Abstract
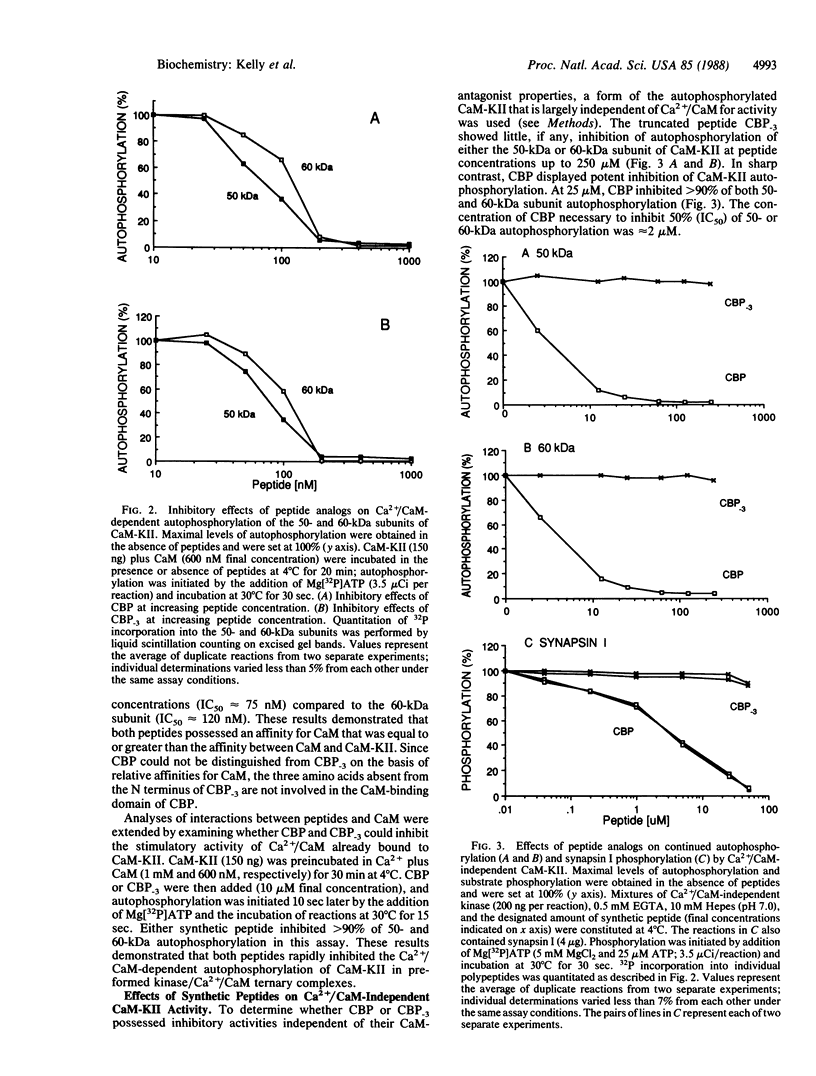
The activation of Ca2+/calmodulin (CaM)-dependent protein kinase II (CaM-KII) by Ca2+/CaM results in autophosphorylation and the generation of Ca2+/CaM-independent enzyme activity. We postulated that CaM binding and subsequent autophosphorylation alters the conformation of CaM-KII and exposes its substrate-binding and catalytic site(s). Previous peptide mapping studies on CaM-KII demonstrated the close proximity of CaM-binding and autophosphorylation domains. Analyses of the deduced amino acid sequences encoding CaM-KII have allowed the identification of its CaM-binding domain and have revealed two consensus phosphorylation sites that flank this regulatory domain. We report herein the distinct properties of two synthetic peptides modeled after the CaM-binding domain of CaM-KII. The first peptide binds CaM in a Ca2+-dependent manner and is an antagonist of CaM-KII activation (IC50 approximately equal to 75 nM). It does not, however, inhibit CaM-KII activity. A second peptide containing the same CaM-binding domain plus a putative autophosphorylation sequence at its N terminus displayed bifunctional regulatory properties. In addition to being a CaM antagonist, the latter was a potent inhibitor of Ca2+/CaM-independent kinase activity (IC50 approximately equal to 2 microM). We suggest that this bifunctional peptide represents an active site-directed inhibitory element of CaM-KII. The separation of CaM antagonist and active site-directed inhibitory properties of this peptide distinguishes CaM-KII from other CaM-dependent enzymes in which bifunctional regulatory properties appear to reside in the same peptide domain. These results indicate that the definition of site-directed inhibitory peptides should, in some cases, be expanded to include bona fide phosphorylation sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Krebs E. G. Inhibition of cyclic AMP-dependent protein kinase by analogues of a synthetic peptide substrate. J Biol Chem. 1978 Dec 25;253(24):8968–8971. [PubMed] [Google Scholar]
- Fukunaga K., Yamamoto H., Matsui K., Higashi K., Miyamoto E. Purification and characterization of a Ca2+- and calmodulin-dependent protein kinase from rat brain. J Neurochem. 1982 Dec;39(6):1607–1617. doi: 10.1111/j.1471-4159.1982.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of a calmodulin in postsynaptic densities. II. Presence of a calmodulin-activatable protein kinase activity. J Cell Biol. 1981 Jun;89(3):440–448. doi: 10.1083/jcb.89.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Montgomery P. R. Subcellular localization of the 52,000 molecular weight major postsynaptic density protein. Brain Res. 1982 Feb 11;233(2):265–286. doi: 10.1016/0006-8993(82)91202-1. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Shenolikar S. Role of autophosphorylation in regulating calmodulin-dependent protein kinases. Methods Enzymol. 1987;139:690–714. doi: 10.1016/0076-6879(87)39121-9. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Yip R. K., Shields S. M., Hay M. Calmodulin-dependent protein phosphorylation in synaptic junctions. J Neurochem. 1985 Nov;45(5):1620–1634. doi: 10.1111/j.1471-4159.1985.tb07235.x. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., Guerriero V., Jr, Bagchi I. C., Means A. R. The calmodulin binding domain of chicken smooth muscle myosin light chain kinase contains a pseudosubstrate sequence. J Biol Chem. 1987 Feb 25;262(6):2542–2548. [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Edelman A. M., Blumenthal D. K., Krebs E. G. Rabbit skeletal muscle myosin light chain kinase. The calmodulin binding domain as a potential active site-directed inhibitory domain. J Biol Chem. 1987 Sep 5;262(25):11958–11963. [PubMed] [Google Scholar]
- Kuret J., Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1985 May 25;260(10):6427–6433. [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Gorelick F., Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of autophosphorylation sites responsible for generation of Ca2+/calmodulin-independence. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5710–5714. doi: 10.1073/pnas.84.16.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou L. L., Lloyd S. J., Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Colbran R. J., Soderling T. R. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986 Jul 5;261(19):8581–8584. [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S. M., Vernon P. J., Kelly P. T. Autophosphorylation of calmodulin-kinase II in synaptic junctions modulates endogenous kinase activity. J Neurochem. 1984 Dec;43(6):1599–1609. doi: 10.1111/j.1471-4159.1984.tb06084.x. [DOI] [PubMed] [Google Scholar]


