result in gene expression unless coupled to transcriptional enhancer elements (Campisi et al., 1999). In addition, a translational enhancer element from tobacco etch virus (TEV) was incorporated that has been shown to increase gene expression by facilitating ribosome binding (Carrington and Freed, 1990).
Promoter sequences from 1,816 to 123 bp upstream of the CmGAS1 ORF were fused to the 35S-60 basal promoter and the uidA reporter gene, and were analyzed for expression pattern and relative promoter strength in transgenic Arabidopsis. Systematic truncations of this element were made by PCR and site-directed mutagenesis and were similarly tested in transgenic Arabidopsis (Fig. 1B).
On the basis of these truncations, we established that the smallest element necessary and sufficient for minor-vein expression was a 351-bp block located between nucleotides –1,333 and –983 (Fig. 1B). This element conferred the same pattern and approximate strength (within a factor of two) of uidA activity as all other constructs derived from the –1,816 to –123 element fused to the 35S-60 basal promoter. Sequences from –1,816 to –1,334, and from –982 to –123 are therefore dispensable for both tissue specificity and promoter strength. Further truncation of the minimal 351-bp element to –1,203 on the 5′ side or –1,032 on the 3′ side eliminated minor-vein activity; however, plants harboring the –1,333 to –1,032 fragment retained X-GlcA staining in the hydathodes (data not shown).
All constructs derived from the –1,816 to –123 element fused to the 35S-60 basal promoter demonstrated reduced activity relative to the construct pSGXho101. The promoter in pSGXho101 extends from –1,762 to +12 of the CmGAS1 sequence, and sequences from –122 to+12 may therefore contain enhancer elements that contribute significantly to promoter strength without affecting tissue specificity. This premise is supported by the presence of highly conserved sequences in the same region of CmGAS1 orthologs from other species in the Cucurbitaceae (see below). However, it is noted that the constructs are derived from different, albeit related, plasmid backbones (pGPTV-KAN versus pBi101.2; pGPTV-KAN is derived from pBi101.2; Becker et al., 1992).
High-Resolution Mutagenesis
To pinpoint more precisely the cis-acting elements responsible for the minor-vein expression pattern, sequences within the –1,333 to –983 region were mutated as consecutive stretches of 10 nucleotides. Nucleotides were mutated in stretches of 10 to coincide roughly with the anticipated size of transcription factor-binding sites (six to 12 nucleotides). A SalI recognition site was incorporated in the center of each mutagenized sequence for convenient identification, and the remaining nucleotides were converted to noncomplementary transversions (i.e. A↔C, G↔T), which were rationalized to be the most disruptive. This created 35 mutant constructs designated 1 to 35 (Fig. 2A), each of which was fused to the 35S-60 basal promoter and functionally tested in transgenic Arabidopsis. Each construct was scored for uidA expression pattern and relative strength by histochemical staining (Fig. 2, A–E).
Figure 2.
High-resolution mutagenesis of the CmGAS1 promoter and analysis of resulting expression patterns. A, CmGAS1 mutations and resulting expression patterns. CmGAS1 promoter sequences –1,333 to –984 are indicated by the black text. The corresponding mutant sequences, indicated 1 to 35 and separated by spaces, are indicated above in red, italicized text. Blocks I, II, and III, which resulted in altered expression patterns, are enclosed in gray boxes. Within each block, underscored mutant sequences (17, 22, and 32) abolished expression in the minor vein, but retained expression in hydathodes and root apices, overscored mutations (21 and 31) abolished gene expression in all tissues, and the mutation underscored with asterisks (18) resulted in gene expression in all vascular tissue. Mutations surrounding block II resulting in a approximately 50% reduction in staining intensity are enclosed in a white box; n > 20 seedlings. B, Representative plant with uidA expression controlled by the –1,333 to –984 promoter. The observed expression pattern is characteristic of all mutations excluding the blocks enclosed by gray boxes in A. C, uidA expression in hydathodes without minor-vein expression. The imaged plant harbors mutation 17, but is characteristic of those with mutations 22 and 32. D, uidA expression in all veins resulting from mutation 18. E, Absence of uidA expression in all tissues. A plant harboring mutation 21 is shown and is also representative of plants harboring mutation 31.
As demonstrated by the histochemical assays, six mutations altered the tissue specificity of reporter gene expression from that observed with the parent construct, pGPTV-TT(–1,333–983; Fig. 2B), and clustered into three “blocks”. Block I was defined by mutations 17 and 18, and includes nucleotides –1,173 to –1,154 (Fig. 2A). Mutation 17 abolished expression in minor veins, but expression was retained in hydathodes (Fig. 2C) and root apices (data not shown). Mutation 18 had minimal effect on staining intensity in the minor veins and hydathodes, but resulted in strong expression throughout the vascular network of the leaf, stem, and root, where it is otherwise not observed (Fig. 2D). Block II (Fig. 2A) was defined by mutations 21 and 22, and spans nucleotides –1,133 to –1,124. Both of these mutations abolished minor-vein expression. In addition to minor veins, mutation 21 abolished expression in all tissues (Fig. 2E), whereas mutation 22 retained expression in the hydathodes and root apices. Mutations flanking block II—particularly 20, 23, 24, and 25—exhibited reduced X-GlcA staining in the minor veins, however, the overall pattern was not altered from that observed with pGPTV-TT(–1,333–983). Block III (Fig. 2A) was defined by mutations 31 and 32, and consists of nucleotides –1,033 to –1,014. As with block II, mutation 31 abolished activity in all tissues and mutation 32 abolished activity only in the minor veins.
Taken together, blocks I, II, and III are required for and work cooperatively to regulate expression from the CmGAS1 promoter in minor veins. One-half of block I, and both blocks II and III, are together required for expression in vascular tissues. The other half of block I is required to repress expression in the larger veins of the leaf as well as in the vascular bundles of stems and roots (Fig. 2D).
Phylogenetic Footprinting
Arabidopsis, although a convenient heterologous host for functional analysis of a tissue-specific promoter, is not closely related to melon (Soltis et al., 1999) and does not load photoassimilate by the polymer trap mechanism (Haritatos et al., 2000b). Therefore, we chose to confirm by an independent approach that the sequences essential in Arabidopsis are also important for activity in the Cucurbitaceae. Phylogenetic footprinting is a comparative approach for identifying putative transcription factor-binding sites in a promoter and is based on the fact that essential sequences are conserved over evolutionary time (Fickett and Wasserman, 2000; Hardison, 2000; Loots et al., 2000). Sequence data were obtained for regions upstream of CmGAS1 orthologs in representative Cucurbitaceae species, using a PCR-based “genome walking” methodology (Siebert et al., 1995). The gene-specific primers used were intended to preferentially amplify CmGAS1 orthologs. To ensure that the obtained upstream sequence did correspond to the promoter region of the CmGAS1 ortholog, sufficient sequence from the ORF was also obtained to allow comparative alignments (data not shown) with the CmGAS1 (NCBI accession no. AY077642) and CmGAS2 (NCBI accession no. AY077641) ORFs.
Sequence divergence among the species was initially unknown, and a broad sampling was therefore selected based on classifications derived from molecular and morphological data and on geographical distribution (Jeffrey, 1990; Jobst et al., 1998). Within the genus Cucumis, sequence conservation upstream of the CmGAS1 orthologs was too great to provide meaningful insight into sequences that were conserved through functional necessity (data not shown), and comparisons were instead performed at the family level. Six Old World species, melon, watermelon (Citrullus lanatus), Luffa cylindrica, Trichosanthes kirilowii, Momordica charantia, and Seyrigia humbertii, and two New World species, Sechium edule and squash (Cucurbita pepo), were selected. The greatest divergence among the species analyzed, based on DNA sequence comparisons, is between melon and squash, and the remaining species are intermediate between these two. Watermelon is relatively close to melon, and S. edule is relatively distant. However, S. edule appears to be closer to the Old World species than it is to its New World counterpart, squash. S. humbertii is worthy of note: It is a leafless xerophyte and therefore does not have minor veins in the canonical sense.
Local alignments in the GAS1 promoter regions of each representative specie were sought with the Gibbs sampler algorithm of the MACAW software package (Schuler et al., 1991; Lawrence et al., 1993). Sequences were selected as being significantly conserved and potentially involved in regulating gene expression if similarity was roughly 90% across all species for at least eight consecutive nucleotides. Numerous sequences meeting this criterion were identified along the 3,081 bp of the CmGAS1 promoter (Fig. 3A and supplemental data, found in the online version of this article at http://www.plantphysiol.org). These values were selected because we anticipated individual transcription factor recognition sites to be six to 12 nucleotides in length and tolerant of some degeneracy. Reduced stringency resulted in “hits” that appeared spurious based on their scattered distribution throughout the promoters.
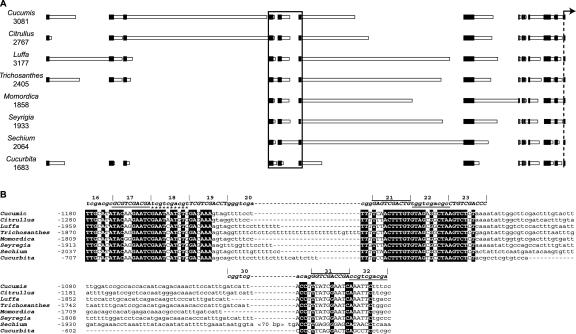
Figure 3.
Alignment of conserved GAS1 promoter sequences among eight genera in the Cucurbitaceae. A, Graphic representation of alignment constructed with MACAW. Horizontal bars represent sequences from each ortholog, with the location of the ATG start codon indicated by the dashed line with an arrow on the right. Total analyzed sequence lengths upstream of the start codons are indicated on the left. Sequences demonstrating roughly 90% similarity over at least 8 bp among the genera are indicated by vertically aligned black boxes. Gaps introduced to bring sequences into alignment are indicated by the lack of horizontal bars. Aligned sequences enclosed by the box are presented in detail in B. B, Alignment of sequences enclosed by the box in A. Corresponding mutations are indicated above the alignments, using the same pattern of symbols as in Figure 2A. Sequential mutants alternate in upper and lowercase solely to emphasize boundaries. Sequences selected as significant blocks of conservation are indicated in uppercase text. Sequences that are identical among all eight genera are indicated by white text on a black background. See also supplemental data.
Three of the regions conserved among the species correlated with the three blocks determined by mutagenesis (Fig. 3, A and B). Sequence identity among the species was particularly high for blocks I and II and extended for several nucleotides beyond the block boundaries, as defined by site-directed mutagenesis (Fig. 3B). A T-rich stretch between blocks I and II was also conserved, but demonstrated considerable variation in length. The extent of sequence identity among the species in block III was not as pronounced, especially between the New and the Old World species (Fig. 3B).
Upstream of block I, three regions of prominent sequence conservation were identified among melon, watermelon, L. cylindrica, T. kirilowii, and squash. These regions are immediately upstream of block I in L. cylindrica, T. kirilowii, and squash but are separated by roughly 1 kb in melon and watermelon (Fig. 3A). There is relatively little homology in these intervening regions. One of the upstream conserved regions (–2,366 to –2,345 of CmGAS1) was used as an anchor to obtain sequence information from M. charantia, S. humbertii, and S. edule. The upstream regions of homology are therefore not represented in these latter species. However, block I was within 100 bp of this conserved anchor, and these species therefore appear to have an upstream promoter architecture resembling that of L. cylindrica, T. kirilowii, and squash.
Three stretches of near identity greater than 20 bp in length were found between block III and the GAS1 ORF, along with four closely clustered, shorter sequences (Fig. 3A and supplemental data). The distance between block III and the first of these sites varied greatly, from 176 bp in squash to 1,347 bp in S. edule. Within this region, there is some interspersed homology between melon and watermelon, and between L. cylindrica and T. kirilowii (data not shown). Further downstream are additional stretches of variable-length, non-conserved sequences interspersed among those that are conserved. Generally, sequence similarity between the species analyzed increases closer to the ORF.
It is noteworthy that the blocks demonstrate substantial variation in spacing among the species, but that the order and orientation of individual blocks is constant, implying that over evolutionary time, the architecture of the promoter has been influenced substantially by insertion and deletion events.
Comparison with Known Transcription Factor-Binding Sites
The sequences identified as being functionally essential for minor-vein expression and conserved among the species analyzed were used to challenge the TRANSFAC database of characterized transcription factors (Hehl and Wingender, 2001). The semipalindromic sequence GAATCGAAT(G/C) within block I, which was disrupted in both mutations 17 and 18, resembles the recognition sequence of AtHB-9 (GTAAT(G/C) ATTAC). AtHB-9 is a homeodomain transcription factor of the Leu zipper class, of which closely related members are involved in regulating gene expression in vascular tissues (Sessa et al., 1998; Baima et al., 2001). Block II contains the sequence CTTT that is characteristic of the core recognition sequence of the dof family of transcription factors. Members of the dof family are also demonstrated to be involved in controlling vascular tissue gene expression (Baumann et al., 1999; Gualberti et al., 2002; Papi et al., 2002). Due to the low level of sequence conservation in block III, insufficient sequences are identical among the species to identify a clear pattern, however, among the Old World species, the presence of a CTTT motif also suggests the binding site for a dof transcription factor.
DISCUSSION
In the Cucurbitaceae, GAS1 catalyzes the synthesis of galactinol, which is the first committed step in the synthesis of RFO transport sugars and is an integral part of the polymer trap mechanism of phloem loading. Consistent with this, GAS1 activity localizes to the minor veins where RFOs are synthesized and loading occurs (Beebe and Turgeon, 1992). However, the CmGAS1 promoter confers gene expression to the minor veins in plants that do not use galactinol as an intermediate in the phloem-loading process, and where there is no a priori reason to expect this promoter to be recognized. This implies that the regulatory machinery governing minor-vein expression is conserved across a broad range of eudicotyledons, whereas the types of genes regulated by this machinery have changed.
As an initial step in unraveling this genetic program, sequence elements within the galactinol synthase promoter that are necessary and sufficient for its precise tissue specificity were functionally characterized in transgenic Arabidopsis as a heterologous host. Promoter truncations revealed that sequences conferring minor-vein specificity were situated on a 351-bp fragment located 1,333 to 983 nucleotides upstream of the galactinol synthase ORF. The essential sequences were then mapped with 10-bp resolution by site-directed mutagenesis, and three blocks were characterized as being functionally significant: block I spans nucleotides –1,163 to –1,144 (mutations 17 and 18); block II spans nucleotides –1,133 to –1,114 (mutations 21 and 22); and block III spans nucleotides –1,033 to –1,014 (mutations 31 and 32). Promoter truncations also indicated that an essential element is located between nucleotides –1,333 and –1,203, however, this element was not identified by high-resolution mutagenesis or by comparative sequence analysis. The –1,333 to –1,203 element has an AT content of 84% that may be involved in chromatin structure and affect gene expression when deleted entirely, but may not be affected by smaller mutations.
The genera Arabidopsis and Cucumis are not closely related (Soltis et al., 1999), and they load photoassimilate by different mechanisms. A comparative approach, phylogenetic footprinting, was used to support and expand the results obtained from the functional analysis (Fickett and Wasserman, 2000; Hardison, 2000). This technique is attracting increased attention as more genomic sequences from related species are deposited in sequence databases, and as techniques for isolating unknown sequences upstream of known genes improve. Comparisons on the genomic scale have been reported among mammals (Loots et al., 2000; Dermitzakis et al., 2002), nematodes (Thacker et al., 1999), plants (Colinas et al., 2002), and other organisms. Analysis on individual promoters in plants has been performed among the Poaceae (Wang et al., 1999; Kaplinsky et al., 2002) and the Brassicaceae (Hill et al., 1998; Koch et al., 2001), as well as a broad sampling of both higher and lower plants (Arguello-Astorga and Herrera-Estrella, 1996; Arguello-Astorga and Herrera-Estrella, 1998). A recent analysis of cereal promoters compared several software programs for phylogenetic footprinting, but did not include the one used here (Guo and Moose, 2003). Selection of species for comparison is essential for success: Sequences from species that are closely related exhibit significant nonfunctional conservation, whereas conservation in distantly related species may be difficult to identify among spurious background homologies. In our studies, there was insufficient sequence divergence within the genus Cucumis, however, comparisons across the Cucurbitaceae proved more fruitful.
Representative species were selected based primarily on guide trees established by comparison of ribosomal internal transcribed spacer sequences with consideration of classifications based on morphological data (Jeffrey, 1990; Jobst et al., 1998). Detailed classification of this economically important family is sadly lacking, and grouping of genera into monophyletic tribes is particularly poor. Based on geographical distribution of natural populations, Cucumis, Citrullus, Momordica, Luffa, Trichosanthes, and Seyregia are Old World genera (with exceptions; Jeffrey, 1990), whereas Cucurbita and Sechium are New World genera. The greatest evolutionary distance, based on internal transcribed spacer data, was between Cucumis and Cucurbita. The geographical distribution of these species has led some to speculate that the two diverged with the separation of the continents, 80 to 100 million years ago, however, the fossil record suggests that the family may only be 40 million years old (Jobst et al., 1998). Seyrigia humbertii is a xerophyte from Madagascar that is leafless, and therefore does not have minor veins in the canonical sense. It would be interesting to determine where the ortholog of CmGAS1 is expressed in S. humbertii to establish where galactinol synthesis and phloem loading occur in its photosynthetic stem.
Local alignment of sequences upstream of the CmGAS1 orthologs from the representative species identified regions of sequence conservation all along the 3,081 bp of the CmGAS1 promoter. However, based on functional analysis in Arabidopsis, sequences upstream of nucleotide –1,333 and downstream of nucleotide –983 are not essential for minor-vein expression. It is thus not clear why these other sequences are so strongly conserved. The two regions closest to the GAS1 ORF are either partially or fully downstream of CmGAS1 nucleotide –123, and may account for the difference in uidA expression observed between constructs derived from the –1,816 to –123 fragment, and pSGXho101 (Fig. 1). Similarly, regions upstream of nucleotide –1,762 may also be involved in promoter strength, because histochemical staining of transformed plants suggest that the promoter fragment contained on pSG3K101 is qualitatively stronger than that of pSGXho101 (data not shown).
In melon, CmGAS1 is expressed in maturing seeds (Volk et al., 2003) in addition to minor veins, and may also be expressed in spatial and temporal ways of which we are currently unaware. The “extra” conserved sequences in the promoters may therefore be necessary and conserved for these other expression patterns. Furthermore, the conserved sequences may not be involved in regulating GAS1, but may instead regulate adjacent genes (an adjacent ORF was not evident in any of the sequences obtained) or have long-range effects on gene expression. It is also possible that these sequences play an essential role in chromatin structure and have no direct effect on gene expression. It is unlikely that the sequences are conserved by mere chance because comparison of melon and squash GAS1 (NCBI accession no. AR014709) coding sequences, where constraints on base substitutions are expected to be greater, do not show equivalent stretches of sequence identity (data not shown).
Due to our analysis of numerous species and our criterion for 90% similarity over at least eight consecutive nucleotides, the resolution at each site is higher than what is commonly reported in phylogenetic footprinting studies, in that each site is within the size range expected for the binding sites of individual transcription factors. By comparison, phylogentic footprinting studies among mammalian species frequently cite cis-acting elements that are 70% homologous over 100 nucleotides (Loots et al., 2000; Dermitzakis et al., 2002) and therefore do not define individual potential binding sites. Conversely, high-resolution comparisons complemented and extended our functional analysis to an extent that we recommend incorporating this approach into similar studies in gene expression.
It is worthy of note that there is no comparable homology with the promoter region of CmGAS2 (Volk et al., 2003; data not shown), indicating complete divergence of the promoters since the two paralogs diverged from a single ancestral gene. In turn, this indicates that the paralogous promoters are probably not bound directly by any common transcription factors.
Three conserved regions were identified among the orthologs between nucleotides –1,333 and –983 of CmGAS1 that correlate with the three blocks identified by site-directed mutagenesis and functional analysis in Arabidopsis. Sequence identity was strong among the species analyzed in the vicinity of blocks I and II, and in both cases, this conservation extended beyond the boundaries of sequences that are functionally essential (Fig. 3B). Notwithstanding, sequence comparisons revealed nucleotides within each block that are not conserved among all species. These nucleotides are therefore not essential in the absolute sequence context, but may be essential for proper spacing within or between cis-acting promoter elements. In this important regard, phylogenetic footprinting improved the resolution of the promoter analysis beyond that obtained by site-directed mutagenesis alone. Sequence conservation was not as prominent in block III, particularly between the New World species, S. edule and squash, and the Old World species. Without the support of the mutagenesis data, the limited conservation in this region would have been dismissed as spurious background homology among the Old World species. This emphasizes the importance of selecting a range of species for analysis, as well as the requirement for a second, functional means to identify important sequences.
The first block does not display a clear sequence similarity to characterized transcription factor-binding sites, but does have remote homology to sequences recognized by a large class of transcription factors characterized by a homeodomain adjacent to a Leu zipper (HD-Zip). HD-Zip transcription factors form homo- and potentially heterodimers through the Leu zipper domain and tend to bind DNA at palindromic sequences (Sessa et al., 1998). The semipalindromic sequence GAATCGAAT(A/C) within block I therefore has the characteristics of a site recognized by such a complex. This sequence was partially disrupted by mutation 17, which abolished gene expression in the minor veins, and by mutation 18, which promoted expression in all vascular tissues. This also correlates with HD-Zip characteristics because HD-Zip proteins can function as either activators or repressors, and several members are expressed in vascular tissues (Sessa et al., 1998; Baima et al., 2001). It may be that block I binds proteins of the HD-Zip class, and mutating either half of the site determines whether it functions as an enhancer or a silencer by determining which protein complex is able to bind. We cannot formally exclude the possibility that sequences introduced by mutation 18 act as a serendipitous enhancer by binding transcription factors in the major veins that would not normally interact with the CmGAS1 promoter. However, we feel this is unlikely as all mutations contained the same core sequence, GTCGAC, and some had very similar flanking sequences (i.e. mutation 19; Fig. 2A), yet only mutation 18 demonstrated prominent expression throughout the vascular network.
In block II, mutation 21 disrupted sequences that correlate with the core recognition sequence of the dof family of transcription factors (CTTT; Yanagisawa and Schmidt, 1999) and abolished uidA activity in all tissues. Mutation 22 prevented expression in the minor veins, but not hydathodes or root apices. One interpretation of this is that different dof factors interact with block II in different tissues. Mutating the core recognition sequence (mutation 21) may prevent binding in all tissues, whereas mutating the flanking sequences (mutation 22) prevents binding of the vascular-tissue factor, but not the hydathode or root factor. That block II contains a potential dof factor-binding site is particularly interesting because members of the dof family are expressed in vascular tissues, and are demonstrated to be necessary for phloem-specific expression from other promoters (Baumann et al., 1999; Gualberti et al., 2002; Papi et al., 2002).
In block III, sequence conservation is strong among the Old World Cucurbitaceae, but is not as pronounced among the New World species. For this reason, a class of transcription factor that potentially interacts with this site cannot be assigned. As in blocks I and II, block III sequences were disrupted by two adjacent mutations, 31 and 32, each of which resulted in different expression patterns. Mutation 31 abolished activity in all tissues, and mutation 32 abolished activity only in the minor veins. Block III may therefore constitute the binding site for a single class of transcription factor, as proposed for block II, in which one mutation destroys the binding site core and the second disrupts those necessary only for binding minor-vein factors. Alternatively, block III may be a composite site that binds a complex, such as the dimer proposed for block I, and the different expression patterns may represent which constituents are able to bind.
Few other characterized promoters drive gene expression in mature minor veins, with exclusion from larger vascular bundles. One such promoter is from a stachyose synthase gene in Alonsoa meridionalis (AJ487031; Voitsekhovskaja, 2001). As with GAS, stachyose synthase is involved in the synthesis of RFOs (Keller and Pharr, 1996), and similarly to melon, the genus Alonsoa appears to phloem load by the polymer trap mechanism because it has intermediary cells and translocates primarily RFOs (Turgeon et al., 1993). However, Alonsoa and Cucumis are only distantly related (Soltis et al., 1999) and almost certainly adopted the polymer trap mechanism of phloem loading independently from ancestors that did not have intermediary cells and translocated primarily Suc (Turgeon et al., 2001). Nonetheless, the sequence TTTTCTTTTTTTCCCTTTGTGTAG, which is present 1,293 nucleotides upstream of the A. meridionalis stachyose synthase ORF, is very similar to the TTTTCCTTTTTCAACTTTGTGTAG sequence that overlaps with block II and is conserved among the Cucurbitaceae. Whether these sequences have similar functions in the two species is not yet established. The Arabidopsis Suc transporter gene AtSUC4 also appears to be expressed in minor veins and not larger bundles (Weise et al., 2000), but promoter elements matching those in the CmGAS1 promoter were not identified (data not shown).
On the basis of these analyses, highly specific gene expression restricted to the companion cells of minor veins is attributable to the combinatorial activity of several trans-acting factors that function as either positive or negative regulators. The positive regulators must act together, because preventing any one of them from interacting with the promoter abolishes gene activity. Each one must therefore be present in, but not necessarily limited to, the minor veins where expression is ultimately observed. The negative regulator that interacts with block I to prevent expression in the major veins, is presumably absent from the minor veins, or is in some way prevented from interacting with the DNA in these tissues. This factor may instead be present in, and interact with, the CmGAS1 promoter in the major veins and other phloem tissues to repress activity. To directly test this model for regulating gene expression in the minor veins, DNA-binding proteins that interact with each sequence block are being sought. Knowledge of both the cis- and trans-acting factors that operate at the CmGAS1 promoter will provide a strong framework for identifying other genes that are coordinately regulated by the same pathway.
MATERIALS AND METHODS
Promoter Truncations
All plasmid constructions were by standard procedures (Ausubel et al., 1995). Enzymes and reagents were obtained from New England Biolabs (Beverly, MA), Invitrogen (Carlsbad, CA), Applied Biosystems (Foster City, CA), and TaKaRa (distributed by PanVera; Madison, WI) and used according to the manufacturers' instructions. The Cornell BioResource Center performed sequencing reactions.
Plasmid pSG3K101 was previously described (Haritatos et al., 2000a) and consists of 3,081 bp of promoter and 12 bp of CmGAS1 ORF fused to the uidA reporter gene in pBi101.2. The promoter was truncated to 1,762 bp in pSGXho101 by digesting pSG3K101 with XhoI and XbaI, filling in the ends with Klenow, and ligating the resulting blunt ends. The promoter was similarly truncated to 1,077 bp in pSGBam101 by using BamHI in place of XhoI.
Plasmid pGemTT was constructed to contain a multiple cloning site upstream of a basal promoter consisting of the first 60 bp of the CaMV 35S promoter and a translational enhancer from TEV (TT, transcription initiation/translational enhancer), both derived from pIBT210 (Haq et al., 1995). The XhoI, XbaI, and BamHI restriction sites of pIBT210 were removed sequentially by digesting with each enzyme, filling in the ends with Klenow, and ligating the blunt-end products. The –60 region of the CaMV 35S promoter and the TEV translational enhancer element (35s-60) were PCR amplified from the resulting construct with a forward primer that incorporates recognition sites for XbaI, XhoI, and BamHI (tct aga ctc gag gga tcc cac tat cct tcg caa gac c) and a reverse primer corresponding to sequences in the polyadenylation signal in pIBT210 (aag ccg gta aga gac aac aac). This PCR product was gel purified with a QIAquick Gel Extraction Kit (Qiagen USA, Valencia, CA), digested with SacI, phosphorylated with polynucleotide kinase, and the fragment corresponding to the minimal promoter and translational enhancer was gel purified and sub-cloned into SacI- and HincII-digested pGEM3zf+ (Promega, Madison, WI) to create pGemTT.
The CmGAS1 promoter fragments represented in Figure 1B were PCR-amplified with appropriate combinations of the following oligonucleotides using the Elongase polymerase preparation (Invitrogen) and pSG3K101 as template. The numeric in each oligonucleotide name indicates the first complementary sequence in the promoter. The forward oligonucleotides were GAS-1816F (tta agt aag ctt tgt gat cga tgg tat tag atg agt tcc), GAS-1333F (cat gtt aag ctt tag aac taa acc taa gtt atg tta tgt gtc), and GAS-678F (aga act aag ctt taa tca tta tca aaa aca cta cc). The reverse oligonucleotides were GAS-1149R (agg agg tct aga ttc tcc aca tta ttc gat tct tg), GAS-634R (cct aaa cat cta gat ttg att tca att ctg ttt taa ggt agt g), and GAS-123R (agc ttg tct aga gaa ggg atg gga agg agg c). The PCR products were extracted with chloroform and precipitated with ethanol, digested with HindIII and XbaI, gel purified, and sub-cloned into the same sites of pGemTT to create pGemTT(–1,816–123), pGemTT(–1,816–1,149), pGemTT(–1,333–634), and pGemTT(–678–123). The basal promoter (i.e. the –60 region of the 35S promoter with the translational enhancer element from TEV), and the promoter fusions were isolated as HindIII-SmaI fragments, and sub-cloned into the same sites of the binary vector pGPTV-KAN (Becker et al., 1992). This created pGPTV-TT, pGPTV-TT(–1,816–123), pGPTV-TT(–1,816–1,149), pGPTV-TT(–1,334–634), and pGPTV-TT(–678–123).
The fragment corresponding to CmGAS1 sequences –1,333 to –634 contained sequences necessary and sufficient for uidA expression in minor veins of Arabidopsis, and pGemTT(–1,333–634) was therefore used for further truncations. Sequences within this fragment were altered to SalI recognition sites by site-directed mutagenesis (Kunkel et al., 1987) with the oligonucleotides GAS-776 (gta aaa atg gtg tcg aca acc aat tc), GAS-983 (cag aag gtc gac ggt atg ttc), GAS-1032 (gaa ttt gag tcg aca aag gta atg), and GAS-1203 (tca atg atg tcg aca gct aat at), creating pGemTT-1203, pGemTT-1032, pGemTT-983, and pGemTT-776. The last three were digested with HindIII and SalI, and the intervening promoter sequences were sub-cloned into the HindIII and XhoI sites of pGPTV-TT to create pGPTV-TT(–1,333–1,032), pGPTV-TT(–1,333–983), and pGPTV-TT(–1,333–776). To create pGPTV-TT(–1,203–634), pGemTT-1203 was digested with HindIII and SalI, made blunt with Klenow, and ligated back together. The plasmid was then digested with HindIII (recreated in the HindIII-SalI fusion) and XhoI, and the truncated promoter fragment was sub-cloned into pGPTV-TT.
High-Resolution Mutagenesis
From the promoter truncation experiments, CmGAS1 promoter sequences between –1,333 and –983 were necessary and sufficient for uidA expression in minor veins of Arabidopsis, and therefore pGemTT(–1,334–983) was constructed for high resolution mutagenesis. pGemTT(–1,338–983) was created by digesting pGemTT-983 with SalI and XhoI and ligating the compatible cohesive ends. The sequences between positions –1,333 and –983 were mutagenized as consecutive blocks of 10 bp by site-directed mutagenesis. The sequences of the 35 mutagenic oligonucleotides used are available from the authors upon request. In general, each was 30 to 40 nucleotides in length, and introduced a SalI restriction site into the center of the target sequence along with two noncomplementary transversions on each side. The mutated sequences in the resulting 35 constructs are depicted in Figure 2A. Each of the mutagenized promoters were sub-cloned into pGPTV-KAN as HindIII-XmaI fragments.
Transformations and Analysis of GUS Activity
pGPTV-KAN-derived binary vectors harboring promoter constructs were introduced to Agrobacterium tumefaciens strain GV3101 pMP90 by heat shock and selected on 2× YT medium supplemented with rifampicin and gentamycin, each at 50 mg L–1, and kanamycin at 100 mg L–1 (Koncz and Schell, 1986). Arabidopsis ecotype Columbia was transformed by the floraldip transformation procedure (Clough and Bent, 1998). The floral dip procedure ensured that each plant was independently transformed and hemizygous for the uidA reporter gene (Desfeux et al., 2000). Harvested seeds were sterilized with chlorine gas (Clough and Bent, 1998), and transgenic seedlings were selected on Murashige and Skoog medium (Invitrogen) supplemented with Gamborg's vitamins (Sigma-Aldrich, St. Louis), 1% (w/v) Suc, 100 mg L–1 kanamycin sulfate, and 2.5 g L–1 Gel-Rite (Sigma-Aldrich) as solidifying agent. Twenty-one days after seeding, a minimum of 20 kanamycin resistant plants, including roots, were histochemically stained for GUS enzyme activity with X-GlcA (Rose Scientific, Edmonton, Canada) for 24 h by standard procedures (Gallagher, 1992). Quantitative fluorescent assays for GUS enzyme activity with 4-methylumbelliferyl β-d-glucuronide hydrate as substrate were similarly performed by standard procedures (Gallagher, 1992) on a minimum of 12 pooled seedlings.
Phylogenetic Footprinting
Phylogenetic footprinting was employed to identify conserved sequences upstream of CmGAS1 orthologs in representative Cucurbitaceae species. Melon (Cucumis melo; Hales's Best Jumbo), watermelon (Citrullus lanatus; Burpee's Fordhook), and squash (Cucurbita pepo; Prolific Straight Neck) were obtained from W. Atlee Burpee & Co. (Warminster, PA). Cucumber (Cucumis sativum; Burpless Orient Express) was obtained from Germania Seed Company (Chicago). Momordica charantia and Seyrigia humbertii were obtained from the Cornell Conservatory. Luffa cylindrica and Trichosanthes kirilowii were obtained from Horizon Herbs, LLC (Williams, OR). Sechium edule was obtained from a local grocery. High Mr DNA was isolated by standard procedures (Doyle and Doyle, 1990).
Galactinol synthase constitutes a small gene family, and steps were taken to ensure that sequences were obtained from CmGAS1 orthologs. Initially, GAS cDNA sequences were obtained from GenBank (rice [Oryza sativa], D26537; Vitis riparia, AF178569; common bugle [Ajuga reptans], AJ237693; pea [Pisum sativum], AJ243815; squash, AR014709; soybean [Glycine max], AR014710; canola [Brassica napus], AF106954; and Arabidopsis, AB062848) and aligned with ClustalX (Thompson et al., 1997) to identify conserved sequences with CmGAS1 (AY077642) and CmGAS2 (AY077641). From this analysis, the oligonucleotides GASamino (cca aga ggg cgt acg tga cgt tct; starting 50 nucleotides downstream of the CmGAS1 ATG initiation codon) and ALLGAS3 (cca tgt ttt ttc ica gaa rca rtc cat; starting at nucleotide 599 of the genomic sequence) were designed and used to PCR amplify GAS ORF sequences from cucumber, watermelon, and M. charantia. Major bands were sequenced and along with sequences from squash (AR014709) were classified as either orthologs or paralogs of CmGAS1 by comparison with CmGAS1 and CmGAS2. Nested reverse oligonucleotides specific for CmGAS1 orthologs were then designed: LAGASR1 (cat cag gaa gga cag cka caa tga gag ggt ag; starting at nucleotide 175) and LAGASR2 (gag acc ctt tgc caa tcc aac tac acc ttt cca; starting at nucleotide 126).
Sequence information for regions upstream of CmGAS1 orthologs in watermelon, squash, L. cylindrica, and T. kirilowii was obtained using the genome walking method (Siebert et al., 1995), as described by BD Biosciences Clontech (Palo Alto, CA), using Ex-Taq polymerase (TaKaRa). Promoter fragments obtained were sub-cloned into pGem-T Easy (Promega) and sequenced. Blocks of eight or more nucleotides demonstrating roughly 90% similarity based on the degree of segment pair overlap among the species were identified with a Gibbs sampler algorithm and assembled into a composite multiple alignment with the MACAW software package (Schuler et al., 1991; Lawrence et al., 1993). An oligonucleotide corresponding to sequences that are highly conserved among the species sampled and located upstream of those necessary and sufficient for minor-vein expression (GAS2345, cta agg tga tts aay cgy gtg g, starting at nucleotide –2,366 of CmGAS1) was used sequentially with LAGAS1 and LAGAS2 to amplify promoter regions from M. charantia, S. humbertii, and S. edule, which were then incorporated into the alignment.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor. Novel sequences reported in this manuscript were submitted to NCBI GenBank: C. lanatus, AY37977; L. cylindrica, AY379778; T. kirilowii, AY379779; M. charantia, AY379780; S. humbertii, AY379781; S. edule, AY379782; and C. pepo, AY379783.
Supplementary Material
Acknowledgments
We thank Róisín C. McGarry and Ashlee R. McCaskill for reviewing the manuscript and Jian Hua for helpful discussion.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027714.
This work was supported by the U.S. Department of Agriculture/Cooperative State Research, Education, and Extension Services/National Research Initiative Competitive Grants Program (proposal no. 2001–35318–10893 to R.T.).
The online version of this article contains Web-only data.
References
- Arguello-Astorga GR, Herrera-Estrella LR (1996) Ancestral multipartite units in light-responsive plant promoters have structural features correlating with specific phototransduction pathways. Plant Physiol 112: 1151–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello-Astorga G, Herrera-Estrella L (1998) Evolution of light-regulated plant promoters. Annu Rev Plant Physiol Plant Mol Biol 49: 525–555 [DOI] [PubMed] [Google Scholar]
- Ausubel F, Brent R, Kingston RE, Moore DD, Seidmam JG, Smith JA, editors (1995) Short Protocols in Molecular Biology, Ed 3. John Wiley and Sons, New York
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G (2001) The Arabidopsis AtHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol 126: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K, De-Paolis A, Costantino P, Gualberti G (1999) The DNA binding site of the dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 11: 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195–1197 [DOI] [PubMed] [Google Scholar]
- Beebe DU, Turgeon R (1992) Localization of galactinol, raffinose, and stachyose synthesis in Cucurbita pepo leaves. Planta 188: 354–361 [DOI] [PubMed] [Google Scholar]
- Berman BP, Nibu Y, Pfeiffer BD, Tomancak P, Celniker SE, Levine M, Rubin GM, Eisen MB (2002) Exploiting transcription factor binding site clustering to identify cis-regulatory modules involved in pattern formation in the Drosophila genome. Proc Natl Acad Sci USA 99: 757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi L, Yang YZ, Yi Y, Heilig E, Herman B, Cassista AJ, Allen DW, Xiang HJ, Jack T (1999) Generation of enhancer trap lines in Arabidopsis and characterization of expression patterns in the inflorescence. Plant J 17: 699–707 [DOI] [PubMed] [Google Scholar]
- Carrington JC, Freed DD (1990) Cap-independent enhancement of translation by a plant potyvirus 5′ nontranslated region. J Virol 64: 1590–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colinas J, Birnbaum K, Benfey PN (2002) Using cauliflower to find conserved non-coding regions in Arabidopsis. Plant Physiol 129: 451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermitzakis ET, Reymond A, Lyle R, Scamuffa N, Ucla C, Deutsch S, Stevenson BJ, Flegel V, Bucher P, Jongeneel CV et al. (2002) Numerous potentially functional but non-genic conserved sequences on human chromosome 21. Nature 420: 578–582 [DOI] [PubMed] [Google Scholar]
- Desfeux C, Clough SJ, Bent AF (2000) Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol 123: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J, Lukens L (1998) Transcriptional regulators and the evolution of plant form. Plant Cell 10: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15 [Google Scholar]
- Fickett JW, Wasserman WW (2000) Discovery and modeling of transcriptional regulatory regions. Curr Opin Biotechnol 11: 19–24 [DOI] [PubMed] [Google Scholar]
- Gallagher SR, ed (1992) GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, San Diego
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak MA, Beebe DU, Turgeon R (1996) Phloem loading. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker, New York, pp 209–227
- Gualberti G, Papi M, Bellucci L, Ricci L, Bouchez D, Camilleri C, Costantino P, Vittorioso P (2002) Mutations in the dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 14: 1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Moose SP (2003) Conserved noncoding sequences among cultivated cereal genomes identify candidate regulatory sequence elements and patterns of promoter evolution. Plant Cell 15: 1143–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq TA, Mason HS, Arntzen CJ, Clements J (1995) Production of an orally immunogenic bacterial protein in transgenic plants. J Cell Biochem 2588904319
- Hardison RC (2000) Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet 16: 369–372 [DOI] [PubMed] [Google Scholar]
- Haritatos E, Ayre BG, Turgeon R (2000a) Identification of phloem involved in assimilate loading in leaves by the activity of the galactinol synthase promoter. Plant Physiol 123: 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R (2000b) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211: 105–111 [DOI] [PubMed] [Google Scholar]
- Hehl R, Wingender E (2001) Database-assisted promoter analysis. Trends Plant Sci 6: 251–255 [DOI] [PubMed] [Google Scholar]
- Hill TA, Day CD, Zondlo SC, Thackeray AG, Irish VF (1998) Discrete spatial and temporal cis-acting elements regulate transcription of the Arabidopsis floral homeotic gene APETALA3. Development 125: 1711–1721 [DOI] [PubMed] [Google Scholar]
- Iyer VR, Horak CE, Scafe CS, Botstein D, Snyder M, Brown PO (2001) Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409: 533–538 [DOI] [PubMed] [Google Scholar]
- Jeffrey C (1990) Appendix: Classification of the Cucurbitaceae. In DM Bates, RW Robinson, C Jeffrey, eds, Biology and Utilization of the Cucurbitaceae. Comstock Publishing Associates, Ithaca, NY, pp 449–463
- Jobst J, King K, Hemleben V (1998) Molecular evolution of the internal transcribed spacers (ITS1 and ITS2) and phylogenetic relationships among species of the family Cucurbitaceae. Mol Phylogenet Evol 9: 204–219 [DOI] [PubMed] [Google Scholar]
- Kaplinsky NJ, Braun DM, Penterman J, Goff SA, Freeling M (2002) Utility and distribution of conserved noncoding sequences in the grasses. Proc Natl Acad Sci USA 99: 6147–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Pharr DM (1996) Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker, New York, pp 157–183
- Koch MA, Weisshaar B, Kroymann J, Haubold B, Mitchell-Olds T (2001) Comparative genomics and regulatory evolution: conservation and function of the CHS and APETALA3 promoters. Mol Biol Evol 18: 1882–1891 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of Ti-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA (1987) Rapid and efficient site-specific mutagenesis without phenotypic selection. Method Enzymol 154: 367–382 [DOI] [PubMed] [Google Scholar]
- Lawrence CE, Altschul SF, Boguski MS, Liu JS, Neuwald F, Wootton JC (1993) Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science 262: 208–214 [DOI] [PubMed] [Google Scholar]
- Loots GG, Locksley RM, Blakespoor CM, Wang ZE, Miller W, Rubin EM, Frazer KA (2000) Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science 288: 136–140 [DOI] [PubMed] [Google Scholar]
- Papi M, Sabatini S, Altamura MM, Hennig L, Schafer E, Costantino P, Vittorioso P (2002) Inactivation of the phloem-specific dof zinc finger gene DAG1 affects response to light and integrity of the testa of Arabidopsis seeds. Plant Physiol 128: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler GD, Altschul SF, Lipman DJ (1991) A workbench for multiple alignment construction and analysis. Proteins 9: 180–190 [DOI] [PubMed] [Google Scholar]
- Sessa G, Steindler C, Morelli G, Ruberti I (1998) The Arabidopsis AtHB-8, -9 and -14 genes are members of a small gene family coding for highly related HD-ZIP proteins. Plant Mol Biol 38: 609–622 [DOI] [PubMed] [Google Scholar]
- Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA (1995) An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res 23: 1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE, Chase MW (1999) Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402: 402–404 [DOI] [PubMed] [Google Scholar]
- Thacker C, Marra MA, Jones A, Baillie DL, Rose AM (1999) Functional genomics in Caenorhabditis elegans: an approach involving comparisons of sequences from related nematodes. Genome Res 9: 348–359 [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R (1989) The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40: 119–138 [Google Scholar]
- Turgeon R (1996) Phloem loading and plasmodesmata. Trends Plant Sci 1: 418–423 [Google Scholar]
- Turgeon R, Beebe DU, Gowan E (1993) The intermediary cell - minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191: 446–456 [Google Scholar]
- Turgeon R, Medville R, Nixon KC (2001) The evolution of minor-vein phloem and phloem loading. Am J Bot 88: 1331–1339 [PubMed] [Google Scholar]
- Voitsekhovskaja OV (2001) On the role of sugar compartmentation and stachyose synthesis in symplastic phloem loading. PhD thesis. University of Goettingen, Goettingen
- Volk GM, Haritatos EE, Turgeon R (2003) Galactinol synthase gene expression in melon. J Am Soc Hortic Sci 128: 8–15 [Google Scholar]
- Wang RL, Stec A, Hey J, Lukens L, Doebley J (1999) The limits of selection during maize domestication. Nature 398: 236–239 [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kuhn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends Plant Sci 5: 283–290 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Schmidt RJ (1999) Diversitiy and similarity among recognition sequences of dof transcription factors. Plant J 17: 209–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.