We refer to the publication by Lamb et al (2009). This report describes the Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA), encoded in the pathogenicity island (PAI), as essential for the rapid activation of the transcription factor nuclear factor κB (NF-κB) in epithelial cells. The authors reported fast NF-κB activation in AGS (human gastric carcinoma) cells infected with H. pylori G27 wild type, but not with the isogenic CagA-deficient strain, determined by IκBα phosphorylation and degradation, RelA phosphorylation, NF-κB DNA-binding, and interleukin 8 (IL-8) mRNA expression.
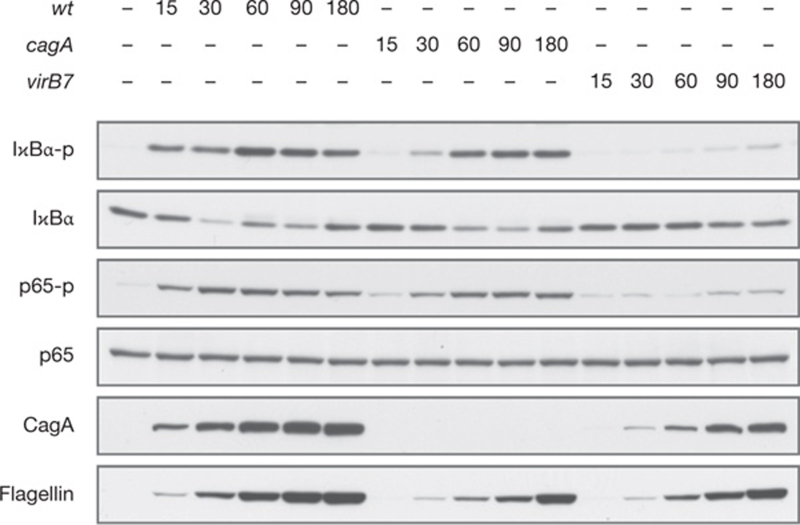
However, the profound CagA dependency of NF-κB activation, representing the centrepiece of the study by Lamb et al (2009), is in clear contradiction to previously published data including data from our laboratory. Early documentation in the literature indicates type IV secretion system/PAI-dependent—but CagA-independent—rapid activation of NF-κB by H. pylori in epithelial cells, determined by NF-κB DNA-binding, NF-κB transactivation activity or IL-8 secretion (for example: Sharma et al 1995; Censini et al 1996; Fischer et al 2001; Neu et al 2002; Foryst-Ludwig et al 2004). For more than 10 years, our lab has routinely monitored NF-κB activation in H. pylori-infected cells, and we find that IκBα phosphorylation and degradation, RelA phosphorylation (Fig 1), DNA-binding and induction of IL-8 are reproducibly induced by H. pylori wild-type and isogenic CagA-deficient strains to an almost equal extent. A slight delay in the induction of NF-κB by the CagA-deficient strain can be explained, for example, by its slightly delayed attachment to host cells. In our opinion, a single CagA-deficient H. pylori strain that is able to rapidly activate NF-κB with a potency similar to the wild-type strain represents strong evidence against an essential requirement for CagA, irrespective of the genetic background of the strain. Although few studies suggest a contributory role of CagA in late H. pylori-induced NF-κB activation (for example, Suzuki et al 2009), these studies address long-term effects involving indirect processes, but not direct NF-κB activation within the first hour of infection.
Figure 1.
Rapid NF-κB activation by Helicobacter pylori is type IV secretion system (T4SS)-dependent and CagA-independent. AGS cells were infected (MOI of 100) with the P1 H. pylori wild-type (wt) strain, the CagA-deficient (cagA) or T4SS-deficient (virB7) isogenic mutant strains. At the indicated times after infection, whole cell lysates were prepared and analysed by SDS-PAGE and western blotting. For immunodetection, protein-specific or phosphoprotein-specific antibodies (phospho-IκBα Ser32/36; phospho-p65 Ser536) were used, as indicated.
As a possible explanation for the conflicting data, we would like to refer to the publication by Fischer et al (2001). Therein, the authors stated that great care needs to be taken in the generation and propagation of H. pylori isogenic mutant strains to prevent accidental functional inactivation of the PAI concomitant with the deletion of single genes encoded in it: a phenomenon named ‘polar effect'. Functional inactivation of the PAI due to the ‘polar effect' has been documented in the literature (Naumann et al 1999). Thus, complementation of the cagA-deficient H. pylori strains with cagA could have unequivocally proven the suggested function of CagA in NF-κB activation.
We think our comment offers fair, putative reasons for the conflicting data and helps to diminish uncertainty in the scientific community.
Footnotes
The authors declare that they have no conflict of interest.
References
- Censini S et al. (1996) Proc Natl Acad Sci USA 93: 14648–14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W et al. (2001) Mol Microbiol 42: 1337–1348 [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A et al. (2004) Biochem Biophys Res Commun 316: 1065–1072 [DOI] [PubMed] [Google Scholar]
- Lamb A et al. (2009) EMBO Rep 10: 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M et al. (1999) J Biol Chem 274: 31655–31662 [DOI] [PubMed] [Google Scholar]
- Neu B et al. (2002) Am J Gastrointest Liver Physiol 283: G309–G318 [DOI] [PubMed] [Google Scholar]
- Sharma SA et al. (1995) Infect Immun 63: 1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suziki M et al. (2009) Cell Host Microbe 5: 23–34 [DOI] [PubMed] [Google Scholar]