Abstract
Antiphospholipid syndrome (APS) is defined by recurrent pregnancy loss and thrombosis in the presence of antiphospholipid (aPL) Ab’s. Currently, therapy for pregnant women with APS is focused on preventing thrombosis, but anticoagulation is only partially successful in averting miscarriage. We hypothesized that complement activation is a central mechanism of pregnancy loss in APS and tested this in a model in which pregnant mice receive human IgG containing aPL Ab’s. Here we identify complement component C5 (and particularly its cleavage product C5a) and neutrophils as key mediators of fetal injury, and we show that Ab’s or peptides that block C5a–C5a receptor interactions prevent pregnancy complications. The fact that F(ab)′2 fragments of aPL Ab’s do not mediate fetal injury and that C4-deficient mice are protected from fetal injury suggests that activation of the complement cascade is initiated via the classical pathway. Studies in factor B–deficient mice, however, indicate that alternative pathway activation is required and amplifies complement activation. In contrast, activating FcγRs do not play an important role in mediating aPL Ab–induced fetal injury. Our findings identify the key innate immune effectors engaged by pathogenic autoantibodies that mediate poor pregnancy outcomes in APS and provide novel and important targets for prevention of pregnancy loss in APS.
Introduction
Antiphospholipid syndrome (APS) is characterized by thrombosis and pregnancy loss that occur in the presence of antiphospholipid (aPL) Ab’s (1). Over the last two decades, APS has emerged as a leading cause of pregnancy loss and pregnancy-related morbidity. It is now recognized that recurrent miscarriage occurs in 1% of couples (2–4) and that up to 20% of women with recurrent miscarriage have aPL Ab’s. In the majority of these otherwise normal women, aPL Ab’s are the sole explanation for fetal loss (5, 6). The primary treatment for these patients, anticoagulation throughout pregnancy, is fraught with potential complications, including hemorrhage and osteoporosis. Moreover, treatment can prove to be ineffective. Identification of the mechanisms of pregnancy loss in women with aPL Ab’s would permit development of safer and more effective therapies. Here we identify blockade of the receptor for a proteolytic fragment of complement component 5a (C5a) as a particularly effective treatment in mice and a potentially important target for treatment of patients.
Several murine models have been developed to study mechanisms of fetal loss in APS. In one model, passive transfer of human IgG isolated from aPL Ab–positive sera from women with recurrent fetal loss, as well as murine and human monoclonal aPL Ab’s, induce fetal loss and growth restriction in pregnant mice, demonstrating the direct pathogenic role of aPL Ab’s (7–10). While the specific antigenic reactivity of aPL Ab’s is critical for their effects, the pathogenic mechanisms that mediate aPL Ab–induced vascular thrombosis, tissue injury, and recurrent fetal loss remain incompletely understood (11–14).
We hypothesized that complement activation is a necessary in vivo intermediary step for the clinically relevant deleterious effects of aPL Ab’s on endothelial and inflammatory cells, platelets, and trophoblast cells within the placenta. We investigated this mechanism because it is well established that activated complement fragments themselves have the capacity to bind and activate inflammatory and endothelial cells as well as to induce a prothrombotic phenotype (15, 16). The validity of this hypothesis was demonstrated in our recent studies showing that in the murine model of APS, blockade of C3 activation prevents fetal loss and growth restriction induced by passive aPL Ab transfer (17). Nevertheless, the effectors of tissue injury, the role of individual complement activation pathways, and the precise targets for treatment have remained unknown.
In addition to causing complement activation, aPL Ab’s may induce injury through inflammatory pathways involving activating Fcγ receptors (FcγRs) and neutrophils. These mediators could also link production of pathogenic IgG to development of overt clinical disease. In the current work we examined these three mechanisms to determine their relative importance in aPL Ab–mediated fetal loss. The results of these studies provide a conceptual framework within which rational therapeutic strategies and interventions can be developed.
Methods
Mice.
Adult mice (2–3 months old) were used in all experiments. BALB/c mice were purchased from Taconic Farms (Germantown, New York, USA). FcRγ–/– mice backcrossed to BALB/c mice were provided by Jeffrey Ravetch (Rockefeller University, New York, New York, USA) (18). C4–/– mice were generated by homologous recombination and backcrossed to C57BL/6 for 17 generations (19, 20). C5–/– (B10.D2-H2dH2-T18c Hco/o2Sn) and the C5+/+ background-strain mice (B10.D2-H2dH2-T18c Hco/oSnJ) were obtained from The Jackson Laboratories (Bar Harbor, Maine, USA). C5a receptor–deficient (C5aR-deficient) mice were generated by targeted deletion of the murine C5aR gene and determined to be completely C5aR deficient by PCR, Northern blot, and immunohistochemistry analyses (T.J. Hollman and R.A. Wetsel, data not shown). C5aR-deficient animals were backcrossed with C57BL/6J mice. Heterozygous C5aR+/– backcrossed mice were interbred, and the resulting C5aR+/+ and C5aR–/– littermates were used for studies. Mice deficient in factor B (fB) were generated by targeted deletion (21). The fB–/– mice were generated by intercrossing of fB+/– and then maintained as a homozygous deficient strain. Procedures that involved mice were approved by the local Committee on Animal Use in Research and Education and were conducted in strict accordance with guidelines for the care and use of laboratory research animals promulgated by the NIH.
Preparation of aPL and other Ab’s.
Human IgG containing aPL Ab’s (aPL-IgG) were obtained from three patients with APS characterized by high-titer aPL Ab’s (>140 GPL units), thromboses, and/or pregnancy losses (1). IgG was purified by affinity chromatography using protein G-Sepharose chromatography columns (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). Human IgG from healthy non-autoimmune individuals (NH-IgG) was purified by an identical method. All IgG samples were treated to deplete endotoxin with Centriprep ultracentrifugation devices (Millipore Corp., Bedford, Massachusetts, USA) and determined to be free of endotoxin using the limulus amebocyte lysate assay. F(ab)′2 fragments were obtained by digestion of purified aPL-IgG pooled from patients 2 and 3 using immobilized pepsin (Pierce Chemical Co., Rockford, Illinois, USA). The digested supernatants were passed through protein G-Sepharose to remove remaining intact IgG, and their purity was assessed by Western blot analysis using an Ab specific for the F(ab)′2 fragment (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). The F(ab)′2 fragments were demonstrated to have aPL reactivity similar to the intact aPL-IgG by using ELISA (Sigma-Aldrich, St. Louis, Missouri, USA). The generation, structure, and specificity of the human IgG1 mAb’s aPL (mAb 519), anti-DNA (mAb 412.67), and anti-rabies virus (mAb 57) were previously described (10, 22–24).
Murine pregnancy model.
Females were mated with previously isolated males. The presence of a vaginal plug defined day 0 of pregnancy. On days 8 and 12 of pregnancy, mice were treated with intraperitoneal injections of aPL-IgG (10 mg), aPL-IgG F(ab)′2 (10 mg), NH-IgG (10 mg), and human mAb’s (aPL, anti-DNA, or anti-rabies) (1 mg) (7, 17). To inhibit C5, mice were treated on days 8 and 10 of pregnancy with anti-C5 mAb (1 mg, intraperitoneally) or murine IgG as a control (25). To block C5aR, mice received a C5aR antagonist peptide (AcPhe[L-ornithine-Pro-D-cyclohexylalanine-Trp-Arg]) (50 μg) on day 8, 30 minutes before treatment with aPL-IgG (26, 27). To deplete neutrophils, mice were treated on day 7 with rat anti-mouse granulocyte RB6-8C5 mAb (PharMingen, San Diego, California, USA) (100 μg, intraperitoneally) that reacts with Ly6G (Gr-1 myeloid differentiation antigen); an IgG2b mAb was the isotype control. The level of Ly6G antigen expression in bone marrow correlates with granulocyte maturation, and in peripheral blood, rat anti-mouse granulocyte RB6-8C5 mAb recognizes neutrophils and eosinophils (28–30). Neutrophil depletion was observed 24 hours after administration of anti-mouse granulocyte mAb (anti-Gr) and persisted through day 15. Mice were sacrificed on day 15 of pregnancy, uteri were dissected, fetuses and placentas were weighed, and fetal resorption rates were calculated (number of resorptions per total number of formed fetuses and resorptions). Resorption sites are easily identified and result from loss of a previously viable fetus. Functional C3 activity in serum was measured using the previously described zymosan assay (31).
Immunohistochemistry.
Deciduas were removed from mice on day 8 of pregnancy, 60 minutes after administration of aPL-IgG, frozen in OCT compound, and cut into 10-μm sections. After quenching endogenous peroxidase with 1% H2O2 in methanol and blocking nonspecific binding sites with normal goat serum (Cappel, ICN Pharmaceuticals, Aurora, Ohio, USA), sections were incubated with goat anti-mouse C3, goat anti-human IgG (Cappel, ICN Pharmaceuticals), or goat anti-human F(ab′)2 (Jackson ImmunoResearch Laboratories Inc.), followed by anti-goat IgG conjugated to HRP (Sigma-Aldrich). To detect infiltrating granulocytes, sections were incubated with rat anti-mouse granulocyte RB6-8C5 mAb (PharMingen), followed by rabbit anti-rat IgG conjugated to HRP (Sigma-Aldrich). Bound HRP was detected with 3,3-diaminobenzidine (DAB). Sections were counterstained with hematoxylin. The intensity of staining of C3 within decidual and embryo tissue was scored on a semiquantitative scale (0 to 5+) by three observers who were blinded to the experimental condition. Data are expressed as the mean of 5–8 mice for each experimental condition. Sections of frozen tissue were also stained with H&E.
Western blot analysis.
Deciduas, including embryos, were removed from mice on day 8 of pregnancy, 60 minutes after administration of aPL-IgG, and immediately frozen at –70°C. The tissue was homogenized in RIPA lysis buffer containing 1% Triton X-100, 0.5% deoxycholic acid, 150 mM NaCl, 20 mM β-glycerophosphate, 20 mM Tris-HCl (pH 8.0), 5 mM EGTA, 3 mM MgCl2, 0.1% SDS, 1 mM DTT, 50 μM Na3VO4, and EDTA-free protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany). Lysates (50 μg/sample) were resolved by electrophoresis with a 10% Bis-Tris polyacrylamide gel (Invitrogen Corp., Carlsbad, California, USA) and transferred to a nitrocellulose membrane. The membrane was probed with HRP-conjugated goat anti-mouse C3 (Cappel, ICN Pharmaceuticals) and was visualized using a chemiluminescence detection kit (Amersham International, Amersham, United Kingdom). The proteolytic cleavage product C3-α′ band was identified by comparing serum incubated with or without zymosan.
Statistical analyses.
Data are expressed as mean ± SD. A Student’s t test was used to compare fetal resorption rates and fetal weights between groups. A Mann-Whitney U test was used to compare values for semiquantitative scoring of immunohistochemistry. P values of less than 0.05 were used to reject the null hypothesis.
Results
Activating FcγRs are not required for aPL Ab–induced pregnancy complications.
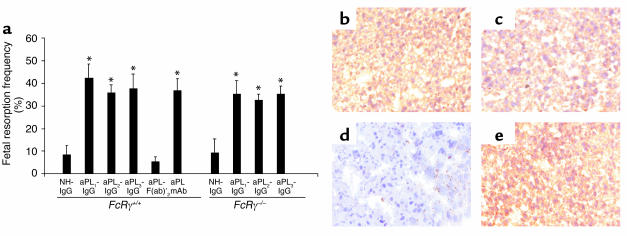
The Fc domain of pathogenic IgG may initiate tissue damage by binding FcγR on effector cells and/or initiating activation of complement. As a first approach to determine the role of FcγR in pregnancy loss induced by aPL Ab’s we compared the consequences of treating pregnant mice with polyclonal IgG isolated from APS patients and F(ab)′2 fragments prepared from the same IgG source. Passive transfer of IgG from three different patients with high-titer aPL Ab’s (aPL-IgG) (>140 GPL units) consistently caused a fourfold increase in the frequency of fetal resorption (Figure 1a). In contrast, treatment with F(ab)′2 fragments of aPL-containing IgG did not affect the frequency of fetal loss (Figure 1a). Fetal loss in mice treated with F(ab)′2 fragments of aPL-IgG was similar to that observed in mice treated with NH-IgG (Figure 1a). In addition, growth restriction induced by treating pregnant mice with intact aPL-IgG was also absent in surviving fetuses of mice treated with aPL-IgG F(ab)′2 [average fetal weight: aPL-IgG, 213 ± 42 mg; aPL-IgG F(ab)′2, 343 ± 43 mg; NH-IgG, 326 ± 32 mg; aPL-IgG F(ab)′2 versus aPL-IgG, P < 0.05]. Importantly, deposition of human F(ab)′2 IgG in decidual tissues was similar in mice treated with aPL-IgG and aPL-IgG F(ab)′2 (Figures 1, b and c), and no human IgG deposition was observed in deciduas from mice treated with NH-IgG (Figure 1d).
Figure 1.
Activating FcγRs are not required for aPL Ab–mediated pregnancy loss. Pregnant FcRγ+/+ and FcRγ–/– mice were treated with IgG from a healthy non-autoimmune individual (NH-IgG), three different patients with APS (aPL-IgG1, aPL-IgG2, aPL-IgG3), F(ab)′2 fragments from a pool of aPL-IgG from patients 2 and 3 [aPL-F(ab)′2], or human monoclonal aPL Ab (aPL mAb) on days 8 and 12 of pregnancy. Mice were sacrificed on day 15 of pregnancy, fetuses were weighed, and frequency of fetal resorption calculated (n = 4–7 mice/group). (a) Treatment with all intact aPL-IgG preparations and aPL mAb caused an increase in fetal resorptions in FcRγ+/+. *P < 0.05 versus NH-IgG, Student’s t test. Administration of aPL-F(ab)′2 did not affect pregnancy outcome. FcRγ–/– mice were not protected from fetal loss induced by intact aPL-IgG. *P < 0.05 versus NH-IgG, Student’s t test). In surviving fetuses from FcRγ –/– mice there was 36% decrease in weight. (b–e) Immunohistochemical analysis of decidual tissue from day 8 of pregnancy. Sections were stained with goat anti-human IgG, the chromogen was DAB (brown), and the counterstain was hematoxylin. Human IgG was deposited in deciduas from FcRγ+/+ mice within 60 minutes of administration of aPL-IgG (b) or aPL-F(ab)′2 (c), whereas no IgG was detected in deciduas from FcRγ+/+ mice treated with NH-IgG (d). Deposition of human IgG was similar after treatment with aPL-IgG in FcRγ–/– (e) and FcRγ+/+ mice (b). Data are representative of observations from three to six decidua from mice in each experimental group. Original magnification was ×200.
Given our finding that the Fc portion of IgG is necessary for aPL Ab–mediated injury, we considered the possibility that aPL Ab’s deposited in the decidua initiate inflammation, thrombosis, and fetal demise by cross-linking stimulatory FcγRs expressed on monocytes, neutrophils, platelets, or mast cells. To examine the role of the FcγR in aPL Ab-induced pregnancy loss, we studied mice with targeted deletion of the common γ subunit (FcRγ–/–) that is required for signaling by activating FcγRs, high-affinity FcγRI, and low-affinity FcγRIII (18). Although FcRγ-deficient mice are reported to have less-severe or undetectable Ab-dependent experimental hemolytic anemia, thrombocytopenia, and glomerulonephritis (32), we found that FcRγ–/– mice were not protected from poor pregnancy outcomes after passive transfer of aPL-IgG (Figure 1a). To exclude the possibility that FcRγ deficiency altered the localization of aPL-IgG, we performed immunohistochemical analyses of deciduas from FcRγ +/+ and FcRγ –/– at day 8 of pregnancy (harvested 60 minutes after treatment with aPL-IgG). Comparable amounts of human IgG were present in FcRγ-sufficient and FcRγ-deficient mice (Figures 1, b and e). Thus, in our murine model of APS, aPL-IgG targeted to the placenta can initiate fetal damage in the absence of activating FcγRs, while F(ab)′2 fragments of aPL-IgG do not mediate such injury.
Blockade of C4 or C5 activation protects mice from aPL Ab–induced pregnancy loss.
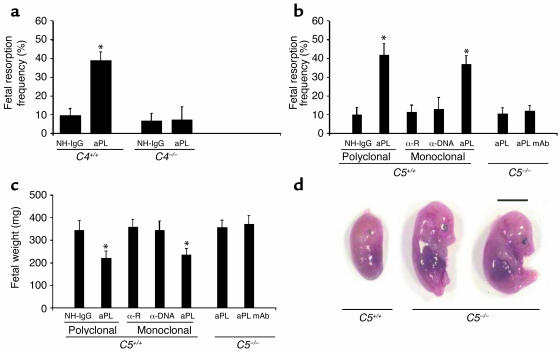
The complement pathway presents a second Fc-dependent means of effecting Ab-mediated injury, and our initial studies showed that blocking C3 prevents fetal loss in murine APS (17). To assess the importance of the classical pathway of complement activation, we treated pregnant C4-deficient mice with aPL-IgG. C4–/– mice were protected from fetal loss (Figure 2a) and growth restriction (average fetal weight in aPL-IgG–treated mice: C4+/+ versus C4–/– 248 ± 19 mg versus 413 ± 30 mg; P < 0.001, n = 5 mice/group), suggesting that aPL Ab’s trigger the complement cascade through either the classical or lectin pathways. That the classical pathway is required as an initiator of complement activation by aPL-IgG is supported by our finding that F(ab)′2 fragments of aPL-IgG, which lack the Fc portion necessary to activate the classical pathway, do not cause pregnancy loss (Figure 1a).
Figure 2.
C4 or C5 deficiency prevents aPL Ab–induced fetal loss and growth restriction. (a) Pregnant C4+/+ and C4–/– mice were treated with aPL-IgG (aPL) (10 mg, intraperitoneally) or NH-IgG on days 8 and 12 of pregnancy, and fetal resorption frequencies were determined on day 15 (n = 5 mice/group). *P < 0.001, aPL versus control. (b–d) Pregnant C5+/+ and C5–/– mice were treated intraperitoneally with aPL-IgG (10 mg), monoclonal human aPL Ab (1 mg), monoclonal human anti-DNA (α-DNA; 1 mg), or their respective controls (NH-IgG or monoclonal human anti-rabies Ab, α-R) on days 8 and 12 of pregnancy. Fetal resorption frequencies and fetal weights were determined on day 15 of pregnancy (n = 5–11 mice/group). (b and c) C5–/– mice were protected from fetal loss (b) and growth restriction (c), whereas in the C5+/+ mice background strain aPL-IgG or aPL mAb caused pregnancy complications. *P < 0.01, aPL versus control. (d) Day 15 fetuses from C5–/– and C5+/+ mice treated with aPL-IgG. Scale bar: 1 cm.
Following initiation of the complement cascade, any of several complement activation fragment-derived ligand-receptor interactions could mediate fetal injury such as those that we have observed. To define which elements of the complement cascade mediate pregnancy loss, we initially focused on complement component 5. C5 is a pivotal member of the complement system because all three initiating pathways converge to activate C5 and two effector pathways lead from it. To determine whether activation of C5 is required for aPL Ab–induced fetal loss, we treated pregnant C5-deficient and C5-sufficient mice with aPL-IgG, control IgG, human aPL mAb, human anti-DNA mAb, or control human IgG1 mAb. In C5+/+ mice, both APS patient-derived polyclonal aPL-IgG and human aPL mAb caused a fourfold increase in the frequency of fetal resorption and a significant decrease in embryo weight as compared with control IgG (Figure 2, b–d). Treatment with anti-DNA Ab, an autoantibody often present in patients with APS, had no effect on pregnancy outcome. That the results from experiments with human aPL mAb were similar to results obtained with polyclonal aPL Ab’s also indicates that Ab’s reactive with aPL, rather than xenoreactive Ab’s that may be present in polyclonal human IgG, are sufficient to initiate complement activation and fetal damage in this model.
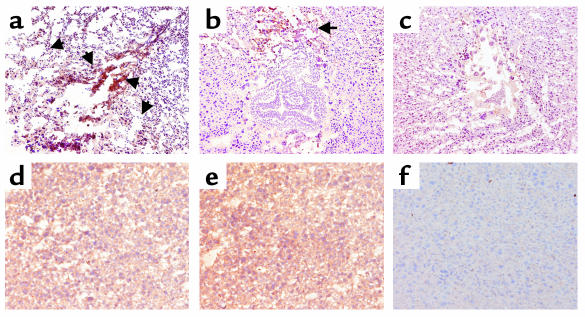
In contrast to C5+/+ mice, mice lacking C5 were protected from aPL Ab-induced pregnancy complications (Figure 2, b–d). Immunohistochemical analyses of deciduas from day 8 of pregnancy obtained 60 minutes after treatment with aPL-IgG, showed extensive deposition of human IgG and C3 and focal necrosis and neutrophil infiltration in C5+/+ mice (Figure 3, a and d). In C5–/– mice there were no inflammatory infiltrates, and deciduas and embryos had normal morphology despite the presence of human IgG within decidual tissue (Figure 3, b and e). Importantly, in C5–/– mice there was less C3 deposition, as shown by comparing the representative sections shown in Figure 3, a and b, and by grading the intensity of C3 staining in embryos and decidua from aPL-IgG–treated mice on a semiquantitative scale of 0–5+ (C5–/– versus C5+/+: 2.5 ± 0.5 versus 4.3 ± 0.6; P < 0.001, n = 5 mice/group). Taken together, these results demonstrate that C5 activation is a critical proximal effector for the induction of fetal loss by aPL Ab’s and implicate C5 activation and its downstream effects in amplifying local C3 deposition.
Figure 3.
C5 deficiency limits inflammation, necrosis, and activation of C3 by aPL Ab’s. Pregnant C5+/+ and C5–/– mice were treated with aPL-IgG (a, b, d, and e) or NH-IgG (c and f) as described in the legend to Figure 2, and immunohistochemical analysis was performed on decidual tissue from day 8 of pregnancy. (a–c) Detection of C3 in day-8 deciduas from aPL-IgG– and NH-IgG–treated mice. The deciduas were stained with anti-mouse C3, the chromogen was DAB (brown), and the counterstain was hematoxylin. Decidua from C5+/+ mice (a) had extensive C3 deposition (arrows), inflammatory cell infiltrates, and necrotic fetal debris, whereas embryos from C5–/– mice (b) treated with aPL-IgG appeared normal, and there was limited C3 deposition in deciduas at the maternal-fetal interface compared with that of C5+/+ treated with NH-IgG (c). Original magnification was ×50. (d–f) Detection of human IgG in deciduas. Sections were stained with goat anti-human IgG, the chromogen was DAB (brown), and the counterstain was hematoxylin. Within 60 minutes of administering aPL-IgG, human IgG was detectable in deciduas from C5+/+ mice (d) and C5–/– mice (e), whereas no IgG was detected in deciduas from C5+/+ mice treated with NH-IgG (f). Data are representative of observations from three to six mice in each experimental group. Original magnification was ×200.
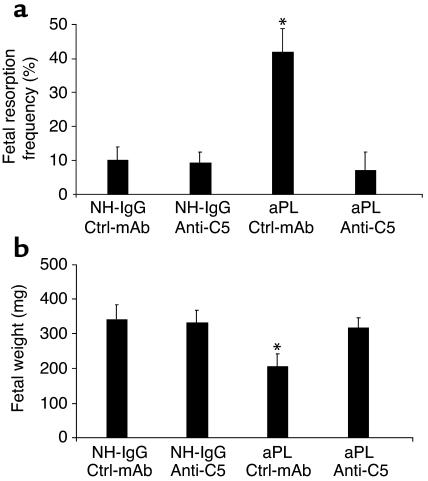
As an alternative strategy to confirm that C5 activation is required for fetal loss, we investigated the outcome of blocking C5 activation with anti-C5 mAb (25). These experiments may prove particularly relevant because a similar anti-human C5 mAb is in phase II studies in patients with rheumatoid arthritis and phase I studies in patients with active lupus nephritis (33, 34). We administered anti-C5 mAb before treatment with either NH-IgG or aPL-IgG. The ensuing blockade of C5 cleavage (35) prevented aPL Ab–induced pregnancy loss and growth restriction (Figure 4, a and b). Indeed, pregnant mice treated with anti-C5 mAb were protected to an extent similar to C5-deficient mice (Figure 2, b and c, and Figure 4).
Figure 4.
Inhibition of C5 activation with anti-C5 mAb prevents aPL Ab–induced pregnancy complications. (a and b) Pregnant BALB/c mice were treated with aPL-IgG (10 mg, intraperitoneally) or NH-IgG (10 mg, intraperitoneally) at days 8 and 12 of pregnancy. Mice also received either anti-C5 mAb (1 mg, intraperitoneally) or control murine IgG (Ctrl-mAb; 1 mg, intraperitoneally) on days 8 and 10 (n = 5–11 mice/group). Pregnancies were assessed as described in the legend for Figure 1. Administration of anti-C5 mAb prevented fetal resorption (a) and growth restriction (b). *P < 0.05 versus NH-IgG plus Ctrl-mAb.
C5a-C5aR interactions are critical mediators of aPL Ab–induced pregnancy complications.
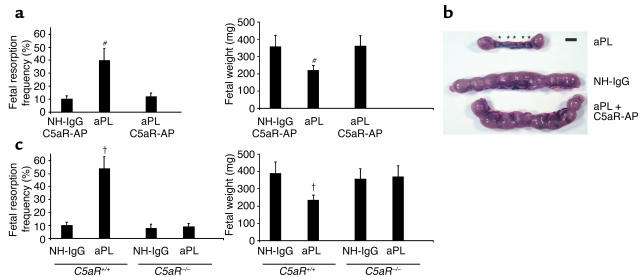
Two complement effector pathways are initiated by cleavage of C5: C5a, a potent anaphylatoxin and cell activator, and C5b, which leads to formation of the C5b-9 membrane attack complex (MAC). We used two methods to distinguish the role of C5a and the C5aR from that of MAC seeded by C5b. First, we treated pregnant mice that had received aPL-IgG with a highly specific peptide antagonist of C5aR, AcPhe[L-ornithine-Pro-D-cyclohexylalanine-Trp-Arg], which possesses potent in vivo anti-inflammatory activities in murine models of endotoxic shock, renal ischemia-reperfusion injury, and the Arthus reaction (26, 27, 36, 37). Administration of C5aR antagonist peptide prevented aPL-Ab–induced pregnancy loss and growth restriction, but had no effect on either frequency of fetal resorption or fetal size in the absence of aPL Ab’s (Figure 5, a and b). Fetal protection conferred by the C5aR antagonist was comparable to that seen with anti-C5 mAb and in mice lacking C5 (Figures 2 and 4), suggesting that downstream pathogenic effects are mediated predominantly by C5a-C5aR interactions. Immunohistological analysis of decidual tissue from mice treated with aPL-IgG and C5aR antagonist peptide yielded results similar to those in C5–/– mice. There was minimal C3 deposition surrounding normal-appearing fetuses and no evidence of inflammation.
Figure 5.
Blockade of C5a-C5aR interaction protects pregnancies from aPL Ab–associated injury. (a and b) Pregnant BALB/c mice were given aPL-IgG (10 mg, intraperitoneally) or NH-IgG (10 mg, intraperitoneally) on days 8 and 12, and some also received C5aR antagonist peptide (C5aR-AP) (50 μg, intraperitoneally) on day 8, 30 minutes before administration of aPL-IgG (n = 5–11 mice/group). Pregnancy outcomes were assessed as described in the legend for Figure 1. Treatment with C5aR-AP prevented fetal loss and growth inhibition. #P < 0.01, aPL versus aPL plus C5aR-AP. (b) Uteri from day 15 of pregnancy. There are two small amnion sacs and five resorptions (*) in the uterus from an aPL-IgG–treated mouse, while the uterus of a mouse that received aPL-IgG along with C5aR-AP contained normal size amnionic sacs and no resorptions, similar to that from a mouse treated with NH-IgG. Data are representative of observations in five to seven mice in each experimental group. Scale bar: 1 cm. (c) The effects of effects of aPL-IgG on pregnancy outcomes in C5aR–/– and C5aR+/+ mice were compared (n = 5–11 mice/group). Pregnant mice were treated with aPL-IgG or NH-IgG as described above. C5aR–/– mice were protected from aPL-IgG–induced fetal resorption and growth inhibition. †P < 0.05, aPL-IgG versus NH-IgG.
As a second approach to test the hypothesis that C5a-C5aR interactions mediate aPL-induced pregnancy complications, we performed studies in mice deficient in C5aR. In the background strain, C5aR+/+, there was a fivefold increase in the frequency of fetal resorption after treatment with aPL-IgG (Figure 5c), whereas, as predicted by experiments with the C5aR antagonist peptide, aPL-IgG did not increase the frequency of fetal resorption in C5aR–/– mice (Figure 5c). The protective effects of the total absence of C5aRs were also observed when fetal weights were examined (Figure 5c). Taken together, our experiments with C5aR–/– mice and C5aR antagonist peptide identify the C5a-C5aR interaction as a critical effector of aPL Ab–induced injury.
Depletion of neutrophils protects against aPL-induced pregnancy complications.
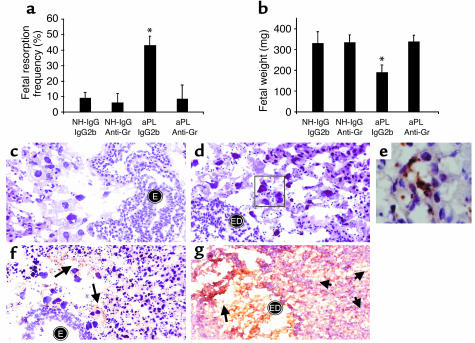
C5a is a potent chemotactic factor and activator of neutrophils. Since we observed neutrophil infiltration at sites of fetal resorption and demonstrated that the C5a-C5aR interaction is necessary for aPL Ab–induced pregnancy loss, we hypothesized that neutrophils were the critical cellular effectors of fetal damage. Indeed, neutrophils have been implicated as effectors in pathogenic Ab-induced arthritis and in Ab-independent murine models of pregnancy loss (38, 39). To examine the relative importance of these cells in aPL Ab–initiated damage, we depleted neutrophils on day 7 of pregnancy by treating mice with rat anti-Gr RB6-8C5; IgG2b Ab served as the isotype control. In the absence of neutrophils, treatment with aPL-IgG did not cause pregnancy loss or growth restriction, nor were there inflammatory infiltrates within the deciduas (Figure 6, a–e). Furthermore, without neutrophil infiltration (Figure 6, c–e) there was less C3 deposition, as shown in Figure 6f compared with Figure 6g and by grading intensity of C3 staining in embryos and decidua from aPL-IgG–treated mice (anti-Gr versus IgG2b: 2.4 ± 0.7 versus 4.3 ± 0.6; P < 0.001, n = 5 mice/group). These findings are similar to the limited C3 deposition we observed in C5–/– mice and in C5aR blockade.
Figure 6.
Neutrophil depletion protects mice from aPL Ab–induced pregnancy complications and limits C3 deposition. BALB/c mice received anti-mouse granulocyte RB6-8C5 mAb (anti-Gr) (100 μg, intraperitoneally) or IgG2b isotype control mAb on day 7 of pregnancy. On days 8 and 12, mice were treated with aPL-IgG or NH-IgG (n = 5–11 mice/group). (a and b) Neutrophil depletion protected mice from (a) fetal resorption (*P < 0.01, aPL-IgG plus anti-Gr versus aPL-IgG plus IgG2b) and (b) growth restriction (*P < 0.01, aPL-IgG plus anti-Gr versus aPL-IgG plus IgG2b). (c and d) Histologic sections of deciduas from day 8 of pregnancy were stained with H&E. In deciduas from mice treated with anti-Gr plus aPL-IgG (c) there were intact embryos (E) and no inflammatory infiltrates, while in deciduas from mice treated with aPL-IgG plus IgG2b (d) there was extensive neutrophilic infiltration (stained with anti-Gr shown in e) surrounding embryonic debris (ED). Original magnification was ×400. (e) Immunohistochemistry to detect infiltrating granulocytes in decidua from an aPL-IgG plus IgG2b–treated mouse. Original magnification was ×1,000. (f and g) Immunohistochemistry for C3 deposition in decidual tissue. Staining for C3 (arrows) was less intense and limited to the fetal-maternal interface in deciduas from mice that had received anti-Gr before treatment with aPL-IgG (f), compared with that of mice treated with aPL-IgG plus IgG2b (g). In the presence of infiltrating neutrophils, C3 deposits were present throughout decidual tissue (g), with particularly intense staining at the fetal-maternal interface surrounding the necrotic residual embryonic debris (arrows). Original magnification was 0400.
To exclude the possibility that neutrophil depletion due to treatment with anti-Gr mAb caused complement consumption, we measured circulating functional C3 levels using a zymosan activation assay before and after treatment with anti-Gr mAb (n = 4 mice) (31). Functional C3 measured at nine time points from 6 to 32 hours after anti-Gr treatment ranged from 95% to 107% of pretreatment levels. At no point was there evidence for a significant decrease in C3, indicating protection against aPL Ab-induced pregnancy loss afforded by anti-Gr mAb treatment is not due to complement consumption by IgG-opsonized neutrophils. Rather, our results are consistent with the conclusion that neutrophils contribute directly to fetal injury. Thus, while among its many effects C5a can activate platelets, endothelial cells, and mononuclear phagocytes, it appears that C5a-mediated recruitment (and likely activation) of neutrophils in the placenta is critical for the development of pregnancy loss and fetal damage.
Alternative pathway of complement activation contributes to aPL Ab–induced fetal loss.
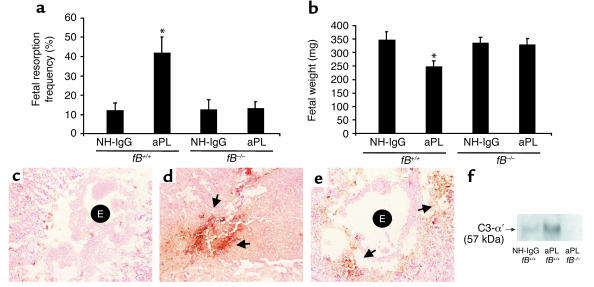
In the absence of neutrophil infiltration in decidual tissue, whether as a consequence of blockade of C5a-C5aR interactions or neutrophil depletion, we observed limited activation of C3 and improved pregnancy outcomes (Figure 3, b and e, and Figure 6, c and f). It has been suggested that neutrophils promote complement deposition by causing tissue damage that triggers complement activation and by secreting C3 and/or properdin at sites of inflammation to amplify complement activation via the alternative pathway (40, 41). Given the importance of neutrophils in our model of APS and their potential role as activators of the alternative pathway, we examined the contribution of this pathway of complement activation in aPL Ab–induced pregnancy loss by performing studies in mice deficient in fB. We found that fB–/– mice were protected from fetal resorption and growth restriction caused by aPL Ab’s. The frequency of pregnancy loss in fB–/– mice treated with aPL-IgG was comparable to that observed in mice treated with control IgG (Figure 7a). In contrast, fetal wastage and growth restriction was evident in fB+/+ (background strain) mice treated with aPL-IgG (Figure 7, a and b). Immunohistochemical analyses showed substantially less C3 deposition in decidual tissues and embryos from fB–/– mice treated with aPL Ab’s than in fB+/+ mice, as shown by comparing the representative sections in Figure 7, d and e, and by grading intensity of C3 staining in embryos and deciduas (fB–/– versus fB+/+: 2.4 ± 0.5 versus 4.0 ± 0.6; P < 0.001, n = 5–7 mice/group). We confirmed the immunohistochemistry results with analysis of lysates from decidual cells by Western blot analysis probed with anti-mouse C3 (Figure 7f). Taken together, these findings indicate that the alternative pathway is an amplifier of complement activation triggered by aPL Ab’s targeted to the deciduas.
Figure 7.
The absence of fB protects mice from aPL Ab–induced fetal loss and extensive C3 deposition within deciduas. fB+/+ and fB–/– mice were treated with aPL-IgG (10 mg, intraperitoneally) or NH-IgG (10 mg, intraperitoneally) on days 8 and 12 of pregnancy. Fetal resorption frequencies and fetal weights were determined on day 15 of pregnancy (n = 4–8 mice/group). (a and b) In contrast to fB+/+ mice, those deficient in fB were protected from fetal resorption (*P < 0.05, fB+/+ aPL-IgG versus NH-IgG) and growth restriction (P < 0.001, fB+/+ aPL-IgG versus NH-IgG). (c–e) Immunohistochemistry for C3 deposition in decidual tissue from day 8 of pregnancy following aPL-IgG administration. In deciduas from fB+/+ mice treated with NH-IgG (c), there was minimal C3 deposition and an intact embryo (E). In fB+/+ mice treated with aPL-IgG (d), C3 deposits were present throughout decidual tissue surrounding the necrotic residual embryonic debris (arrows). In contrast, in fB–/– mice treated with aPL-IgG (e), C3 deposition was limited (arrows) and the embryos remained intact (E). (f) Detection of C3 by Western blotting. Lysates from deciduas of fB+/+ mice and fB–/– mice were resolved by electrophoresis and blotted with anti-murine C3 Ab. C3 deposition was greater in deciduas from aPL-IgG–treated fB+/+ mice than in fB–/– mice, as evidenced by the presence of the cleaved C3-α′ chain.
In summary, our results show that factor B, C3, C5, and C5aR are required for pregnancy complications triggered by aPL Ab’s and that neutrophils are critical effector cells in our model of APS. That aPL-IgG can initiate fetal damage in the absence of activating FcγRs, but not in the absence of C4, and that F(ab)′2 fragments of aPL-IgG do not mediate such injury, suggest that initiation of the complement cascade occurs via the classical pathway. Our observation that factor B is required for fetal death and that its presence is associated with increased C3 deposition shows, however, that the alternative pathway amplifies local complement activation and also plays a critical role in the induction of fetal loss.
Discussion
We have shown in a murine model of APS induced by passive transfer of human aPL Ab’s that complement activation plays an essential and causative role in fetal loss and tissue injury and, in contrast to other models of Ab-mediated disease, that activating FcγRs are not required for aPL Ab–induced effects. Specifically, we have identified the proinflammatory sequelae of C5a-C5aR interactions and the recruitment of neutrophils as the critical intermediates linking pathogenic aPL Ab’s to fetal damage. Our conclusions are based on the fetal protective effects of C5aR deficiency and C5aR antagonist peptide, the similar findings with anti-C5 mAb and in C5–/– mice, where C5a generation is prevented, and on the effects of neutrophil depletion.
Our observations that C4–/– mice are protected from aPL Ab–induced pregnancy loss and that F(ab)′2 fragments of aPL-IgG do not cause fetal injury indicate that the classical pathway is the initiator of complement activation and is required for tissue damage. Generation of C5a, through activation of the classical complement pathway, amplifies the effects of aPL Ab’s targeted to the placenta. C5a attracts and activates neutrophils, monocytes, and mast cells, and stimulates the release of inflammatory mediators, including reactive oxidants, proteolytic enzymes, chemokines, and cytokines, as well as complement components. Proteases secreted by inflammatory cells, particularly neutrophils, can also increase C5a generation by directly cleaving C5 (42), leading to autocrine and paracrine stimulation and further recruitment of leukocytes.
Depletion of neutrophils prevents aPL Ab–mediated fetal injury, indicating that these cells are essential effectors of tissue damage. Although neutrophil recruitment and activation are most likely a direct C5a effect, we cannot exclude the possibility that they occur secondarily to C5a-C5aR–mediated activation of mast cells (43). Mast cells play a critical role in murine arthritis and bullous pemphigoid induced by passive transfer of Ab’s (43, 44). In these Ab-mediated diseases, as in our model of aPL Ab–mediated fetal loss, C5 and neutrophils are required for tissue damage (38, 43, 45, 46).
In addition to myeloid cells, C5aR is expressed on endothelial cells, where it triggers production of macrophage inflammatory protein-2, a potent neutrophil chemotactic factor, and monocyte chemoattractant protein-1, which recruits monocytes and lymphocytes (47, 48). Release of chemokines in the presence of C5a increases transmigration of neutrophils and amplifies inflammatory cell–mediated tissue damage. C5aR has also been identified on lung, kidney, and liver parenchymal cells, where it contributes to inflammatory damage (27, 49). Whether C5aR is expressed by trophoblast cells or natural killer cells (abundant in deciduas; ref.50) and whether it alters their function to impair pregnancy is unknown.
C5a-C5aR interactions can also trigger thrombosis, the classic pathologic feature of APS. C5aR induces tissue factor expression on monocytes and production of plasminogen activator inhibitor-1 by mast cells (51). In a rat model of Ab-mediated thrombotic glomerulonephritis, C5aR blockade prevents thrombus formation and leukocyte accumulation, and, similar to our findings, depletion of neutrophils prevents glomerular thrombosis, despite the presence of C3 and MAC (52). These studies underscore the link between complement activation and thrombophilia in inflammatory diseases.
One of our most striking findings is that C3 deposition in decidual tissue of aPL Ab-treated mice is diminished in the absence of C5 activation and C5a release. We observed this phenomenon in mice treated with anti-C5 mAb, mice lacking C5 or C5aR, and mice treated with C5aR antagonist peptide. While decreased C3 deposition as a consequence of C5 activation blockade may appear counterintuitive because C3 activation precedes C5 activation, we believe this finding is explained by the coincident inhibition of neutrophil infiltration. In each setting where C5 activation was blocked, neutrophils were absent from decidual tissues, and in neutrophil-depleted mice, C3 deposition was substantially decreased. Thus, C3 activation and deposition do not appear to be solely dependent on complement components because these are ample in the plasma and extracellular fluid; rather, in the absence of neutrophils, there is limited amplification of the cascade and cleavage of C3.
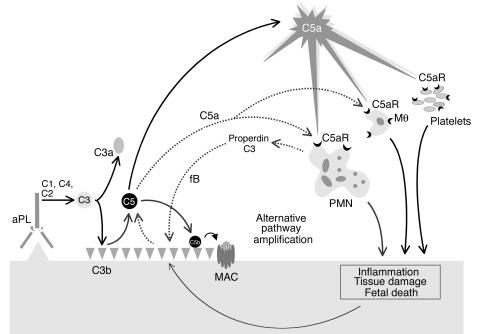
Because apoptotic and necrotic cells activate alternative and classical pathways, neutrophil-induced cell damage may in and of itself increase C3 deposition in decidual tissues (53). In addition, neutrophils can enhance complement activation by releasing complement components, including C3 and properdin, a critical positive regulator of the alternative pathway. Properdin functions by stabilizing the interaction of fB with spontaneously generated initial C3(H2O) and the formation of the C3 convertase C3bBb (40, 41). Such positive regulatory activity permits properdin to significantly enhance alternative pathway C3 activation resulting either from initiation of this pathway directly by C3(H2O) formation or indirectly through the amplification loop, which uses C3b generated from the classical pathway C3 convertase C4b2a. Thus, properdin and C3 secretion by neutrophils may accelerate alternative pathway activation at sites of leukocyte infiltration, enhancing C3 activation and deposition (40). Our results suggest that initial C3 deposition catalyzed by classical pathway activation leads to C5a generation, attracting neutrophils and potentially triggering properdin release (Figure 8). Furthermore, the experiments in the fB–/– mice support the possibility that properdin and the alternative pathway generate most C3 at sites of injury and initiate a positive feedback loop that generates additional C5a (Figure 8).
Figure 8.
Mechanism of aPL Ab–induced fetal damage. APL Ab’s are preferentially targeted to the placenta where they activate complement via the classical pathway leading to the generation of potent anaphylatoxins and mediators of effector cell activation, particularly C5a. C5a attracts and activates neutrophils, monocytes, and platelets and stimulates the release of inflammatory mediators, including reactive oxidants, proteolytic enzymes, chemokines, cytokines, and complement factors C3 and properdin. Secretion of C3 and properdin by neutrophils, as well as the presence of apoptotic and necrotic decidual tissue, may accelerate alternative pathway activation (dashed line), creating a proinflammatory amplification loop at sites of leukocyte infiltration that enhances C3 activation and deposition and generates additional C5a. This results in further influx of neutrophils, inflammation within the placenta, and, ultimately, fetal injury. Depending on the extent of damage, either death in utero or fetal growth restriction ensues.PMN, neutrophil; Mθ, monocyte/macrophage.
The linkage of alternative pathway activation with neutrophil infiltration may also account for the resistance of mice deficient in fB, C3, C5/C5aR, or neutrophils to joint damage after treatment with arthritogenic Ab’s, a phenotype that parallels our model (38, 45, 46, 54, 55). There are, however, fundamental differences in the mechanisms of tissue damage in these two experimental models of Ab-mediated injury. Arthritogenic Ab’s act through both FcγR and C5a, the latter generated exclusively through the alternative complement pathway with classical pathway components entirely dispensable (38, 46). In contrast, our studies clearly show that fetal injury caused by aPL Ab’s requires the classical complement pathway as an initiator and is independent of FcγR. Nonetheless, a common and unexpected finding emerged in both experimental models — the importance of the alternative pathway for injury. Our findings are novel in that they link alternative pathway activation to neutrophil infiltration and raise the possibility that infiltrating cells regulate local complement activation.
That blockade of C5 or C5aR is effective in preventing fetal injury in APS has important therapeutic implications. Blocking the complement cascade at C5 inhibits mediators and effectors of tissue injury while preserving the complement-derived immunoprotective functions of C3. Complement inhibitors are now being tested in patients with inflammatory, ischemic, and autoimmune diseases. Identifying complement-related markers that predict high risk for fetal loss will allow us to translate insights about the mechanisms of complement-mediated disease to interventions that may prevent, arrest, or modify the deleterious effects of aPL Ab’s.
Acknowledgments
This research was supported by the Alliance of Clinical Research (J.E. Salmon and V.M. Holers), Mary Kirkland Center for Lupus Research (J.E. Salmon), S.L.E. Foundation Inc. (J.E. Salmon), National Kidney Foundation (J.M. Thurman), and NIH grants AI-31105 (to V.M. Holers), AI-25011 (to R.A. Wetsel), and GM-62134 (to J.D. Lambris).
Footnotes
See the related Commentary beginning on page 1639.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: antiphospholipid syndrome (APS); antiphospholipid (aPL); complement component 5a (C5a); Fcγ receptor (FcγR); C5a receptor (C5aR); factor B (fB); human IgG containing aPL Ab’s (aPL-IgG); human IgG from healthy individuals (NH-IgG); anti-mouse granulocyte mAb (anti-Gr); 3,3-diaminobenzidine (DAB); membrane attack complex (MAC).
References
- 1.Wilson WA, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome. Arthritis Rheum. 1999; 42:1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am. J. Obstet. Gynecol. 1996; 174:1584–1589. doi: 10.1016/s0002-9378(96)70610-5. [DOI] [PubMed] [Google Scholar]
- 3.Rai R, Cohen H, Dave M, Regan L. Randomized controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies. Br. Med. J. 1997; 314:253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum. Reprod. 1994; 9:1328–1332. doi: 10.1093/oxfordjournals.humrep.a138703. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil. Steril. 1994; 66:24–29. [PubMed] [Google Scholar]
- 6.Yetman DL, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertil. Steril. 1996; 66:540–546. doi: 10.1016/s0015-0282(16)58565-3. [DOI] [PubMed] [Google Scholar]
- 7.Branch DW, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am. J. Obstet. Gynecol. 1990; 163:210–216. doi: 10.1016/s0002-9378(11)90700-5. [DOI] [PubMed] [Google Scholar]
- 8.Piona A, et al. Placental thrombosis and fetal loss after passive transfer of mouse lupus monoclonal or human polyclonal anti-cardiolipin antibodies in pregnant naive BALB/c mice. Scand. J. Immunol. 1995; 41:427–432. doi: 10.1111/j.1365-3083.1995.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 9.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of antiphospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal antibodies. Proc. Natl. Acad. Sci. U. S. A. 1991; 88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikematsu W, et al. Human anticardiolipin monoclonal autoantibodies cause placental necrosis and fetal loss in BALB/c mice. Arthritis Rheum. 1998; 41:1026–1039. doi: 10.1002/1529-0131(199806)41:6<1026::AID-ART9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Simantov R, et al. Activation of cultured vascular endothelium by antiphospholipid antibodies. J. Clin. Invest. 1995; 96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.di Simone N, et al. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered beta2-glycoprotein I. Arthritis Rheum. 2000; 43:140–150. doi: 10.1002/1529-0131(200001)43:1<140::AID-ANR18>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierangeli SS, et al. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999; 99:1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 14.Rand JH, et al. Pregnancy loss in the antiphospholipid-antibody syndrome — a possible thrombogenic mechanism. N. Engl. J. Med. 1997; 337:154–160. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 15.Wetsel RA. Structure, function, and cellular expression of complement anaphylatoxin receptors. Curr. Opin. Immunol. 1995; 7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 16.Shin ML, Rus HG, Nicolescu FI. Membrane attack by complement: assembly and biology of terminal complement complexes. Biomembranes. 1996; 4:123–149. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holers VM, et al. Complement C3 activation is required for anti-phospholipid antibody-induced fetal loss. J. Exp. Med. 2002; 195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 1994; 76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 19.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. U. S. A. 1995; 92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer MB, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 1996; 157:549–556. [PubMed] [Google Scholar]
- 21.Matsumoto M, et al. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. U. S. A. 1997; 94:8720–8724. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Schettino EW, Padlan E, Ikematsu H, Casali P. Structure-function analysis of a lupus anti-DNA autoantibody: central role of the heavy chain CDR3 Arg in dsDNA and ssDNA binding. Eur. J. Immunol. 2000; 30:2015–2026. doi: 10.1002/1521-4141(200007)30:7<2015::AID-IMMU2015>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikematsu H, Harindranath N, Ueki Y, Notkins AL, Casali P. Clonal analysis of a human antibody response. II. Sequences of the VH genes of human monoclonal IgM, IgG and IgA to rabies virus reveal preferential utilization of the VH3 segments and somatic hypermutation. J. Immunol. 1993; 150:1325–1337. [PMC free article] [PubMed] [Google Scholar]
- 24.Ikematsu W, Kobarg J, Ikematsu H, Ichiyoshi Y, Casali P. Clonal analysis of a human antibody response. III. Nucleotide sequences of human monoclonal IgM, IgG and IgA to rabies virus reveal Vκ gene utilization, junctional Vκ-Jκ and Vλ-Jλ heterogeneity, and somatic hypermutation. J. Immunol. 1998; 161:2895–2905. [PubMed] [Google Scholar]
- 25.Frei Y, Lambris JD, Stockinger B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol. Cell Probes. 1987; 1:141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 26.Finch AM, et al. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 1999; 42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 27.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001; 166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 28.Hestdal K, et al. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 1991; 147:22–28. [PubMed] [Google Scholar]
- 29.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods. 1996; 197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 30.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 1994; 179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley S, Li B, Dehoff M, Molina H, Holers VM. Mouse Crry/p65 is a regulator of the alternative pathway of complement activation. Eur. J. Immunol. 1993; 23:1381–1384. doi: 10.1002/eji.1830230630. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 1998; 16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 33.Quigg RJ. Use of complement inhibitors in tissue injury. Trends Mol. Med. 2002; 8:430–436. doi: 10.1016/s1471-4914(02)02386-9. [DOI] [PubMed] [Google Scholar]
- 34.Tesser J, et al. Safety and efficacy of the humanized anti-C5a antibody h5G1.1 in patients with rheumatoid arthritis. Arthritis Rheum. 2001; 44:S274. (Abstr.) [Google Scholar]
- 35.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. U. S. A. 1995; 92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strachan AJ, Woodruff TM, Haaima G, Fairlie DP, Taylor SM. A new small molecule C5a receptor antagonist inhibits the reverse-passive Arthus reaction and endotoxic shock in rats. J. Immunol. 2000; 164:6560–6565. doi: 10.4049/jimmunol.164.12.6560. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam TV, et al. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003; 63:134–142. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- 38.Grant EP, et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J. Exp. Med. 2002; 196:1461–1471. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA × DBA/2 mice is mediated by the procoagulant fgl2 [erratum 1999, 162:3105] J. Immunol. 1998; 160:545–549. [PubMed] [Google Scholar]
- 40.Schwaeble WJ, Reid KB. Does properdin crosslink the cellular and the humoral immune response? Immunol. Today. 1999; 20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 41.Wirthmueller U, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J. Immunol. 1997; 158:4444–4451. [PubMed] [Google Scholar]
- 42.Huber-Lang M, et al. Generation of C5a by phagocytic cells. Am. J. Pathol. 2002; 161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J. Clin. Invest. 2001; 108:1151–1158. doi:10.1172/JCI200111494. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee DM, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002; 297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 45.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001; 167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 46.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002; 16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 47.Czermak BJ, et al. In vitro and in vivo dependency of chemokine generation on C5a and TNF-alpha. J. Immunol. 1999; 162:2321–2325. [PubMed] [Google Scholar]
- 48.Laudes IJ, et al. Expression and function of C5a receptor in mouse microvascular endothelial cells. J. Immunol. 2002; 169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- 49.Haviland DL, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J. Immunol. 1995; 154:1861–1869. [PubMed] [Google Scholar]
- 50.Moffett-King A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002; 2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 51.Wojta J, et al. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood. 2002; 100:517–523. doi: 10.1182/blood.v100.2.517. [DOI] [PubMed] [Google Scholar]
- 52.Kondo C, et al. The role of C5a in the development of thrombotic glomerulonephritis in rats. Clin. Exp. Immunol. 2001; 124:323–329. doi: 10.1046/j.1365-2249.2001.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998; 188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 2002; 169:454–459. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, et al. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J. Immunol. 2000; 164:4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]